Abstract
Aim
To evaluate the efficacy and safety of the restricted protein diet supplemented with keto analogues when applied in end-stage renal disease (ESRD).
Methods
The Cochrane Library, PubMed, Embase, CBM and CENTRAL databases were searched and reviewed up to January 2017. Clinical trials were analyzed using RevMan 5.3 software.
Results
Five randomized controlled trials were selected and included in this study according to our inclusion and exclusion criteria. Changes in serum albumin, PTH, triglyceride, cholesterol, calcium, phosphorus, hemoglobin, Kt/v and CRP before and after treatment were analyzed. Meta-analysis results indicated that, compared with normal protein diet, low-protein diet (LPD) supplemented with keto analogues (sLPD) could improve serum albumin (P < 0.00001), hyperparathyroidism (P < 0.00001) and hyperphosphatemia (P = 0.008). No differences in triglyceride, cholesterol, hemoglobin, Kt/v and CRP were observed between different protein intake groups.
Conclusion
Restricted protein diet supplemented with keto analogues (sLPD) may improve nutritional status and prevent hyperparathyroidism in ESRD patients. The current data were mainly obtained from short-term, single-center trails with small sample sizes and limited nutritional status indexes, indicating a need for further study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
End-stage renal disease (ESRD) represents the final stage of various causes of chronic kidney disease (CKD). With the development of economy, the prevalence of ESRD has received increasing focus as one of the leading public health problems [1,2,3,4]. Renal replacement therapy works as one of the most significant methods for ESRD patients; however, it cannot work as normal renal simulating physiological state absolutely [5]. Evidence tells that most ESRD patients who need maintenance dialysis suffer from malnutrition to some extent. Many researchers have attributed malnutrition to restricted protein diet which tends to moderate the symptoms of azotemia and acid–base disturbances. Some studies support restricted protein diet supplemented with keto analogues in the early stages of CKD patients helps gain smaller possibility of malnutrition in the following stage of maintenance dialysis [6, 7]. We previously conducted a meta-analysis with stage 3–5 CKD patients who did not enter the maintenance dialysis stage, and proved that restricted protein diet supplemented with keto analogues could delay the progression of CKD, prevent hyperphosphatemia, hyperparathyroidism and benefit blood pressure control without causing malnutrition [8]. However, whether it could still be available in ESRD patients remains unknown and that is why we carry out this study.
Methods
Search strategy
A literature search was performed in PubMed, Embase, Cochrane Library, China Biology Medicine (CBM) and China National Knowledge Infrastructure (CNKI). PubMed (1966–January 2017), Embase (1974–January 2017), the Central Register of Controlled Trials (1999–January 2017), the Cochrane Renal Group (1999–January 2017), CBM and CNKI were searched for the identification of relevant trials. The following search terms were used: keto acid, α-keto acid, keto analogue, ESRD (end-stage renal disease), ESKD (end-stage kidney disease), RRT (renal replacement therapy), dialysis, hemodialysis, peritoneal dialysis, low-protein diet, very low-protein diet, LPD, vLPD, sLPD and svLPD.
Inclusion criteria and risk of bias
Articles were selected and subsequently screened based on the patient problem intervention comparison outcome (PICO) principle. Five randomized controlled trials (RCTs) were finally selected. Inclusion criteria were: (1) study subjects were ESRD adults receiving maintenance dialysis therapy; (2) study subjects were treated with LPD/vLPD supplemented with keto analogues or free diet; (3) laboratory test indexes such as serum albumin (Alb), serum triglyceride (TG), cholesterol (CHO), calcium (Ca) and phosphorus (P) were clearly reported; (4) study subjects were followed up for at least 2 months. The exclusion criteria applied were as follow: (1) study subjects were children or animals; (2) old low-quality studies (before 1980); (3) studies without detailed observe indexes; (4) studies whose full texts are unavailable. Full texts of all potential articles were retrieved and reviewed independently by at least 2 investigators. Risk of bias tables recommended by Cochrane network was relied upon to assess the risk of bias.
