Abstract
Major depressive disorder (MDD) is a neurasthenic disease, which is the second-largest burden of disease globally. Increasing studies have revealed that depression is associated with abnormalities in gut microbiota and metabolites. Several species of bacteria have been classified as psychobiotics, which confer mental health benefits through interactions with commensal gut microbiota. Therefore, it is essential to identify new psychobiotics and elucidate their mechanisms in the treatment of depression. This study aims to evaluate the antidepressant effect of Akkermansia muciniphila (AKK) in a mouse model of depression induced by chronic restraint stress (CRS). C57BL/6 male mice were divided into three groups: mice subjected to CRS, mice not subjected to CRS, and mice treated with AKK for 3 weeks. Behavioral tests were performed, and hormone, neurotransmitter, and brain-derived neurotrophic factor (BDNF) levels were measured. Cecal microbiota was analyzed using 16S rRNA gene sequencing, and serum metabolites were detected using untargeted metabolomics. In addition, correlations between altered gut microbiota and metabolites with significant variations in serum associated with AKK ameliorating depression were analyzed using Pearson’s correlation coefficient. The results revealed that AKK significantly ameliorated depressive-like behavior and restored abnormal variations in depression-related molecular (corticosterone, dopamine, and BDNF). Moreover, AKK altered chronic stress–induced gut microbial abnormalities. Untargeted metabolomics analysis revealed 23 potential biomarkers in serum that could be associated with the mechanisms underlying CRS-induced depression and the therapeutic effects of AKK. Pearson’s correlation coefficient analysis revealed that AKK predominantly upregulated β-alanyl-3-methyl-l-histidine and edaravone to relieve depression. Furthermore, β-alanyl-3-methyl-l-histidine and edaravone exhibited the antidepressant phenotype in mice subjected to CRS. In conclusion, the study demonstrated that AKK ameliorates chronic stress–induced depressive symptoms in mice by regulating gut microbiota and metabolites.
Key points
• AKK reduces depressive-like behaviors induced by chronic stress.
• AKK regulates the gut microbial structure and metabolomics of serum under the chronic stress.
• Antidepressant effect of AKK correlates with the increase of β-alanyl-3-methyl-l-histidine and edaravone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Major depressive disorder (MDD) is a neurasthenic disease that is characterized by depressed mood, diminished interests, impaired cognitive function, and vegetative symptoms, including disturbed sleep or decreased appetite (Otte et al. 2016). MDD severely influences personal work, learning, and social interaction, causing disability and suicide among patients. Being a clinically prevalent mental disorder, depression is reported that 1 in 5 adults is affected by it throughout their lives, which is the second leading cause of disability in the USA (GBD 2016 Disease and Injury Incidence and Prevalence Collaborators 2017).
Based on the World Health Organization (WHO) report, approximately 350 million people suffered depression globally in 2015, and the prevalence rate was as high as 4.4% (Smith 2014). Additional research have predicted that depression will be the second-largest burden of disease globally by 2020 (Murray et al. 2013). Notably, the pathogenesis of depression has not been fully understood.
Over the last few decades, a few studies have implicated genetic factors, personality traits, and environmental factors in the pathogenesis of depression. Gut microbiota are considered a “second brain,” which can regulate brain development and function (Franzosa et al. 2015). Moreover, gut microbiota and the central nervous system exchange information in two ways via nerve, endocrine, and immune pathways (Cryan and Dinan, 2012). Studies have demonstrated that gut microbes modulate the development of depression (Fung et al. 2017). While acknowledging a strong link between gut microbiota and depression, the precise microbial strain and mechanism of interaction between gut microbiota and metabolites remain obscure.
Akkermansia muciniphila, a gram-negative anaerobic bacteria, belonging to the phylum Verrucomicrobia, is a symbiotic bacterium widely distributed in the human intestinal mucus layer, accounting for 1–5% of human intestinal microorganisms (Derrien et al. 2008). The potential application of AKK as a next-generation probiotic drug has attracted the attention of researchers (Zhai et al. 2019; Hagi and Belzer 2021; Zhang et al. 2021). Several studies presently investigate the ability of AKK to ameliorate metabolic disorders (Depommier et al. 2019). Previous studies revealed that the abundance of AKK is inversely correlated with progeria, inflammatory bowel disease (IBD), colitis-associated colorectal cancer (CAC), and hypertension (Bárcena et al. 2019; Li et al. 2017; Png et al. 2010; Wang et al. 2020). In addition, AKK could exhibit a protective effect on neurological diseases such as epilepsy, amyotrophic lateral sclerosis (ALS), Alzheimer’s disease (AD), and autism (Blacher et al. 2019; Li et al. 2019; Olson et al. 2018; Wang et al. 2011).
Recent studies reported that AKK is negatively correlated with depression (McGaughey et al. 2019). Nonetheless, the role of AKK in the treatment of depression and the underlying mechanism of anti-depression remain unclear. Therefore, this study aimed to investigate the effect of AKK on regulation of gut microbiota, metabolites, and the mechanism of reducing depression symptoms in mice.
