Abstract
The aim of this study was to investigate the effects of Artemisia princeps (AP) extract on bone metabolism and its potential role in the prevention of osteoporosis in ovariectomized rats. Twenty-six female Sprague-Dawley rats were divided into five groups and treated as follows: sham-operated control group (SHAM); ovariectomized control group (OVX), ovariectomized group treated by gavage with 10 mg/kg/day alendronate (ALEN); ovariectomized group treated by gavage with 100 mg/kg/day Artemisia princeps (AP100); ovariectomized group treated by gavage with 300 mg/kg/day Artemisia princeps (AP300). Treatment of ovariectomized rats with AP extracts for 15 weeks prevented the reduction in bone thickness and trabecular bone mineral density caused by urinary Ca and Cr excretion, and also prevented the increase in bone turnover by maintaining the serum Ca/P ratio. As a result, the microarchitecture of the trabecular bone and cortical bone after ovariectomy was markedly improved by administration of AP extracts. In conclusion, AP prevented bone loss and osteoclast activity associated with high bone turnover in ovariectomized rats by controlling the serum Ca/P ratio and through anti-inflammatory and anti-oxidant properties. Our data implicate AP as a promising therapeutic option for the improvement of postmenopausal osteoporosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is characterized by a reduction in bone strength and an increased risk for low-trauma fracture and is one of the most common public health problems worldwide (1). In particular, postmenopausal osteoporosis is considered a major concern for women (2-3). Postmenopausal women are at a higher risk of developing osteoporosis than men because estrogen deficiency is considered to be an important cause of pathological bone loss after menopause (4). Deficiency of estrogen is reported to accelerate bone turnover, with the rate of osteoclastic bone resorption exceeding that of osteoblastic regeneration, thus causing an imbalance between bone formation and resorption (5). Complex metabolic and biochemical changes lead to a dramatic reduction in bone mass after menopause, which is related to an increased risk of fragility fractures (6-7). Recently, many studies have shown that oxidative stress (OS) plays a role in the pathogenesis of osteoporosis, and several risk factors for osteoporosis, such as aging, abdominal obesity, diabetes, and cardiovascular diseases, are associated with high levels of OS (8-9). Low bone mineral density (BMD) is associated with increased reactive oxygen species (ROS) production, especially in postmenopausal osteoporotic women because of the deficiency in estrogen (10). A decline in estrogen levels leads to loss of protective action against OS and ROS, which is followed by depletion of antioxidant enzymes. The consequent increase in OS and ROS suppresses bone formation by inhibiting osteoblast differentiation and decreasing the survival of these cells, and stimulates osteoclastic activity, ultimately leading to development of osteoporosis (11). The relationship between bone mass and ROS has been investigated through clinical research. An increase in superoxide formation by osteoclasts that accompanies the activated bone resorption and loss of bone density was shown to be a common factor in the pathogenesis of osteoporosis (12). Alendronate, a widely used anti-osteoporotic agent, has been shown to increase bone formation and BMD, with concurrent improvement in OS markers of osteoporotic fractures in mice and in postmenopausal women (13). Estrogen replacement therapy (ERT) has also been shown to play an important beneficial role in the improvement of anti-oxidative activity and prevention of bone loss (14). However, it is difficult for menopausal women to comply with long-term ERT because it causes side effects including nausea, headaches, breast tenderness, a rise in blood pressure, and weight gain (15). Moreover, when ERT is stopped the bone loss is likely to increase again at the same rate as immediately after menopause. In recent years, there has been a trend towards studying herbal foods related to bone metabolism and their anti-oxidative effects (16).
