Abstract
Probiotics, postbiotics, and n-3 polyunsaturated fatty acids (PUFA) have antidepressant-like effects. However, the underlying mechanisms of the dopaminergic pathway are unclear. The present study investigated the hypothesis that probiotics and postbiotics combined with n-3 PUFA synergistically improve depression by modulating the dopaminergic pathway through the brain-gut axis. Rats were randomly divided into seven groups: non-chronic mild stress (CMS) with n-6 PUFA, and CMS with n-6 PUFA, n-3 PUFA, probiotics, postbiotics, probiotics combined with n-3 PUFA, and postbiotics combined with n-3 PUFA. Probiotics, postbiotics, and n-3 PUFA improved depressive behaviors, decreased blood concentrations of interferon-γ, and interleukin-1β, and increased the brain and gut concentrations of short chain fatty acids and dopamine. Moreover, probiotics, postbiotics, and n-3 PUFA increased the brain and gut expression of glucocorticoid receptor and tyrosine hydroxylase; brain expression of l-type amino acid transporter 1 and dopamine receptor (DR) D1; and gut expression of DRD2. The expression of phosphorylated protein kinase A/protein kinase A and phosphorylated cAMP response element-binding protein/cAMP response element-binding protein increased in the brain, however, decreased in the gut by the supplementation of probiotics, postbiotics, and n-3 PUFA. There was synergistic effect of probiotics and postbiotics combined with n-3 PUFA on the depressive behaviors and dopaminergic pathway in blood, brain, and gut. Moreover, no significant difference in the dopaminergic pathways between the probiotics and postbiotics was observed. In conclusion, probiotics and postbiotics, combined with n-3 PUFA have synergistic antidepressant-like effects on the dopaminergic pathway through the brain-gut axis in rats exposed to CMS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depression is increasing and leads to suicide, which is the fourth major cause of death worldwide [1]. Depression downregulates the serotonergic pathway [2], which in turn modulates the dopaminergic pathway [3]. A decrease in the concentration of dopamine (DA) was observed in the brain of depressed rodents exposed to chronic mild stress (CMS) [4,5,6], and in the gut of depressed mice exposed to cold stress [7].
Our previous studies reported that supplementation of biotics including probiotics and postbiotics, and n-3 polyunsaturated fatty acids (PUFA) had antidepressant-like effects by modulating the serotonergic pathway in the brain and gut of rats exposed to CMS [8, 9]. Administration of n-3 PUFA increased the concentration of DA and the expression of tyrosine hydroxylase (TH), the rate-limiting enzyme of DA, in the brain of depressed mice exposed to the forced swimming test (FST) [10, 11]. Moreover, administration of probiotics increased the concentration of DA and the expression of TH, dopamine receptor, and protein kinase A (PKA) in the brain of depressed rodents induced by obesity [12], and exposed to CMS [13, 14], social frustration stress [15], and restraint stress [16]. Administration of postbiotics also increased the DA concentration in the brain of depressed mice treated with corticosterone (CORT) [17] and Salmonella [18]. Since previous studies suggested that both probiotics and postbiotics modified gut microbiome composition and metabolites such as short chain fatty acids (SCFA), probiotics and postbiotics could have similar effects [19]. However, the effects of probiotics, postbiotics, and n-3 PUFA on the brain and gut dopaminergic pathway have not been studied yet.
Our previous studies have shown that supplementation of probiotics and postbiotics combined with n-3 PUFA had a synergistic effect on the serotonergic pathway in the brain and gut of depressed rats exposed to CMS [8, 9]. Since n-3 PUFA, as prebiotics, modified fecal level of SCFA and gut microbiome, n-3 PUFA could have synbiotic effect with probiotics or postbiotics [8]. Valent et al. [20] also showed that supplementation of probiotics combined with n-3 PUFA significantly increased the brain concentration of total DA, compared to probiotics alone in obese mice. However, the synergistic effect of biotics combined with n-3 PUFA on the dopaminergic pathway in the brain and gut has not yet been investigated. Therefore, this study aimed to investigate the hypothesis that supplementation of biotics combined with n-3 PUFA has a synergistic antidepressant-like effect on the dopaminergic pathway through the brain-gut axis in rats exposed to CMS. Additionally, we hypothesized that probiotics and postbiotics would have similar effects on the dopaminergic pathway in the brain and gut of depressed rats exposed to CMS.
Materials and Methods
Animals, and Diet and Oral Supplementation
The experimental protocol was approved by the Institutional Animal Care and Use Committee of the Hanyang University (HY-IACUC-21-0092). The Wistar rats (Central Lab. Animal Inc., Seoul, Korea) were housed in a ventilated air-conditioned room maintained at 22 ± 1 °C with a 12 h light/dark cycle and 40–50% humidity. The experimental diets were isocaloric modified American Institute of Nutrition-93G diet containing 16% fat of the total energy intake (E%) with either 0 E% (n-6 PUFA diet) or 1 E% eicosapentaenoic acid (EPA) and DHA in a ratio of 6:4 (n-3 PUFA diet; Supplementary Table 1). The n-6 PUFA and n-3 PUFA diets were made up of grape seed oil (Sajo Haepyo, Seoul, Korea) with 0 g and 7.33 g of re-esterified triglyceride fish oil (Epax, Ålesund, Norway), respectively. The biotics consisted of Bifidobacterium longum, Lactobacillus helveticus, and Lactobacillus plantarum in a 1:1:1 ratio (Lactomason, Jinju, Korea), and to prepare postbiotics, the probiotics were heat killed. Sterile water (1 mL), with or without 6 × 109 colony-forming units/day of biotics was administered orally.
Experimental Design
One week after acclimatization, five-week-old rats were supplemented with experimental diet and oral supplementation for 12 weeks (Supplementary Fig. 1A). Animals were randomly divided into seven groups (n = 6 per group): n-6 PUFA diet without CMS (NS), n-6 PUFA diet with CMS (N6), n-6 PUFA diet and probiotics with CMS (Pro), n-6 PUFA diet and postbiotics with CMS (Post), n-3 PUFA diet with CMS (N3), n-3 PUFA diet and probiotics with CMS (N3Pro), and n-3 PUFA diet and postbiotics with CMS (N3Post). After six weeks of supplementation, rats were exposed to CMS for five weeks, as described in a previous study [21]. The procedure of CMS included eight different mild stresses with a weekly rotation plan: food deprivation, water deprivation, cage tilting (45°), soiled cage (300 mL of 25 ± 1 °C water), group housing, no bedding, stroboscopic illumination (130 flashes/min), and intermittent illumination every 2 h (Supplementary Fig. 1B). The rats in the NS group were housed separately from those in the CMS groups to avoid CMS disturbances. After CMS, a pre-sucrose preference test (SPT) was performed for four days, and the SPT was performed the following day. The pre-FST and FST were respectively performed the day before and on the day of euthanasia.