Data extraction and management
Two reviewers independently assessed the eligibility of each article using standard data extraction forms, and any disagreement was further reviewed by a third party. For studies from which detailed data could not be extracted, the authors were contacted by emails. Basic information such as first author name, year of publication, study design, inclusion criteria, study sample size, basic characteristics of study subjects, intervention regimen, drug dosage, follow-up duration, outcome data and adverse effects was recorded for each study included. All authors reviewed the final list of articles and supervised the overall search process.
Statistical analysis
Meta-analysis was performed using Review Manager 5.3 software. Risk ratios (RR) and 95% CIs were used to express the results of dichotomous outcomes. Mean difference (MD) was used for results with continuous scales, and standardized mean difference (SMD) was used when different scales were used. Heterogeneity was analyzed using Cochran Q test (n – 1 df), with P < 0.05 denoting statistical significance and I 2 measuring the proportion of variation in efficacy estimates due to heterogeneity beyond chance. Random-effects analysis (I 2 > 50%) and fixed-effect analysis (I 2 < 50%) were performed in meta-analysis according to the protocol. Z test was conducted to analyze the overall effect, with P < 0.05 denoting statistical significance.
Study selection and trial characteristics
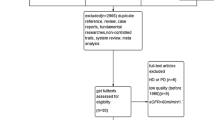
We identified 1790 articles during the first search. Of these, after careful examination of the title and abstract, 1778 articles were excluded because of duplicate references, reviews, case reports, basic researches, non-controlled studies, systematic reviews and meta-analyses. Full texts of the remaining 12 articles were retrieved for further selection. An additional 7 articles were excluded after further review because patients did not receive dialysis therapy in four studies and the other three studies were low quality (before 1980). Eventually, five studies were included in this meta-analysis and systematic review [9,10,11,12,13]. The article search strategy used in our review is described in Fig. 1.
Peritoneal dialysis was performed in the studies of Jiang and Chen [11, 12], and hemodialysis was applied in the other three studies [9, 10, 14]. In addition, protein and keto analogues intake level differed from each study, which may affect heterogeneity of studies. In the study reported by Li, the outcomes of some crucial indexes, such as albumin, calcium, phosphorus, mid-arm muscular circumference, were only shown as histograms without accurate data [13]. We sent an e-mail to the author enquiring the data about histogram, but there is no reply yet. Characteristics of the included studies are listed in Table 1. The risk of bias assessment was performed using a risk of bias table recommended by Cochrane (Table 2).
sLPD would not cause malnutrition in ESRD patients
In order to evaluate the nutritional status of ESRD patients with restricted protein diet, we analyzed nutritional factors in this study. Comparison of serum albumin level (Alb) between sLPD and regular diet group included 4RCTs. Meta-analysis was performed using a fixed-effect model because tests for heterogeneity indicated I 2 = 28%. Result suggested a significantly higher serum albumin level in sLPD group than that in control group (MD 3.84, 95% CI 2.61, 5.06, P < 0.00001). Comparison of cholesterol (CHOL) included 3 RCTs. A fixed-effect model was applied to compare CHOL level in 3RCTs because I 2 = 41%. Result suggested no significance between two groups (MD − 0.21, 95% CI − 0.52, 0.11, P = 0.20). Comparison of triglyceride (TG) included 3 RCTs. The randomized-effects models were used because heterogeneity tests indicated I 2 = 85%. No significant difference was observed between two groups (MD 0.04, 95% CI − 0.28, 0.36, P = 0.81) (Fig. 2). Moreover, Li’s study assessed arm muscle circumferences, skin-fold thicknesses, BMI and Mini-Nutritional Assessment (MNA, Nestlé Nutrition Institute). Results showed that all of these indexes were within the normal range, and no remarkable changes could be observed before and after treatment [13]. In Chen’s study, comparison of mid-arm circumference (MAC) and triceps skin-fold thickness (TSF) revealed no difference between sLPD and control group. However, the level of leptin was decreased in sLPD group after treatment (P < 0.01) which may refer to the reduction in energy wasting and improvement of nutrition status [12].