Artemisia princeps (AP), a member of the composite family of plants, has been widely used as a traditional medicinal herb in Korea, China, and Japan for the treatment of various symptoms including vomiting, diarrhea, and irregular uterine bleeding (17-18). Above all, through in vitro and in vivo experiments anti-infammatory activities and anti-oxidant properties on flavonoids isolated from AP are being studied. Jaceosidin, eupatilin, eupafolin and apigenin, which are flavonoid components of AP, are known to have pharmacological properties; anti-diabetic, anti-allergic, anti-inflammatory, anti-atherosclerosis, anti-oxidant, and anticancer (19-21). In addition, recent studies have reported the use of Artemisia species as a supplement for wound healing and for bone and cartilage regeneration (22-24). Based on this information, the aim of this study was to evaluate whether supplementation with AP extracts has beneficial effects on bone metabolism and bone biomarkers using ovariectomized rats as a model for postmenopausal women.
Methods and materials
Preparation of Artemisia princeps
Artemisia princeps (AP) was obtained from a local supplier (MANI F&B, Incheon, Korea). A mixture of raw plant material and ethanol (1:20) was sonicated in an ultrasonic bath (3210, Branson Ultrasonics, Connecticut, USA) at 60 kHz for 1 hour at 60°C and filtered by vacuum filtration. Ethanol was added to the residue, which was subjected to another round of extraction and filtration. This procedure was repeated two more times. Distilled water was added to the final residue, followed by further sonication and filtration. The resultant AP extract was concentrated in an evaporator (Rotatory evaporator, N-1N, EYELY Sunil, Korea) and freeze-dried (FD8512, Ilshin, Korea).
Animal Models and Experimental Diet
All experimental procedures were approved by and conducted in accordance with guidelines of the Animal Care and Use Committee of Kyung Hee University (KHUASP (SU) 13-01).
Twenty-six Sprague-Dawley rats (5-week-old females; body weight, 152.8 ± 3.9 g) were purchased from SLC, Inc. (Shinzuoka, Japan) and housed in cages with climate control to maintain an adequate temperature (22 ± 2°C), photocycle (12-h light: 12-h dark), and relative humidity of 60% with 24-h air circulation via forced ventilation. Pellet chow diet and water were provided ad libitum during a 1-week acclimatization period. After this period, the rats were randomly divided into five groups as follows: sham-operated control (SHAM, n = 4); ovariectomized control (OVX, n = 5); ovariectomized group treated by gavage with 10 mg/kg/day alendronate (ALEN, n = 5); ovariectomized group treated by gavage with 100 mg/kg/day Artemisia princeps (AP100, n = 6); ovariectomized group treated by gavage with 300 mg/kg/day Artemisia princeps (AP300, n = 6). Animals in the SHAM and OVX control groups were given an oral injection of distilled water. Rats in the ovariectomized groups were anesthetized using isoflurane inhalation (3% dissolved in oxygen) and bilateral ovariectomy was performed via a single longitudinal skin incision on the dorsal midline at the level of the kidneys. The ovaries were ligated and removed. The sham group underwent a similar surgery in which the ovaries were exposed but left intact.
After a recovery period of at least 7 days, the experimental diet (AIN 93G pellets, USA) was provided with water ad libitum during a 12-week preliminary period. This was followed by a 15-week experimental period, during which the rats were given distilled water or the appropriate test agent by oral gavage. At the end of the experimental period, the rats were fasted overnight for 12 h and sacrificed under anesthesia with ethyl ether. Blood was collected by cardiac puncture into serum-separating tubes. Serum was obtained by centrifugation at 3,000 rpm for 15 min at 4°C, and stored at -70°C until required for analysis.
Body Weight and Food Consumption
Food consumption and weight gain of the rats were measured weekly using a balance. During the 15-week experimental period, body weight was measured twice a week and food intake was measured daily. The food efficiency ratio (FER) was calculated using the following equation: [gain of body weight (g)/ day]/ [amount of food consumed (g)/ day].
Blood Analysis
Serum calcium (Ca) and phosphorus (P) levels were determined using a chemistry auto-analyzer (ADVIA 1650, Bayer, Japan). Serum superoxide dismutase (SOD) levels were measured by enzyme-linked immunosorbent assay (ELISA) using a SIRIO-S microplate photometer at 450 nm/ 620 nm. Serum pro-inflammatory cytokines IL-1, IL-6 and TNF-α were measured using a Millipore’s MILLIPLEX rat cytokine panel (Millipore).