Behavior Test
During the pre-SPT, rats were provided with a bottle of sterile water and 1% sucrose solution. The bottles were weighed daily and switched on every day to avoid side bias. Rats were deprived of water and food for 10 h before the SPT, and then provided with a bottle of sucrose solution and a bottle of sterile water with food for 12 h. The sucrose preference index (%) was calculated as follows: (grams of sucrose solution intake/total grams of liquid intake) × 100.
During the pre-FST and FST, rats were individually forced to swim inside a cylinder (height, 50 cm and diameter, 20 cm) containing 25 ± 1 °C water up to a height of 30 cm. The pre-FST and FST were performed for 15 and 5 min, respectively, and videotaped. The duration of immobility, swimming, and climbing were measured by three raters who were blinded to the test conditions.
Blood and Tissue Collection
The rats were anesthetized with Zoletil and Rompun (30 mg/kg and 10 mg/kg body weight, respectively). Serum was collected, and the dissected brain (hippocampus) and gut (colon) were rinsed with saline, weighed, and immediately frozen in liquid nitrogen. Blood and tissue samples were then stored at -80 °C for further analysis.
Gas Chromatography
The brain and gut (100–300 mg) were homogenized, total lipids were extracted, and phospholipids were separated using thin-layer chromatography (SIL G-25; Machereynagel GmbH & Co, Duren, Germany) [22]. Phospholipids were methylated, and the composition of long-chain fatty acids (14:0 or more) was analyzed using gas chromatography (Shimadzu 2010 AF; Shimadzu Scientific Instrument, Tokyo, Japan) with a 100 m × 0.25 mm inner diameter and 0.20 μm film capillary column (SP2560; Supelco, Bellefonte, PA, USA) [23]. The carrier gas was hydrogen at a flow rate of 40 mL/min, and the injection and detection temperatures were 230 °C and 240 °C, respectively. The run temperature began at 180 °C, and increased by 5 °C/min to 200 °C and by 10 °C/min to 240 °C. The split ratio was 10:1, and external standards (GLC-OQA; Nu-Check Prep Elysian, MN, USA) were used to identify the fatty acids. The coefficient of variation was 4.3%.
To calculate the concentration of the SCFA, an internal standard (7:0; Cat # 75190, Sigma-Aldrich, Burlington, MA, USA) was added during homogenization of the brain and gut tissues, and NaOH and phosphoric acid were added for stabilization and acidification [24]. The SCFA, including acetic (2:0), propionic (3:0), isobutyric (4:0i), and butyric acids (4:0n), were analyzed using gas chromatography (Shimadzu 2010 AF; Shimadzu Scientific Instrument, Tokyo, Japan) with a 15 m × 0.53 mm inner diameter and 0.50 μm film capillary column (25326; Supelco, Bellefonte, PA, USA). Nitrogen was used as the carrier gas at a flow rate of 40 mL/min, and the injection and detection temperatures were set at 190 °C. The run temperature was set at 80 °C and increased by 5 °C/min up to 190 °C, and the split ratio was 10:1. External standards (WSFA-4 mixture, Matreya, State College, PA, USA) were used to identify the fatty acids.
Enzyme-Linked Immunosorbent Assay (ELISA)
Serum concentrations of interleukin (IL)-1β and interferon (INF)-γ were measured using an ELISA kit (Cat # LS-F5627; LS-F5066-1, LSBio, Seattle, WA, USA). To measure DA concentration, the brain and gut were homogenized, and the supernatants were analyzed using an ELISA kit (Cat # ENZ-KIT188-0001, Enzo Life Science, Farmingdale, NY, USA). All measurements were performed in triplicates and quantified using standards on a spectrophotometer (Multiscan GO, Thermo Scientific, Waltham, MA, USA), according to the manufacturer’s protocol.
Western Blot Analysis
The brain and gut were homogenized and centrifuged at 10,000 × g for 15 min at 4 °C, and protein content was measured using a bicinchoninic acid assay (Cat # 23225, Pierce Biotechnology, Rockford, IL, USA). Protein (30 µg) was separated on 10% polyacrylamide gels, transferred to polyvinylidene fluoride membranes, and blocked for 1–1.5 h at room temperature with 5% skim milk or bovine serum albumin (BSA) in Tris-buffered saline with 0.1% Tween 20 (TBST). The membrane was incubated with primary antibodies against the TH (Cat # LS-C60884; brain, 1:1000; gut, 1:250), dopamine receptor (DR) D1 (Cat # LS-C332275; brain, 1:2000; gut, 1:1000), DRD2 (Cat # LS-C748000; 1:1000), PKA (Cat# LS-C63197; brain, 1:1000; gut, 1:500), phosphorylated PKA (pPKA; Cat # LS-C63197; brain, 1:1000; gut, 1:500), cAMP response element-binding protein (CREB; Cat # 9197; 1:1000), phosphorylated CREB (pCREB; Cat # 9198; 1:1000), glucocorticoid receptor (GR; Cat # 183127; 1:500), and l-type amino acid transporter1 (LAT1; Cat # LS-B15706; 1:1000) in 5% BSA in TBST overnight at 4℃. Antibodies against CREB and pCREB were purchased from Cell Signaling Technology (Danvers, MA, USA), antibody against GR was purchased from Abcam (Cambridge, UK), and other antibodies were purchased from LSBio (Seattle, WA, USA). Membranes were incubated with horseradish-peroxidase-conjugated secondary antibodies, anti-rabbit IgG (Cat # LS-C60884, LSBio, Seattle, WA, USA), with 5% BSA in TBST for 1 h at room temperature. Immunoreactive bands were visualized with the UV setting on a ChemiDoc MP Imaging System (Bio-Rad, Hercules, CA, USA) to estimate the quantity of protein in each lane, and β-actin was used for normalization. Western blot analysis was performed in triplicates.