sLPD could ameliorate MBD in ESRD patients
Two RCTs were included in the comparison of serum phosphorus (P) level between sLPD and regular food treatment groups. Meta-analysis using random-effects models (I 2 = 85%) indicated significantly lower serum phosphorus level in sLPD group (MD − 0.37, 95% CI − 0.64, − 0.09, P = 0.008). Two RCTs involved in the comparison of serum calcium (Ca) level, fixed-effect model (I 2 = 0%) analysis suggested better outcome in sLPD group (MD 0.1, 95% CI 0.09, 0.11, P < 0.00001). Comparison of serum PTH level was performed using random-effects models with 2 RCTs, which showed significant effect of sLPD in preventing hyperparathyroidism (MD − 212.35, 95% CI − 294.28, − 130.42, P < 0.00001) (Fig. 3).
Effects of sLPD on anemia, Kt/v and inflammatory biomarkers in ESRD patients
Assessment of hemoglobin, Kt/v and CRP included 2 RCTs, respectively. No significant difference was observed between sLPD and regular food groups in the present meta-analysis (Fig. 4). Li’s study also suggested that C-reactive protein (hsCRP) was similar between treatment group and control group [13]. Furthermore, in the study by Chen, the comparison of interleukin-1α (IL-1α), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) indicated lower levels in sLPD group, but only the change in CRP level reached statistical significance (P < 0.01) [12]. This result may suggest reduced inflammatory status in sLPD group.
Discussion
Nowadays, ESRD has become one of the most vital threats to health. Not only primary kidney disease induced ESRD, but also obesity, diabetes, hypertension or other cardiovascular disease contribute to the increasing prevalence of ESRD [15, 16]. The adjusted incidence rate of ESRD, which includes both dialysis and transplant patients, remains at 352 per million per year [4]. Moreover, ESRD associated morbidity is rising gradually every year in both developing and developed countries [1,2,3,4, 17]. More and more researches suggested that hyperphosphatemia, CKD-MBD and other complications remarkably increased the mortality of ESRD patients. Considering that application of sLPD/sVLPD in early stage CKD patients could delay the progress of disease and prevent severe complications such as anemia, hypertension, hyperphosphatemia, hyperparathyroidism and malnutrition [8], researchers began to think it may also be helpful for ESRD patients receiving dialysis.
Although it is worried that restricted protein diet may lead to malnutrition because of inadequate nutrient intake, clinical trials and observational studies relieved this problem. In the current study, assessment of serum albumin level (Alb) showed significantly higher level in sLPD group and comparison of cholesterol (CHOL) and triglyceride (TG) showed no difference between two groups, suggesting that sLPD could improve nutritional status of ESRD patients. The mechanism of this effect may lie in the fact that several other factors such as metabolic acidosis, uremic toxin accumulation, chronic inflammation, insulin resistance, hyperparathyroidism and oxidative stress are also contributive to ESRD-related PEW (protein-energy wasting) [6, 18]. Several investigations have pointed that restricted protein diet may reduce amino acid oxidation, decrease protein degradation and prevent metabolic acidosis. Therefore, it is helpful to maintain a better nutrition status and prevent PEW in dialysis patients [19,20,21,22,23]. It was previously reported that sLPD treatment led to clear amelioration of protein synthesis in peritoneal dialysis (PD) patients and maintained lower transport rates [24,25,26].Hence, several studies suggested that sLPD is safe in CKD patients and would not increase mortality [7, 26].
Hyperphosphatemia is the leading cause of CKD-MBD and is associated with increased risk of cardiovascular diseases as well as mortality rates in CKD patients [14, 27]. Our earlier study reported that restricted protein diet supplemented with keto analogues could decrease serum phosphorus and PTH levels in patients of stage 3–5 CKD [8]. For ESRD patients undergoing maintenance dialysis treatment, the current study confirms that sLPD could be beneficial to mineral bone disease by decreasing serum phosphorus, PTH level and avoiding hypocalcemia. Long-term prospective cohort study also indicated that sLPD may improve the primary outcomes of native AVF, reduce the vascular stiffness and calcification, possibly by preserving vascular wall quality through a better serum phosphorus and inflammatory reaction control [28].