Urine Analysis
Prior to termination of the study, the rats were transferred to individual metabolic cages for urine collection over the 12-h fasting period. During this time the rats had free access to water but food was withheld. Instruments used for collection of urine were washed with 0.1 N HCl. Urine samples were centrifuged at 2,000 rpm for 15 min at room temperature and the upper layer was collected and stored at -70°C until analysis. Urinary Ca was determined using a chemistry auto-analyzer (ADVIA 1650, Bayer, Japan). Urinary creatinine, which is a useful marker of bone resorption, was measured using an ELISA kit based on collagen cross-links™ (Metra Biosystems Inc., Mountain View, CA, USA). N-terminal telopeptide of type-I collagen (NTx), a marker of bone turnover, was measured by ELISA using a commercial kit (Osteomark, Wampole Laboratories, USA).
Histomorphometric Analysis by Micro-Computed Tomography
Histomorphometric analysis of the tibia was performed using a SkyScan 1076 (SkyScan, Kontich, Belgium) micro-computed tomography (μ-CT or micro-CT) system. The cortical and trabecular microstructure and 3D images of the left tibiae were scanned at 50 kV and 200 μA with a rotation step of 0.4°. During the analysis, zoletil 50 (Virbac) and rompun (Bayer) were administered intraperitoneally to anesthetize the animals. Analyses of the reconstructed scans were performed using NRecon cone-beam algorithm software (SkyScan), and the files were imported into CTAn software (SkyScan) for 3D analysis and image generation.
Statistical Analysis
Statistical analysis was carried out using SPSS software (Version 21.0, SPSS INC., Chicago, IL, USA). All data were expressed as mean ± standard deviation (SD), and the Duncan’s multiple range test was used to compare different groups. Values of p<0.05 were considered significant.
Results
Body Weight, Weight Gain, Food Intake, and Food Efficiency Ratio
The mean values of body weight, food intake, and food efficiency ratio (FER) are shown in Table 1.
At the start of the experiment, initial body weights were not different among the groups. However, at 4 weeks after ovariectomy, the body weights of OVX rats were significantly increased compared with those of the SHAM group, and this significance was maintained throughout the experimental period (p<0.05). As a result, the weight gain of ovariectomized groups was significantly higher than that of the SHAM group (p<0.05), whereas the weight gain among the ovariectomized groups was not significantly different. The mean values of food intake and FER were significantly lower in the SHAM group than in all other groups (p<0.05). In addition, treatment of ovariectomized rats with AP100 tended to result in a decrease in food intake compared with the other OVX groups.
Effect of AP on Serum and Urinary Biochemistry in Experimental Animals
As shown in Table 2, the serum levels of Ca were generally similar among all groups. The OVX groups tended to show a decrease in serum levels of Ca compared with the SHAM group (p<0.05), but the serum levels of Ca in all groups were within the normal range (7.2–13.0 mg/dl). The serum level of P in the OVX group was significantly lower than the levels in all other groups (p<0.05), which were in the normal range (3.11–11.0 mg/dl) (25). The serum Ca/P ratio in the OVX group was significantly higher than that in all other groups (p<0.05).
The urinary NTx was not significantly different among the five groups, although the SHAM and OVX+treatment (ALEN, AP100, and AP300) groups tended to show decreased urinary NTx compared with the OVX group. The urinary Ca of all OVX+treatment groups was significantly reduced (p<0.05). In addition, the SHAM group had slightly lower urinary Ca than the OVX group, but the difference was not significant. The urinary creatinine of the OVX group was significantly higher than that of all other groups (p<0.05).
Effect of AP on the Antioxidant Enzyme SOD in Experimental Animals
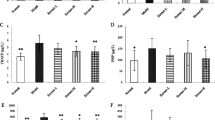
As shown in Fig. 1, the serum levels of SOD were highest in the OVX group and lowest in the SHAM and ALEN groups (p<0.05). In addition, the serum levels of SOD in the AP groups (AP100, AP300) were lower than that in the OVX group, but there was no difference between the AP100 and AP300 groups.