Immunofluorescence Staining
The brain (dentate gyrus region of the hippocampus) and gut (colon) samples were fixed in 4% paraformaldehyde, dehydrated, embedded in paraffin, cut into 10 μm and 4 μm sections, respectively, and mounted on glass slides. The sections on the glass slides were then immersed in xylene followed by alcohol, rehydrated in wash buffer, and heated in sodium citrate buffer (0.01 M, pH 6.0). The sections were blocked with a PAP pen (Enzo Life Science, Farmingdale, NY, USA) and incubated with primary antibodies against the DRD1 (Cat # LS-C332275; 1:50; LSBio, Seattle, WA, USA) and DRD2 (Cat # sc-5303; 1:100; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 4 °C overnight. The sections were incubated with goat anti-rabbit IgG H&L with Alexa Fluor® 488 (Cat # LS-C354919, LSBio, Seattle, WA, USA) and 555 (Cat # ab150078, Abcam, Cambridge, UK) for 2 h at room temperature, washed, and mounted with Fluoroshield containing 4′, 6-diamidino-2- phenylindole (DAPI; Cat # ENZ-53003-M010; Enzo Life Science, Farmingdale, NY, USA). The Leica TCS SP8 X confocal microscope and Leica AF imaging software (Leica Microsystems, Wetzlar, Germany) were used to evaluate immunofluorescence images.
Statistical Analysis
Data were analyzed using SPSS for Windows (version 27.0; SPSS Inc., Chicago, IL, USA). All values are expressed as mean with standard deviations and differences were considered significant at P < 0.05. Data were analyzed using independent t-tests with the CMS factor. The effects of the n-3 PUFA diet and biotics, and their synergistic effects were determined using two-way analysis of variance followed by Duncan’s post hoc test.
Results
Depressive Behaviors, DA, and Inflammatory Cytokines
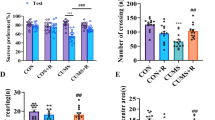
The sucrose preference, the duration of climbing and swimming, and the DA concentration in brain and gut were significantly decreased by CMS, however, the duration of immobility was increased by CMS (Table 1). Probiotics, postbiotics and n-3 PUFA significantly increased the sucrose preference, climbing duration, and the DA concentration in brain and gut. There were no significant differences in depressive behaviors and DA concentration between the probiotics and postbiotics. Supplementation of biotics combined with n-3 PUFA had a significant synergistic effect on the climbing duration and DA concentration in brain and gut. Moreover, no significant effects of diet and biotics on dietary intake, body weight, or organ weight were observed, however, CMS significantly decreased the final body weight (Supplementary Table 2).
The concentrations of IL-1β and INF-γ in the blood were significantly (P < 0.001) increased by the CMS, but decreased by the supplementation of probiotics, postbiotics, and n-3 PUFA (Fig. 1). No significant difference in the cytokines between probiotics and postbiotics was observed. Supplementation of biotics combined with n-3 PUFA had a significant (P < 0.001) synergistic effect on the concentrations of inflammatory cytokines in the blood.
Concentration of cytokines in blood. Values are mean with standard deviation (n = 6/group); *Values are significantly different between non-chronic mild stress (CMS) vs. CMS within the same diet; ** Values are significantly different between probiotics, postbiotics, and n-3 PUFA vs. n-6 PUFA diet within the same CMS condition; *** Values are significantly different between combination of biotics and n-3 PUFA vs. probiotics, postbiotics, and n-3 PUFA within the same CMS condition; IL-1β, interleukin-1β; INF-γ, interferon-γ; NS, n-6 PUFA diet without CMS; Pro, n-6 PUFA diet and probiotics with CMS; Post, n-6 PUFA diet and postbiotics with CMS; N3, n-3 PUFA diet with CMS; N3Pro, n-3 PUFA diet and probiotics with CMS; N3Post, n-3 PUFA diet and postbiotics with CMS
Fatty Acid Composition of Brain and Gut
The concentrations of 2:0 (brain, P = 0.035; gut, P < 0.001), 3:0 (brain, P = 0.036; gut, P < 0.001), 4:0i (brain, P = 0.005, gut, P < 0.001), and 4:0n (P < 0.001) were decreased by CMS (Fig. 2). The concentrations of 2:0 (brain, P = 0.036; gut, P = 0.043), 3:0 (brain, P = 0.037; gut, P < 0.001), and 4:0n (P < 0.001) were increased by the supplementation of n-3 PUFA. Probiotics and postbiotics also increased the concentrations of 2:0 (brain, P = 0.044; gut, P < 0.001), 3:0 (brain, P = 0.011; gut, P < 0.001), and 4:0n (P < 0.001). There were no significant differences between probiotics and postbiotics on the concentrations of SCFA in brain and gut. Supplementation of biotics combined with n-3 PUFA had significant synergistic effects on the concentrations of 2:0 (brain, P = 0.029; gut, P = 0.011), 3:0 (brain, P = 0.006; gut, P < 0.001), and 4:0n (brain, P = 0.003; gut, P < 0.001).
Levels of short chain fatty acids in brain (A) and gut (B). Values are mean with standard deviation (n = 6/group); *Values are significantly different between non-chronic mild stress (CMS) vs. CMS within the same diet; ** Values are significantly different between probiotics, postbiotics, and n-3 PUFA vs. n-6 PUFA diet within the same CMS condition; *** Values are significantly different between combination of biotics and n-3 PUFA vs. probiotics, postbiotics, and n-3 PUFA within the same CMS condition; NS, n-6 PUFA diet without CMS; Pro, n-6 PUFA diet and probiotics with CMS; Post, n-6 PUFA diet and postbiotics with CMS; N3, n-3 PUFA diet with CMS; N3Pro, n-3 PUFA diet and probiotics with CMS; N3Post, n-3 PUFA diet and postbiotics with CMS
Biotics and CMS had no significant effect on the phospholipid composition of long-chain fatty acids in the brain and gut (Supplementary Tables 3 and 4). Supplementation of n-3 PUFA significantly (P < 0.001) increased 20:5n3, 22:5n3, 22:6n3, and total n-3 PUFA, and decreased 18:2n6, 20:2n6, 20:3n6, 20:4n6, 22:4n6, 22:5n6, and total n-6 PUFA.