Although some cohort studies indicated that sLPD may ameliorate anemia, improve dialysis adequacy and inflammatory status [12, 29], our meta-analysis does not show apparent differences between protein restrict group and regular food group. It was previously reported that sLPD could prevent the loss of muscle mass, block the activation of autophagy/mitophagy and decrease inflammation in skeletal muscle in 5/6 nephrectomized rats [30]. Based on this evidence, it is suspected that suboptimal adherence and low patient compliance may be the reasons of compromised nutrition status observed in several studies.
Furthermore, it was reported that sLPD was safe in pregnant patients with advanced CKD and may improve fetal outcomes [31]. Several small sample case reports have suggested that restricted protein diets may favor chronic dialysis discontinuation [32], although the mechanism was still unknown.
Our study had several limitations. The data analyzed in the present meta-analysis were obtained from short-term, small sample sizes, single-center studies. Only 5 RCTs were included in this meta-analysis. And we failed to obtain individual patient and original data, some nutritional status indexes like BMI, mid-arm circumference (MAC) and triceps skin-fold thickness (TSF) were only in one study and some data were presented only as histograms, which may compromise our results. Additionally, our meta-analysis contained heterogeneity in actual protein intake levels which may have an impact on the reliability of our results. Therefore, long-term, large sample, multicenter RCTs are needed to confirm the efficacy and safety of restricted protein diet in ESRD patients.
Conclusion
Our meta-analysis indicated that restricted protein diet supplemented with keto analogues may improve nutritional status and prevent hyperparathyroidism in ESRD patients. However, long-term, large sample, multicenter RCTs are needed to confirm these results in the future.
References
Imai E, Matsuo S (2008) Chronic kidney disease in Asia. The Lancet 371(9631):2147–2148
Kovesdy CP, Kalantar-Zadeh K (2012) Enter the dragon: a Chinese epidemic of chronic kidney disease? The Lancet 379(9818):783–785
Zhang L et al (2012) Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 379(9818):815–822
Saran R et al (2016) US renal data system 2015 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis 67(3 Suppl 1):Svii, S1–Svii S305
Wanner C, Amann K, Shoji T (2016) The heart and vascular system in dialysis. Lancet 388(10041):276–284
Sabatino A et al (2017) Protein-energy wasting and nutritional supplementation in patients with end-stage renal disease on hemodialysis. Clin Nutr 36(3):663–671
Bellizzi V et al (2015) Very low-protein diet plus ketoacids in chronic kidney disease and risk of death during end-stage renal disease: a historical cohort controlled study. Nephrol Dial Transplant 30(1):71–77
Jiang Z et al (2016) Effect of restricted protein diet supplemented with keto analogues in chronic kidney disease: a systematic review and meta-analysis. Int Urol Nephrol 48(3):409–418
Liang J et al (2015) The Effect of α-Ketoacid in Treating Dialysis patients. Chin J of Clin Ration Drug Use 8(5C):120–121 (article in Chinese)
Zhou S et al (2013) Effect of low protein diet combined a-keto acid on calcium and phosphorus metabolism in patients with maintenance hemodialysis. J Clin Med Pract 17(9):111–113 (article in Chinese)
Jiang N et al (2009) Better preservation of residual renal function in peritoneal dialysis patients treated with a low-protein diet supplemented with keto acids: a prospective, randomized trial. Nephrol Dial Transplant 24(8):2551–2558
Chen W et al (2008) Effects of low-protein diet plus α-keto acid on micro-inflammation and the relationship between micro-inflammation and nutritional status in patients performing continuous ambulatory peritoneal dialysis: a randomized controlled trial. J Chin Integr Med 6(5):473–477