Effect of AP on Pro-inflammatory Cytokines in Experimental Animals
As shown in Table 3, the serum levels of pro-inflammatory cytokines IL-1, IL-6, and TNF-α were decreased in the rats treated with AP among all groups. In particular, AP300 treatment significantly reduced the adverse effects of ovariectomy (p<0.05). The serum level of pro-inflammatory cytokines in the ALEN group was significantly higher than the levels in all other groups (p<0.05).
Effect of AP on Histomorphometric Analysis by Micro- Computed Tomography
Bone remodeling is a collective term for the continual counter-balanced processes of bone formation and bone resorption. Bone remodeling activity can be monitored by static histomorphometric analysis (26). To estimate the effects of AP on bone remodeling activity, we analyzed bone histomorphometric indices including bone thickness and BMD in all rats. As shown in Fig. 2, the ratio values per weight for bone thickness and BMD were generally lower in OVX groups compared to the SHAM group. In particular, BMD in the cortical region was significantly reduced in rats with estrogen deficiency induced by ovariectomy. However, bone thickness and trabecular BMD were increased in the rats treated with AP compared with the OVX group. In particular, AP300 treatment significantly reversed the effects of ovariectomy on histomorphometric indices. As shown in Fig. 3, the microarchitecture of the animal trabecular bone and cortical bone was markedly improved by administration of AP after ovariectomy.
Discussion
Our hypothesis was that AP administered via gavage over a 15 week period would have positive effect on bone metabolism, and consequently helps to prevent osteoporosis in ovariectomized rats. We used OVX rats which its ovarian hormone has been removed, to investigate the anti-osteoporosis effect of AP. Treatment of ovariectomized rats with AP extracts for 15 weeks prevented the reduction in bone thickness and trabecular BMD caused by urinary Ca and Cr excretion, and also prevented the increase in bone turnover by maintaining the serum Ca/P ratio. As a result, the microarchitecture of the trabecular bone and cortical bone was markedly improved by administration of AP extracts (Fig. 3).
Oxidative stress (OS) develops in postmenopausal osteoporotic women as a result of estrogen deficiency. The relationship between osteoporosis and OS has been examined in many studies (27-29). The results of the earlier studies on animals showed that osteoclastic functions and differentiations were stimulated by the reactive oxygen species (ROS), especially H2O2 and O2. Oxygen radicals, which appear on cell levels are detoxified by the enzyme superoxide dismutase (SOD). The mechanism of SOD effect is supposed to prevent hematopoietic progenitor cells from death (30-31). In this study, activation of the antioxidant enzyme SOD was significantly increased in the OVX group, consistent with results previously reported in patients suffering from rheumatic diseases and osteoporosis (32-33). Overexpression of SOD is implied as a compensatory action during OS (34) proved by many researchers including Ostalowska et al. (2006). The authors showed increased activity of SOD in the synovial fluid of patients with primary and secondary osteoarthritis of the knee joint. In recent studies, eupatilin, the primary active component of AP, suppressed the expression of inflammatory cytokines and the differentiation of osteoclast in collagen induced arthritis mice (35). In addition, incubation of bone-marrow derived monocytes with eupatilin inhibited their differentiation into osteoclast when these cells were treated (23, 36). Overall findings of previous studies on rat models of osteoporosis indicate that AP extracts improve the collagen content of the bone tissue and the quality of cartilage (37). As in previous reports, the AP-treated groups in our study showed significant improvement in the bone histomophological parameters of the tibia after ovariectomy. Histomorphometric analysis and 3D images from micro-CT revealed that trabecular and cortical bone in AP groups was restored to normal levels compared with the OVX group. The results of the present study are consistent with an earlier report of Kim (2006) showing that AP has protective effects against bone loss in ovariectomized rats.