Dopaminergic Pathway in Brain and gut
Figure 3 showed that CMS decreased the brain expression of DRD1 (P < 0.001), DRD2 (P < 0.001), LAT1 (P = 0.002), TH (P < 0.001), pPKA/PKA (P < 0.001), and pCREB/CREB (P < 0.001). Probiotics, postbiotics, and n-3 PUFA increased the brain expression of DRD1 (P < 0.001), LAT1 (P < 0.001), TH (P < 0.001), pPKA/PKA (diet, P = 0.002; biotics, P < 0.001), and pCREB/CREB (P < 0.001). Supplementation of biotics, combined with n-3 PUFA had significant synergistic effects on the brain expression of DRD1 (P < 0.001), LAT1 (P = 0.003), TH (P < 0.001), pPKA/PKA (P = 0.047), and pCREB/CREB (P = 0.031). Figure 4 showed that CMS significantly (P < 0.001) decreased the expression of DRD2, GR, and TH, and increased the expression of pPKA/PKA and pCREB/CREB in gut. Probiotics, postbiotics, and n-3 PUFA significantly (P < 0.001) increased the expression of DRD2, GR, and TH and decreased the expression of pPKA/PKA and pCREB/CREB in gut. Supplementation of biotics, combined with n-3 PUFA had significant synergistic effects on the gut expression of DRD2 (P = 0.004), TH (P = 0.012), pPKA/PKA (P = 0.042), and pCREB/CREB (P = 0.033). Consistently, immunofluorescence staining showed that probiotics, postbiotics, and n-3 PUFA increased the expression of hippocampus dentate gyrus region DRD1 and colon DRD2 (Fig. 5). There were no differences between probiotics and postbiotics on the expression of the dopaminergic pathway in brain and gut.
The effect of probiotics, postbiotics, and n-3 PUFA on proteins related to dopaminergic pathway in brain. Values are mean with standard deviation (n = 6/group); *Values are significantly different between non-chronic mild stress (CMS) vs. CMS within the same diet; **Values are significantly different between probiotics, postbiotics, and n-3 PUFA vs. n-6 PUFA diet within the same CMS condition; ***Values are significantly different between combination of biotics and n-3 PUFA vs. probiotics, postbiotics, and n-3 PUFA within the same CMS condition; DRD1, dopamine receptor D1; DRD2, dopamine receptor D2; LAT1, l-type amino acid transporter 1; NS, n-6 PUFA diet without CMS; Pro, n-6 PUFA diet and probiotics with CMS; Post, n-6 PUFA diet and postbiotics with CMS; N3, n-3 PUFA diet with CMS; N3Pro, n-3 PUFA diet and probiotics with CMS; N3Post, n-3 PUFA diet and postbiotics with CMS
The effect of probiotics, postbiotics, and n-3 PUFA on proteins related to dopaminergic pathway in gut. Values are mean with standard deviation (n = 6/group); *Values are significantly different between non-chronic mild stress (CMS) vs. CMS within the same diet; **Values are significantly different between probiotics, postbiotics, and n-3 PUFA vs. n-6 PUFA diet within the same CMS condition; ***Values are significantly different between combination of biotics and n-3 PUFA vs. probiotics, postbiotics, and n-3 PUFA within the same CMS condition; CREB, cAMP response element-binding protein; GR, glucocorticoid receptor; pCREB, phosphorylated CREB; PKA, protein kinase A; pPKA, phosphorylated PKA; TH, tyrosine hydroxylase; NS, n-6 PUFA diet without CMS; Pro, n-6 PUFA diet and probiotics with CMS; Post, n-6 PUFA diet and postbiotics with CMS; N3, n-3 PUFA diet with CMS; N3Pro, n-3 PUFA diet and probiotics with CMS; N3Post, n-3 PUFA diet and postbiotics with CMS
Immunofluorescence staining of dopamine receptor D1 (DRD1) and D2 (DRD2) in hippocampus dentate gyrus region (brain) and colon (gut). Expression of DRD1 and DRD2 were visualized using Alexa Fluor 555 (red) and 488 (green), respectively, and DNA was stained blue (DAPI); Staining of DRD1, DRD2, and DAPI, and merged images are shown; NS, n-6 PUFA diet without CMS; Pro, n-6 PUFA diet and probiotics with CMS; Post, n-6 PUFA diet and postbiotics with CMS; N3, n-3 PUFA diet with CMS; N3Pro, n-3 PUFA diet and probiotics with CMS; N3Post, n-3 PUFA diet and postbiotics with CMS
Discussion
The present study demonstrated that supplementation of probiotics and postbiotics, combined with n-3 PUFA exerted synergistic antidepressant-like effects by regulating the dopaminergic pathway through the brain-gut axis in rats exposed to CMS (Fig. 6). Alex et al. [3] showed that serotonergic pathway regulated dopaminergic pathway through serotonin receptor. Consistent with the present study, depression was modulated by the dopaminergic pathway in the brain through decreasing the concentrations of DA and SCFA, and the expression of TH, dopamine receptor, and PKA in depressed rodents exposed to CMS [4,5,6, 8, 9, 25, 26] and restraint stress [16]. Ohtsuki et al. [27] showed that the brain expression of LAT1 decreased in mice with Parkinson’s disease (PD) induced by low concentration of DA. The present study also showed that brain expression of LAT1 decreased in depressed rats exposed to CMS. With respect to the gut dopaminergic pathway, the gut concentration of DA was also decreased in depressed mice exposed to cold stress [7], and the colonic expression of TH and dopamine receptors decreased in PD rats [28, 29]. Previous study showed that relative abundance of Ruminococcaceae in fecal was lower in depressed rat exposed to CMS [8], and PD patients [30], suggesting the relationship between dopaminergic pathway and gut microbiome. The present study is the first study to show that depression is modulated by the gut dopaminergic pathway in depressed rats exposed to CMS.