Li H et al (2011) Effect of short-term low-protein diet supplemented with keto acids on hyperphosphatemia in maintenance hemodialysis patients. Blood Purif 31(1–3):33–40
Chen J (2013) Nutrition, phosphorus, and keto-analogues in hemodialysis patients: a Chinese perspective. J Ren Nutr 23(3):214–217
Liyanage T et al (2015) Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet 385(9981):1975–1982
Murray CJ et al (2013) The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA 310(6):591–608
Robinson BM et al (2016) Factors affecting outcomes in patients reaching end-stage kidney disease worldwide: differences in access to renal replacement therapy, modality use, and haemodialysis practices. The Lancet 388(10041):294–306
Muscaritoli M et al (2009) Malnutrition and wasting in renal disease. Curr Opin Clin Nutr Metab Care 12(4):378–383
Garibotto G et al (2010) Amino acid and protein metabolism in the human kidney and in patients with chronic kidney disease. Clin Nutr 29(4):424–433
Malvy D et al (1999) Effects of severe protein restriction with ketoanalogues in advanced renal failure. J Am Coll Nutr 18(5):481–486
Cianciaruso B et al (2008) Metabolic effects of two low protein diets in chronic kidney disease stage 4–5—a randomized controlled trial. Nephrol Dial Transplant 23(2):636–644
Bernard S et al (1996) Effects of low-protein diet supplemented with ketoacids on plasma lipids in adult chronic renal failure. Miner Electrolyte Metab 22(1–3):143–146
Kalantar-Zadeh K et al (2015) Dietary restrictions in dialysis patients: is there anything left to eat? Semin Dial 28(2):159–168
Jiang N et al (2010) Improved plasma amino acids pattern following 12 months of supplemented low-protein diet in peritoneal dialysis patients. Ren Fail 32(6):709–715
Jiang N et al (2011) Low-protein diet supplemented with keto acids is associated with suppression of small-solute peritoneal transport rate in peritoneal dialysis patients. Int J Nephrol 2011:542704
Duenhas M et al (2013) Reduction of morbidity related to emergency access to dialysis with very low protein diet supplemented with ketoacids (VLPD + KA). Clin Nephrol 79(5):387–393
Shaman AM, Kowalski SR (2016) Hyperphosphatemia management in patients with chronic kidney disease. Saudi Pharm J 24(4):494–505
David C et al (2016) Very low protein diets supplemented with keto-analogues in ESRD predialysis patients and its effect on vascular stiffness and AVF Maturation. BMC Nephrol 17(1):131
Dong J et al (2015) Ketoacid supplementation partially improves metabolic parameters in patients on peritoneal dialysis. Perit Dial Int 35(7):736–742
Zhang YY, Huang J, Yang M, Gu LJ, Ji JY, Wang LJ, Yuan WJ (2015) Effect of a low-protein diet supplemented with keto-acids on autophagy and inflammation in 5/6 nephrectomized rats. Biosci Rep 35(5). doi:10.1042/BSR20150069
Attini R et al (2016) Vegan-vegetarian low-protein supplemented diets in pregnant CKD patients: fifteen years of experience. BMC Nephrol 17(1):132
Piccoli GB et al (2014) Tailoring dialysis and resuming low-protein diets may favor chronic dialysis discontinuation: report on three cases. Hemodial Int 18(3):590–595
Author information
Authors and Affiliations
Contributions
Wei Qin planned the study, analyzed data and assisted in article preparation. Zheng Jiang and Yi Tang searched the literature, selected articles, extracted data, analyzed data and composed the article. Lichuan Yang and Xuhua Mi contributed to the concept, data collection and analysis of this study.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Jiang, Z., Tang, Y., Yang, L. et al. Effect of restricted protein diet supplemented with keto analogues in end-stage renal disease: a systematic review and meta-analysis. Int Urol Nephrol 50, 687–694 (2018). https://doi.org/10.1007/s11255-017-1713-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-017-1713-9