Serum Ca and P levels are widely accepted as biomarkers for bone turnover (38). The observed effects of AP on bone regeneration could be mediated by regulating Ca/P homeostasis. Under stable conditions, serum Ca/P concentrations have a direct influence on bone formation. Thus, in addition to ovary hormone, calcium metabolism plays an important role in bone turnover, and imbalance of Ca/P ratio leads to impair bone deposition (39). Our data proved that ovariectomy led to hypophosphatemia in the OVX rats. Hypophosphatemia can easily cause a defect in the cartilage and bone formation (40-41). Additionally, as expected, calcium imbalance and osteoporosis was documented in the ovariectomized rats (42-43). Our findings suggest that AP can play a crucial role in the regulation of bone mass by maintaining the balance between serum Ca and P levels. Especially, the serum Ca/P ratio, which is used as a clinical marker for defects in bone formation, was high in the OVX group because of an increase in bone resorption. Thus, we suggest that supplementation with AP in ovariectomized rats may help to maintain a suitable balance of serum Ca and P for bone mineralization, and may reduce the high rate of bone turnover. Indeed, after a relatively long treatment period (15 weeks), bone density analysis showed that AP extract had a bone-sparing effect. To ascertain whether bone loss was an inevitable consequence of marked changes in bone turnover, we evaluated specific urinary biochemical markers of bone turnover, 12-h urinary NTx, Ca and Cr. As expected, AP effectively prevented the ovariectomy-induced urinary excretion of Ca and Cr. Our findings indicate that reabsorption of Ca was stimulated in OVX treatment groups leading to a decrease in urinary Ca excretion.
One likely reason for this improvement in bone loss is the decrease in pro-inflammatory markers. The loss of estrogen accelerates the formation of osteoclasts and osteoblasts in the bone marrow by increasing the production and activity of pro-inflammatory cytokines, including IL-6, TNF-α, and IL-1 (44-45). Similar to previous reports, the current study found that expression of the pro-inflammatory cytokines IL-6, TNF-α, and IL-1 was significantly increased in the OVX group. Interestingly, the OVX and ALEN groups showed similar results for pro-inflammatory cytokines. Several reports have warned of the potential side effects from long-term use of alendronate (46). Previous studies have shown similar increases in IL-6, and TNF-α after administration of bisphosphonate (47-49). The increased secretion of IL-1, IL-6, and TNF-α observed in the ALEN group over a relatively long experimental period in our study may explain the potential side effects of alendronate. On the other hand, AP effectively prevented the increase in pro-inflammatory IL-1, IL-6 and TNF-α in ovariectomized rats. As previously observed in similar studies, AP had a positive effect on bone mineralization by reducing the expression of pro-inflammatory cytokines, and by increasing bone density and decreasing bone reabsorption markers (50). This suggests that AP might prevent the side effects of long-term use of alendronate in postmenopausal osteoporotic women.
Most of the findings in the previous study that dealt with the bone biomarker stimulating effect of AP were based on in vitro and in vivo (19-21). The results provided considerable evidence that AP extract have a potential effect to improve bone biomarker. Only a few investigations have been performed as short-term experiments in rats (20, 35, 36, 50). The main strength of this study on the anti-osteoporosis effect of AP is their ability to explain relatively long-term experimental period and the multifaceted intervention. However, it remains unclear whether the activities of AP were caused by a single component or interaction effects. In conclusion, AP prevented bone loss and reduced osteoclast activity and high bone turnover in ovariectomized rats by controlling the serum Ca/P ratio and through anti-inflammatory and the anti-oxidant properties. Our findings suggest that AP might be a promising therapeutic option for the improvement of postmenopausal osteoporosis.
Acknowledgment: This work was supported by Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ009826), Rural Development Administration, Republic of Korea.
Ethical standard: This experiment complies with current laws of the country in which they were performed.
Conflict of interest: The authors declare no conflict of interest.
References
Baron R, Hesse E. Update on bone anabolics in osteoporosis treatment: rationale, current status, and perspectives. J Clin Endocrinol Metab 2012;97(2):311–325.