Abridged general view of the dopaminergic pathway for the antidepressant-like effect of probiotics, postbiotics, and n-3 PUFA. Probiotics, postbiotics, and n-3 PUFA increase the expression of the tyrosine hydroxylase (TH), leading to upregulation of the dopamine (DA) in the brain and gut; In addition, probiotics, postbiotics, and n-3 PUFA increase the gut levels of short chain fatty acids (SCFA) and decrease blood levels of inflammatory cytokines; SCFA can cross the brain-blood barrier and increase dopamine (DA) level in the brain, which attenuates depressive behavior; Probiotics, postbiotics, and n-3 PUFA increase the brain dopamine receptor 1 (DRD1) and gut dopamine receptor 2 (DRD2), which modify protein kinase A (PKA) signaling. CREB, cAMP response element-binding protein; DRD1, dopamine receptor D1; DRD2, dopamine receptor D2; GR, glucocorticoid receptor; LAT1, l-type amino acid transporter1; pCREB, phosphorylated CREB; PKA, protein kinase A; pPKA, phosphorylated PKA; TH, tyrosine hydroxylase
Consistent with the present study, previous studies have shown that n-3 PUFA and krill oil (rich in n-3 PUFA) increase the concentrations of DA and SCFA, and the expression of TH, and pCREB in the brain of depressed rodents exposed to CMS [8, 9, 31] and FST [11]. In the placenta of pregnant women, supplementation of n-3 PUFA during gestation and postpartum increased the gene expression of LAT1 compared to that in the control group [32]. Precursor of DA, L-3,4-dihydroxyphenylalanine (L-DOPA), is known to cross the blood brain barrier by LAT1 [33]. Metz et al. [34] showed that supplementation of fish oil normalized the expression of DRD1 in the brain, which was impaired by amphetamine. Additionally, subcutaneous implantation of fluoxetine, a representative serotonin reuptake inhibitor (SSRI) increased DRD1 expression and serotonin concentration in the brain of depressed mice exposed to the FST [35]. As DA attaches to DRD1, the DRD1 is activated, enhancing the accumulation of cAMP levels and activating PKA [36]. Rashid et al. [37] showed that treatment of DHA metabolites increased the expression of pCREB/CREB and pPKA/PKA in neural stem cell treated with ethanol. The present study is the first study to demonstrate that n-3 PUFA increases the expression of LAT1, pPKA/PKA, and DRD1 in depressed animal model.
Our previous studies showed that n-3 PUFA increased the fecal abundance of Ruminococcaceae, the major butyric acid producer, and the concentration of SCFA, while decreasing the expression of pCREB in the gut of rats exposed to CMS [8, 9]. Patel et al. [38] reported that butyric acid increased the transcription of TH by upregulating gene promoter in the adrenal gland organ cell. As supplementation of n-3 PUFA increased the gut concentration of butyric acid in depressed rats exposed to CMS [8, 9], administration of n-3 PUFA could also increase the expression of TH in the gut. Peng et al. [39] showed that the concentration of DA and the expression of TH decreased in the brain of colonic DRD2 knock out mice with PD compared with those of wild-type mice with PD. Rats with PD showed reduced DRD2 expression and dysmotility in the gut, suggesting the importance of gut DRD2 in the dopaminergic pathway [28]. Magro et al. [40] showed that treatment of INF-γ decreased the L-dopa uptake in Caco-2 cell, and dopamine decarboxylase activity, the enzyme converting L-dopa to DA, increased concentration-dependently by L-dopa in colonic mucosa of colitis rat. Supplementation of fish oil decreased the concentration of INF-γ in blood of mice with listeriosis [41], suggesting that supplementation of n-3 PUFA could increase the concentration of DA in gut. The present study is the first study to demonstrate that n-3 PUFA modulates the gut dopaminergic pathway in depressed animal model by increasing the concentrations of DA and SCFA, and the expression of DRD2, and TH, and by decreasing the expression of pPKA/PKA and pCREB/CREB in the gut.
Consistent with the present study, our previous studies have shown that the administration of probiotics and postbiotics increase the concentration of SCFA and the expression of pCREB in the brain of depressed rats exposed to CMS [8, 9]. Supplementation of probiotics also increased the concentration of DA and the expression of TH, PKA, and DRD1 in the brain of depressed rodents induced by CMS [13], social frustration stress [15], restraint stress [16], and obesity [12]. Wu et al. [42] showed that treatment of probiotics increased the gene expression of LAT1 in intestinal cell exposed to oxidative stress. Consistent with the present study, supplementation of postbiotics increased DA concentration in the brain of depressed mice exposed to Salmonella [18] and treated with CORT [17]. There were no differences between probiotics and postbiotics on DA concentration in the brain of depressed mice exposed to Salmonella [18], suggesting that postbiotics could also modulate the brain dopaminergic pathway. The present study showed that probiotics and postbiotics have similar effects on the brain dopaminergic pathway in depressed animal model.
Our previous studies showed that administration of probiotics and postbiotics increased the fecal abundance of Ruminococcaceae, concentration of SCFA and the expression of GR, and decreased the expression of pCREB in the gut of rats exposed to CMS [8]. It has been well-known that probiotics and postbiotics modified gut microbiome composition by microbiome itself and their metabolites [19]. Gut microbiome and their metabolites including SCFA, urolithin A, and taurine were associated with dopamine transporter and receptor in brain [43,44,45]. Additionally, GR-mediated glucocorticoid actions have known to sustain DA activation during stress via stimulation of TH in the cell body [46], suggesting that probiotics and postbiotics could increase the TH in gut. Cheon et al. [47] also showed that postbiotics increased the expression of TH, the rate-limiting enzyme of DA in human colon adenocarcinoma cell. As DA attaches to DRD2, the DRD2 is activated, inhibiting the production of cAMP levels and PKA [36]. Supplementation of probiotics also normalized the gene expression of PKA, which was impaired by constipation in the colon of mice [48]. The present study is the first study to demonstrate that probiotics and postbiotics modulate the gut dopaminergic pathway in depressed rats exposed to CMS. Supplementation of probiotics decreased the concentration of INF-γ in brain of depressed mice exposed to CMS [49], suggesting that supplementation of probiotics could increase the concentration of DA in gut.
Our previous studies showed that supplementation of probiotics and postbiotics, combined with n-3 PUFA had synergistic effect on the hypothalamic-pituitary-adrenal axis and serotonergic pathway in the brain and gut of rats exposed to CMS [8, 9]. Valent et al. [20] also showed that supplementation of probiotics combined with n-3 PUFA significantly increased the concentration of total DA, including DA and its metabolites, compared to probiotics supplementation alone in obese mice. Our previous study suggested n-3 PUFA as a prebiotics by showing that supplementation of n-3 PUFA combined with probiotics and postbiotics elevated fecal abundance of Ruminococcaceae and gut concentration of butyric acid in depressed rats exposed to CMS [8]. Since postbiotics including fragments of microorganisms and metabolites, particularly SCFA had a beneficial effect to the host [19], the combination of postbiotics and n-3 PUFA could also exert a synergistic effect, which was comparable to those with probiotic and n-3 PUFA. Moreover, supplementation of probiotics combined with dietary fiber significantly decreased depression scores on the hospital anxiety and depression scale compared to probiotics supplementation alone in depressed hemodialysis patients [50]. Additionally, Zhao et al. [51] showed that fecal microbiota transplantation, as a synbiotics for the components of host gut microbiome, bacterial debris, and metabolic products, increased the brain expression of TH and improved gut dysbiosis in mice with PD.
To our knowledge, this is the first study to show antidepressant-like effects of probiotics, postbiotics, n-3 PUFA, and n-3 PUFA combined with probiotics and postbiotics on the dopaminergic pathway through the brain-gut axis. However, the present study has several limitations. First, synergistic antidepressant-like mechanisms of n-3 PUFA, probiotics, and postbiotics have not been demonstrated in DR knockout models. Second, Griffith et al. [52] showed that supplementation of α-linolenic acid, one of n-3 PUFA, improved anxiety behavior, and increased the concentration of DA in brain of diabetic mice, while the diet in the present study only contained EPA and DHA.
In conclusion, the present study suggests that probiotics, postbiotics, and n-3 PUFA exert a synergistic therapeutic effect on depression by modulating the dopaminergic pathway through the brain-gut axis. Further studies are required to confirm the antidepressant-like effects of probiotics, postbiotics, n-3 PUFA, and n-3 PUFA combined with probiotics and postbiotics on the dopaminergic pathway in patients with depression.
Data Availability
No datasets were generated or analysed during the current study.
References
World Health Organization. Depressive disorder (depression). World Health Organization (2023) https://www.who.int/news-room/fact-sheets/detail/depression
Mikulska J, Juszczyk G, Gawrońska-Grzywacz M, Herbet M (2021) HPA Axis in the pathomechanism of Depression and Schizophrenia: new therapeutic strategies based on its participation. Brain Sci 11:1298. https://doi.org/10.3390/brainsci11101298
Alex KD, Pehek EA (2007) Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther 113:296–320. https://doi.org/10.1016/j.pharmthera.2006.08.004
Guo YX, Xia CY, Yan Y, Han Y, Shi R, He J et al (2023) Loganin improves chronic unpredictable mild stress-induced depressive-like behaviors and neurochemical dysfunction. J Ethnopharmacol 308:116288. https://doi.org/10.1016/j.jep.2023.116288
Chen P, Hei M, Kong L, Liu Y, Yang Y, Mu H et al (2019) One water-soluble polysaccharide from Ginkgo biloba leaves with antidepressant activities via modulation of the gut microbiome. Food Funct 10:8161–8171. https://doi.org/10.1039/c9fo01178a
Huang HS, Lin YE, Panyod S, Chen RA, Lin YC, Chai LMX et al (2023) Anti-depressive-like and cognitive impairment alleviation effects of Gastrodia Elata Blume water extract is related to gut microbiome remodeling in ApoE(-/-) mice exposed to unpredictable chronic mild stress. J Ethnopharmacol 302:115872. https://doi.org/10.1016/j.jep.2022.115872
Sun L, Wang X, Zou Y, He Y, Liang C, Li J et al (2023) Cold stress induces colitis-like phenotypes in mice by altering gut microbiota and metabolites. Front Microbiol 14:1134246
Cho H, Jo M, Oh H, Lee Y, Park Y (2023) Synergistic antidepressant-like effect of n-3 polyunsaturated fatty acids and probiotics through the brain-gut axis in rats exposed to chronic mild stress. J Nutr Biochem 116:109326. https://doi.org/10.1016/j.jnutbio.2023.109326
Lee Y, Oh H, Jo M, Cho H, Park Y (2023) Synergistic effect of n-3 PUFA and probiotic supplementation on bone loss induced by chronic mild stress through the brain–gut–bone axis. J Funct Foods 100:105363. https://doi.org/10.1016/j.jff.2022.105363
Jiang L-H, Liang Q-Y, Shi Y (2012) Pure docosahexaenoic acid can improve depression behaviors and affect HPA axis in mice, vol 16. European Review for Medical & Pharmacological Sciences
Takeuchi E, Yamada D, Suzuki S, Saitoh A, Itoh M, Hayashi T et al (2020) Participation of the nucleus accumbens dopaminergic system in the antidepressant-like actions of a diet rich in omega-3 polyunsaturated fatty acids. PLoS ONE 15:e0230647. https://doi.org/10.1371/journal.pone.0230647
Schell M, Wardelmann K, Hauffe R, Rath M, Chopra S, Kleinridders A (2023) Lactobacillus rhamnosus sex-specifically attenuates depressive-like behavior and mitigates metabolic consequences in obesity. Biol Psychiatry Glob Open Sci 3:651–662. https://doi.org/10.1016/j.bpsgos.2023.02.011
Gu F, Wu Y, Liu Y, Dou M, Jiang Y, Liang H (2020) Lactobacillus casei improves depression-like behavior in chronic unpredictable mild stress-induced rats by the BDNF-TrkB signal pathway and the intestinal microbiota. Food Funct 11:6148–6157. https://doi.org/10.1039/d0fo00373e