Dixon, J. M. Hormone replacement therapy and the breast. BMJ 2001;323(7326):1381–1382.
Marcus, R. Post-menopausal osteoporosis. Best Pract Res Clin Obstet Gynaecol 2002;16(3):309–327.
Coxam V. New advances in osteoporosis nutritional prevention. Med Sci(Paris) 2005;21(3):297–301.
Dempster, D. W., & Lindsay, R. Pathogenesis of osteoporosis. Lancet 1993;341(8848):797–801.
Cooper, C., & Aihie, A. Osteoporosis. Baillieres Clin Rheumatol 1995;9(3):555–564.
Riggs, B. L., Jowsey, J., Kelly, P. J., Jones, J. D., & Maher, F. T. Effect of sex hormones on bone in primary osteoporosis. J Clin Invest 1969;48(6):1065–1072.
Park SR, Oreffo RO, Triffitt JT. Interconversion potential of cloned human marrow adipocytes in vitro. Bone 1999;24(6):549–54.
Pittenger, M. F., Mackay, A. M., Beck, S. C., Jaiswal, R. K., Douglas, R., Mosca, J. D., Marshak, D. R. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284(5411):143–147.
Chan KA, Andrade SE, Boles M, Buist DS, Chase GA, Donahue JG, Goodman MJ, Gurwitz JH, LaCroix AZ, Platt R. Inhibitors of hydroxymethylglutaryl-coenzyme A reductase and risk of fracture among older women. Lancet 2000;355(9222):2185–8.
Edwards CJ, Hart DJ, Spector TD. Oral statins and increased bone-mineral density in postmenopausal women. Lancet 2000;355(9222):2218–9.
Meier CR, Schlienger RG, Kraenzlin ME, Schlegel B, Jick H. HMG-CoA reductase inhibitors and the risk of fractures. JAMA 2000;283(24):3205–10.
Lawrence M, Jones L, Lancaster T, Daly E, Banks E. Hormone replacement therapy: patterns of use studied through British general practice computerized records. Fam Pract 1999;16(4): 335–42.
Boulbaroud, S., Mesfioui, A., Arfaoui, A., Ouichou, A., & el-Hessni, A. Preventive effects of flaxseed and sesame oil on bone loss in ovariectomized rats. Pak J Biol Sci 2008;11(13):1696–1701.
Schmidt JW, Wollner D, Curcio J, Riedlinger J, Kim LS. Hormone replacement therapy in menopausal women: Past problems and future possibilities. Gynecol Endocrinol 2006;22(10):564–77.
Liu HC, Chen RM, Jian WC, Lin YL. Cytotoxic and antioxidant effects of the water extract of the traditional Chinese herb gusuibu (Drynaria fortunei) on rat osteoblasts. J Formos Med Assoc 2001;100 (6):383–8.
Geissman TA. Sesquiterpenoid lactones of Artemisia species. I. Artemisia princeps pamp. J Org Chem 31(8):2523-6.
Yamada H, Ohtani K, Kiyo1966;hara H, Cyong JC, Otsuka Y, Ueno Y, Omura S. Purification and chemical properties of anti-complementary polysaccharide from the leaves of Artemisia princeps. Planta Med 1985;(2):121–5.
Kim MJ, Kim DH, Na HK, Oh TY, Shin CY, Surh Ph D Professor YJ. Eupatilin, a pharmacologically active flavone derived from Artemisia plants, induces apoptosis in human gastric cancer (AGS) cells. J Environ Pathol Toxicol Oncol 2005;24(4):261–9.
Boudjelal A, Siracusa L, Henchiri C, Sarri M, Abderrahim B, Baali F, Ruberto G. Antidiabetic Effects of Aqueous Infusions of Artemisia herba-alba and Ajuga iva in Alloxan-Induced Diabetic Rats. Planta Med 2015;81(9):696–704.