Feng S, Meng C, Liu Y, Yi Y, Liang A, Zhang Y et al (2023) Bacillus licheniformis prevents and reduces anxiety-like and depression-like behaviours. Appl Microbiol Biotechnol 107:4355–4368. https://doi.org/10.1007/s00253-023-12580-7
Wu Z, Wang P, Pan D, Zeng X, Guo Y, Zhao G (2021) Effect of adzuki bean sprout fermented milk enriched in γ-aminobutyric acid on mild depression in a mouse model. J Dairy Sci 104:78–91. https://doi.org/10.3168/jds.2020-19154
Rehman MU, Ghazanfar S, Ul Haq R, Ullah S, Khan S, Wu J et al (2022) Probiotics (Bacillus clausii and Lactobacillus fermentum NMCC-14) ameliorate stress behavior in mice by increasing monoamine levels and mRNA expression of dopamine receptors (D(1) and D(2)) and synaptophysin. Front Pharmacol 13:915595. https://doi.org/10.3389/fphar.2022.915595
Wei CL, Wang S, Yen JT, Cheng YF, Liao CL, Hsu CC et al (2019) Antidepressant-like activities of live and heat-killed Lactobacillus paracasei PS23 in chronic corticosterone-treated mice and possible mechanisms. Brain Res 1711:202–213. https://doi.org/10.1016/j.brainres.2019.01.025
Wu Y, Wang Y, Hu A, Shu X, Huang W, Liu J et al (2022) Lactobacillus plantarum-derived postbiotics prevent Salmonella-induced neurological dysfunctions by modulating gut-brain axis in mice. Front Nutr 9:946096. https://doi.org/10.3389/fnut.2022.946096
Żółkiewicz J, Marzec A, Ruszczyński M, Feleszko W (2020) Postbiotics-A step beyond pre- and protiocis. Nutrients 12:2189. https://doi.org/10.3390/nu12082189
Valent D, Arroyo L, Fabrega E, Font IFM, Rodriguez-Palmero M, Moreno-Munoz JA et al (2020) Effects of a high-fat-diet supplemented with probiotics and omega3-fatty acids on appetite regulatory neuropeptides and neurotransmitters in a pig model. Benef Microbes 11:347–359. https://doi.org/10.3920/BM2019.0197
Bravo L (2012) Depressive-like States heighten the aversion to painful stimuli in a rat model of Comorbid Chronic Pain and Depression. Anesthesiology 117(3):613–625. https://doi.org/10.1097/ALN.0b013e3182657b3e
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917. https://doi.org/10.1139/o59-099
Harris WS, von Schacky C, Park Y (2013) In: McNamara RK (ed) Standardizing methods for assessing omega-3 fatty acid biostatus. Hauppauge, New York, USA
Rohde JK, Fuh MM, Evangelakos I, Pauly MJ, Schaltenberg N, Siracusa F et al (2022) A gas chromatography Mass Spectrometry-based method for the quantification of short chain fatty acids. Metabolites 12:170. https://doi.org/10.3390/metabo12020170
van Boxelaere M, Clements J, Callaerts P, D’Hooge R, Callaerts-Vegh Z (2017) Unpredictable chronic mild stress differentially impairs social and contextual discrimination learning in two inbred mouse strains. PLoS ONE 12:e0188537. https://doi.org/10.1371/journal.pone.0188537
Wang L, Guo T, Guo Y, Xu Y (2020) Asiaticoside produces an antidepressant–like effect in a chronic unpredictable mild stress model of depression in mice, involving reversion of inflammation and the PKA/pCREB/BDNF signaling pathway. Mol Med Rep 22:2364–2372. https://doi.org/10.3892/mmr.2020.11305
Ohtsuki S, Yamaguchi H, Kang YS, Hori S, Terasaki T (2010) Reduction of L-type amino acid transporter 1 mRNA expression in brain capillaries in a mouse model of Parkinson’s disease. Biol Pharm Bull 33:1250–1252. https://doi.org/10.1248/bpb.33.1250
Colucci M, Cervio M, Faniglione M, De Angelis S, Pajoro M, Levandis G et al (2012) Intestinal dysmotility and enteric neurochemical changes in a Parkinson’s disease rat model. Auton Neurosci 169:77–86. https://doi.org/10.1016/j.autneu.2012.04.005
Li Y, Zhang Y, Zhang X-L, Feng X-Y, Liu C-Z, Zhang X-N et al (2019) Dopamine promotes colonic mucus secretion through dopamine D5 receptor in rats. Am J Physiology-Cell Physiol 316:C393–C403. https://doi.org/10.1152/ajpcell.00261.2017
Weis S, Schwiertz A, Unger MM, Becker A, Fabbender K, Ratering S et al (2019) Effect of Parkinson’s disease and related medications on the composition of the fecal bacterial microbiota. npj Parkinsons Dis 5:28. https://doi.org/10.1038/s41531-019-0100-x
Choi JE, Borkowski K, Newman JW, Park Y (2021) N-3 PUFA improved post-menopausal depression induced by maternal separation and chronic mild stress through serotonergic pathway in rats-effect associated with lipid mediators. J Nutr Biochem 91:108599. https://doi.org/10.1016/j.jnutbio.2021.108599
Sedlmeier EM, Meyer DM, Stecher L, Sailer M, Daniel H, Hauner H et al (2021) Fetal sex modulates placental microRNA expression, potential microRNA-mRNA interactions, and levels of amino acid transporter expression and substrates: INFAT study subpopulation analysis of n-3 LCPUFA intervention during pregnancy and associations with offspring body composition. BMC Mol Cell Biol 22:15. https://doi.org/10.1186/s12860-021-00345-x
Haddad F, Sawalha M, Khawaja Y, Najjar A, Karaman R (2017) Dopamine and Levodopa Prodrugs for the treatment of Parkinson’s Disease. Molecules 23:40. https://doi.org/10.3390/molecules23010040
Metz VG, Segat HJ, Dias VT, Barcelos RCS, Maurer LH, Stiebe J et al (2019) Omega-3 decreases D1 and D2 receptors expression in the prefrontal cortex and prevents amphetamine-induced conditioned place preference in rats. J Nutr Biochem 67:182–189. https://doi.org/10.1016/j.jnutbio.2019.02.007
Shuto T, Kuroiwa M, Sotogaku N, Kawahara Y, Oh YS, Jang JH et al (2020) Obligatory roles of dopamine D1 receptors in the dentate gyrus in antidepressant actions of a selective serotonin reuptake inhibitor, fluoxetine. Mol Psychiatry 25:1229–1244. https://doi.org/10.1038/s41380-018-0316-x