Kim MJ, Han JM, Jin YY, Baek NI, Bang MH, Chung HG, Choi MS, Lee KT, Sok DE, Jeong TS. In vitro antioxidant and anti-inflammatory activities of Jaceosidin from Artemisia princeps Pampanini cv. Sajabal. Arch Pharm Res 2008;31(4):429–437.
Hwang KE, Choi YS, Choi SM, Kim HW, Choi JH, Lee MA, Kim CJ. (2013) Antioxidant action of ganghwayakssuk (Artemisia princeps Pamp.) in combination with ascorbic acid to increase the shelf life in raw and deep fried chicken nuggets. Meat Sci 2013;95(3):593–602.
Lotfy AA, Ghanem LY, Shennawy AM, Gomaa NA, El-Said HH. Assessment of the toxicity of Artemisia inculta extract on the bone marrow of mice infected with schistosomiasis. Arzneimittelforschung 2006;56(2):104–7.
Ryu R, Jung UJ, Kim HJ, Lee W, Bae JS, Park YB, Choi MS. Anticoagulant and Antiplatelet Activities of Artemisia princeps Pampanini and Its Bioactive Components. Prev Nutr Food Sci 2013;18(3):181–7.
Petterino C, Argentino-Storino A. Clinical chemistry and haematology historical data in control Sprague-Dawley rats from pre-clinical toxicity studies. Exp Toxicol Pathol 2006;57(3): 213–9.
Hornby SB, Evans GP, Hornby SL, Pataki A, Glatt M, Green JR. Long-term zoledronic acid treatment increases bone structure and mechanical strength of long bones of ovariectomized adult rats. Calcif Tissue Int 2003;72(4):519–27.
Salim A, Nacamuli RP, Morgan EF, Giaccia AJ, Longaker MT. Transient changes in oxygen tension inhibit osteogenic differentiation and Runx2 expression in osteoblasts. J Biol Chem 2004;279(38): 40007–16.
Galli F, Piroddi M, Annetti C, Aisa C, Floridi E, Floridi A. Oxidative stress and reactive oxygen species. Contrib Nephrol 2005;149:240–60.
Kim HJ, Chang EJ, Kim HM, Lee SB, Kim HD, Su Kim G, Kim HH. Antioxidant alpha-lipoic acid inhibits osteoclast differentiation by reducing nuclear factor-kappaB DNA binding and prevents in vivo bone resorption induced by receptor activator of nuclear factor-kappaB ligand and tumor necrosis factor-alpha. Free Radic Biol Med 2006;40(9):1483–93.
A. Petkau. Protection of bone marrow progenitor cells by superoxide dismutase. Mol Cell Biochem 1988;84(2):133–40.
Michael J. Smietana, Ellen M. Arruda, John A. Faulkner, Susan V. Brooks, and Lisa M. Larkin. Reactive Oxygen Species on Bone Mineral Density and Mechanics in Cu, Zn Superoxide Dismutase (Sod1) Knockout Mice. Biochem Biophys Res Commun 2010;403(1):149–153.
Colla S, Zhan F, Xiong W, Wu X, Xu H, Stephens O, Yaccoby S, Epstein J, Barlogie B, Shaughnessy JD. The oxidative stress response regulates DKK1 expression through the JNK signaling cascade in multiple myeloma plasma cells. Blood 2007;109(10):4470–7.
Ostalowska A, Birkner E, Wiecha M, Kasperczyk S, Kasperczyk A, Kapolka D, Zon-Giebel A. Lipid peroxidation and antioxidant enzymes in synovial fluid of patients with primary and secondary osteoarthritis of the knee joint. Osteoarthritis Cartilage 2006;14(2):139–45.
Maneesh M, Dutta S, Chakrabarti A, Vasudevan DM. Alcohol abuse-duration dependent decrease in plasma testosterone and antioxidants in males. Indian J Physiol Pharmacol 2006;50(3):291–6.
Kim J, Kim Y, Yi H, Jung H, Rim YA, Park N, Jung SM, Park SH, Ju JH. Eupatilin ameliorates collagen induced arthritis. J Korean Med Sci 2015;30(3):233–9.
Lee JA, Sung HN, Jeon CH, Gill BC, Oh GS, Youn HJ, Park JH. AIP1, a carbohydrate fraction from Artemisia iwayomogi, modulates the functional differentiation of bone marrow-derived dendritic cells. Int Immunopharmacol 2008;8(4):534–41.
Kim MH. The Effects of Artemisia Princeps var. Orientalis Extracts on Serum Lipids and Connective Tissues Collagen in Ovariectomized Rats. Korean J Pharmacogn 2006;37(4):324–330.
de Toro Salas A, Dueñas Díez J, de Jaime Revuelta E. Concentrations of calcium and bone remodeling biomarkers in umbilical cord blood and urine of newborn infants during delivery. An Esp Pediatr 2001;54(3):290–6.
Argenzio RA, Lowe JE, Hintz HF, Schryver HF. Calcium and phosphorus homeostasis in horses. J Nutr 1974;104(1):18–27.
Yoshiko Y, Candeliere GA, Maeda N, Aubin JE. Osteoblast autonomous Pi regulation via Pit1 plays a role in bone mineralization. Mol Cell Biol 2007;27(12):4465–74.
Locker FG. Hormonal regulation of calcium homeostasis. Nurs Clin North Am 31(4):797-803.
B1996;ajammal SS, Zlowodzki M, Lelwica A, Tornetta P, Einhorn TA, Buckley R, Leighton R, Russell TA, Larsson S, Bhandari M. The use of calcium phosphate bone cement in fracture treatment. A meta-analysis of randomized trials. J Bone Joint Surg Am 2008;90 (6):1186–96.
Ebeling PR, Sandgren ME, DiMagno EP, Lane AW, DeLuca HF, Riggs BL. Evidence of an age-related decrease in intestinal responsiveness to vitamin D: relationship between serum 1,25-dihydroxyvitamin D3 and intestinal vitamin D receptor concentrations in normal women. J Clin Endocrinol Metab 1992;75(1):176–82.
Pacifici R, Rifas L, McCracken R, Vered I, McMurtry C, Avioli LV, Peck WA. Ovarian steroid treatment blocks a postmenopausal increase in blood monocyte interleukin 1 release. Proc Natl Acad Sci U S A 1989;86(7):2398–402.
Pacifici R, Brown C, Puscheck E, Friedrich E, Slatopolsky E, Maggio D, McCracken R, Avioli LV. Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proc Natl Acad Sci U S A 1991;88(12):5134–8.
Rizzoli R. Bisphosphonates for post-menopausal osteoporosis: are they all the same? QJM 2011;104(4):281–300.
Sauty A, Pecherstorfer M, Zimmer-Roth I, Fioroni P, Juillerat L, Markert M, Ludwig H, Leuenberger P, Burckhardt P, Thiebaud D. Interleukin-6 and tumor necrosis factor alpha levels after bisphosphonates treatment in vitro and in patients with malignancy. Bone 1996;18(2):133–9.
Adami S, Bhalla AK, Dorizzi R, Montesanti F, Rosini S, Salvagno G, Lo Cascio V. The acute-phase response after bisphosphonate administration. Calcif Tissue Int 1987;41(6):326–31.
Schweitzer DH, Oostendorp-van de Ruit M, Van der Pluijm G, Löwik CW, Papapoulos SE. Interleukin-6 and the acute phase response during treatment of patients with Paget’s disease with the nitrogen-containing bisphosphonate dimethylamino-hydroxy-propylidene bisphosphonate. J Bone Miner Res 10(6):956-62.
Ryu KR, Choi JY, Chung S, Kim DH. Anti-scratching behavioral effect of the essential oil and phytol isolated from Artemisia princeps Pamp. in mice. Planta Med
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cho, HJ., Kim, JW., Ju, SY. et al. Effects of Artemisia princeps supplementation on bone metabolism in ovariectomized rats. J Nutr Health Aging 20, 533–539 (2016). https://doi.org/10.1007/s12603-015-0607-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-015-0607-8