Mishra A, Singh S, Shukla S (2018) Physiological and functional basis of dopamine receptors and their role in neurogenesis: possible implication for Parkinson’s disease. J Exp Neurosci 12:1–8. https://doi.org/10.1177/1179069518779829
Rashid MA, Kim HY (2016) N-Docosahexaenoylethanolamine ameliorates ethanol-induced impairment of neural stem cell neurogenic differentiation. Neuropharmacology 102:174–185. https://doi.org/10.1016/j.neuropharm.2015.11.011
Patel P, Nankova BB, LaGamma EF (2005) Butyrate, a gut-derived environmental signal, regulates tyrosine hydroxylase gene expression via a novel promoter element. Brain Res Dev Brain Res 160:53–62. https://doi.org/10.1016/j.devbrainres.2005.08.005
Peng H, Yu S, Zhang Y, Yin Y, Zhou J (2022) Intestinal dopamine receptor D2 is required for Neuroprotection against 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopaminergic neurodegeneration. Neurosci Bull 38:871–886. https://doi.org/10.1007/s12264-022-00848-3
Magro F, Fraga S, Ribeiro T, Soares-da-Silva P (2004) Decreased availability of intestinal dopamine in transmural colitis may relate to inhibitory effects of interferon-gamma upon L-DOPA uptake. Acta Physiol Scand 180:379–386. https://doi.org/10.1111/j.1365-201X.2004.01260.x
Fritsche K, Byrge M, Feng C (1999) Dietary omega-3 polyunsaturated fatty acids from fish oil reduce interleukin-12 and interferon-gamma production in mice. Immunol Lett 65:167–173. https://doi.org/10.1016/S0165-2478(98)00109-6
Wu Y, Xu H, Cao X, Liu R, Tang L, Zeng Z et al (2020) Bacillus amyloliquefaciens ameliorates H(2)O(2)-Induced oxidative damage by regulating transporters, tight junctions, and apoptosis gene expression in cell line IPEC-1. Probiotics Antimicrob Proteins 12:649–656. https://doi.org/10.1007/s12602-019-09538-5
Hamamah S, Aghazarian A, Nazaryan A, Hajnal A, Covasa M (2022) Role of microbiota-gut-brain axis in regulating dopaminergic signaling. Biomedicines 10:436. https://doi.org/10.3390/biomedicines10020436
Liu J, Jiang J, Qiu J, Wang L, Zhuo J, Wang B et al (2022) Urolithin A protects dopaminergic neurons in experimental models of Parkinson’s disease by promoting mitochondrial biogenesis through the SIRT1/PGC-1α signaling pathway. Food Funct 13:375–385. https://doi.org/10.1039/D1FO02534A
Chen VC-H, Chiu C-C, Chen L-J, Hsu T-C, Tzang B-S (2018) Effects of taurine on striatal dopamine transporter expression and dopamine uptake in SHR rats. Behav Brain Res 348:219–226. https://doi.org/10.1016/j.bbr.2018.04.031
Tan Q, Hu J, Zhou Y, Wan Y, Zhang C, Liu X et al (2021) Inhibitory effect of Lactococcus lactis subsp. lactis HFY14 on Diphenoxylate-Induced Constipation in mice by regulating the VIP-cAMP-PKA-AQP3 signaling pathway. Drug Des Devel Ther 15:1971–1980. https://doi.org/10.2147/dddt.S309675
Cheon MJ, Lee NK, Paik HD (2021) Neuroprotective effects of Heat-killed Lactobacillus plantarum 200655 isolated from Kimchi against Oxidative Stress. Probiotics Antimicrob Proteins 13:788–795. https://doi.org/10.1007/s12602-020-09740-w
Douma EH, de Kloet ER (2020) Stress-induced plasticity and functioning of ventral tegmental dopamine neurons. Neurosci Biobehav Rev 108:48–77. https://doi.org/10.1016/j.neubiorev.2019.10.015
Li N, Wang Q, Wang Y, Sun A, Lin Y, Jin Y et al (2018) Oral Probiotics ameliorate the behavioral Deficits Induced by chronic mild stress in mice via the gut microbiota-inflammation Axis. Front Behav Neurosci 12:266. https://doi.org/10.3389/fnbeh.2018.00266
Haghighat N, Rajabi S, Mohammadshahi M (2021) Effect of synbiotic and probiotic supplementation on serum brain-derived neurotrophic factor level, depression and anxiety symptoms in hemodialysis patients: a randomized, double-blinded, clinical trial. Nutr Neurosci 24:490–499. https://doi.org/10.1080/1028415x.2019.1646975
Zhao Z, Ning J, Bao X-Q, Shang M, Ma J, Li G et al (2021) Fecal microbiota transplantation protects rotenone-induced Parkinson’s disease mice via suppressing inflammation mediated by the lipopolysaccharide-TLR4 signaling pathway through the microbiotia-gut-brain axis. Microbiome 9:1–27. https://doi.org/10.1186/s40168-021-01107-9
Griffith TA, Russell JS, Naghipour S, Helman TJ, Peart JN, Stapelberg NJC et al (2022) Behavioural disruption in diabetic mice: neurobiological correlates and influences of dietary α-linolenic acid. Life Sci 311:121137. https://doi.org/10.1016/j.lfs.2022.121137
Acknowledgements
We would like to thank Editage (www.editage.co.kr) for English language editing. Hyunji Cho is grateful for financial support from the Hyundai Motor Chung Mong Koo Foundation.
Funding
This study was supported by the BK21 FOUR (Fostering Outstanding Universities for Research) project of the National Research Foundation of Korea Grant, and a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (NRF-2021R1A2B5B02002208). We would like to thank Editage (https://www.editage.co.kr ) for English language editing. Hyunji Cho is grateful for financial support from the Hyundai Motor Chung Mong Koo Foundation.
Author information
Authors and Affiliations
Contributions
Experiments and data analysis were performed by Hyunji Cho. The first draft of the manuscript was written by Hyunji Cho. Study design was supervised, and the manuscript was revised by Yongsoon Park. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(docx 107 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cho, H., Park, Y. Synergistic Antidepressant-like Effects of Biotics and n-3 Polyunsaturated Fatty Acids on Dopaminergic Pathway through the Brain-Gut Axis in Rats Exposed to Chronic Mild Stress. Probiotics & Antimicro. Prot. (2024). https://doi.org/10.1007/s12602-024-10332-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s12602-024-10332-1