Materials and methods
Animals
Male C57BL/6 mice (6–8 weeks) were purchased from the Comparative Medicine Centre of Yangzhou University (Approval ID: SCXK-(Su) 2011–0003). Animals were acclimatized for 7 days before experimental procedures began. The mice were maintained under standard laboratory conditions (temperature: 22 ± 2 °C; humidity: 50 ± 10%) for a 12-h light/12-h dark cycle and allowed to freely feed and drink. The experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the Nanjing University of Chinese Medicine.
Treatment and chronic restraint stress
The mice were randomly divided into three groups: (1) control group (n = 6)—200 μl phosphate-buffered saline (PBS) was administered via gavage without application of chronic restraint stress (CRS) for 3 weeks; (2) CRS group (n = 6)—treated with 200 μl PBS via gavage and CRS applied for 3 weeks; (3) CRS + AKK group (n = 6)—200 μl (5 × 108 colony-forming unit (CFU) /mL) of AKK was administered via gavage and CRS applied for 3 weeks (Routy et al. 2018); (4) AKK group (n = 6)—200 μl (5 × 108 CFU/mL) of AKK was administered via gavage and without CRS applied for 3 weeks; (5) CRS + Lactobacillus L group (n = 6)—200 μl (5 × 108 CFU/mL) of Lactobacillus was administered via gavage and CRS applied for 3 weeks; (6) CRS + Lactobacillus H group (n = 6)—200 μl (5 × 109 CFU/mL) of Lactobacillus was administered via gavage and CRS applied for 3 weeks. The body weights and food intake levels were monitored every 3 days.
AKK (ATCC® BAA-835™) was cultured in brain–heart infusion liquid medium (supplemented with 0.1% mucin from porcine stomach) and anaerobically incubated at 37 °C. Lactobacillus plantarum (CICC® 23,133) was cultured in MRS (De Man, Rogosa and Sharpe) broth at 37 °C. Bacteria used for oral administration were prepared by suspending them in PBS. Suspensions of 109 CFU/mL were obtained using a spectrophotometer (NanoDrop 2000c, Thermo, Waltham, MA, USA) at an optical density of 600 nm.
CRS is a method of inducing depressive-like behavior in animals and it is widely used in depression-related research because of its simplicity and superior reproducibility. CRS was conducted as previously described (Luo et al. 2014). An individual mouse was restrained in 50-ml conical centrifuge tube for 3 h daily. Small holes were made in the front part and the periphery of the centrifuge tubes to ensure that the mice could breathe normally while being immobilized. Details of the animal experimental procedures are presented in Fig. 1a.
AKK ameliorates CRS-induced depressive-like behavior in mice. a The timeline of the procedure. b Total distance covered by mice in open-field testing in 5 min. c Measurement of immobility time over the last 4 min during a 6-min testing time in tail suspension test. d Measurement of immobility time over the last 4 min during a 6-min testing time in forced swimming test. Data are presented as means ± SEM and n = 6/group; * and ** indicate P < 0.05 and P < 0.01
Metabolite administration
To evaluate the effect of specific metabolites on behavioral phenotypes, β-alanyl-3-methyl-l-histidine was orally administered at a dose of 10 mg/mouse for 3 weeks (Kaneko et al. 2017), whereas edaravone was administered at a dose of 10 ml/kg per mouse for the last 7 days of restraint stress (Jangra et al. 2017).
Hepatic and renal function
Serum alanine aminotransferase (ALT); aspartate transaminase (AST); creatinine, urea, total cholesterol (TCHO); triglyceride; high-density lipoprotein cholesterol (HDL-C); and low-density lipoprotein cholesterol (LDL-C) were measured by automatic biochemical analyzer (Olympus AU640, Olympus Optical Co., Tokyo, Japan).
Behavioral tests
Open-field test
To examine the locomotor and exploratory activity, mice were placed in the center of an individual open-field arena (40 × 40 × 40 cm) with a video camera fixed at the top and allowed to freely explore the area for 5 min. The total distance traveled and time spent in the central zone was measured. Notably, the arena was thoroughly cleaned using 75% ethanol before each animal entered.
Tail suspension test
Mice were individually suspended 50 cm above the surface of a table with adhesive tape, which was placed 1 cm away from the tip of the tail for 6 min. A camera placed in front of the device was used to record the behavior of mice. Mice were considered immobile when they hung passively motionless. The software recorded the immobility time over the last 4 min of the 6-min testing period.
Forced swimming test
Mice were individually placed in clear Plexiglas cylinders (25 cm high; 10 cm in diameter) filled with water to a depth of 10 cm (23–25° C) for 6 min. A camera was mounted to record the immobility of mice. Immobility in this study was defined as mice floating passively in water without struggling. ANY-maze software (Stoelting, Wood Dale, IL, USA) was used to measure the immobility time over the last 4 min of the 6-min testing period.
Sample collection and preparation
Blood samples were drawn from eyeballs and serum was obtained via centrifugation at 3,000 rpm, 4 °C for 10 min after standing for 1 h. Subsequently, the serum was stored at − 80 °C until UHPLC-QE Orbitrap/MS analysis (1290, Agilent Technologies, Hilden, Germany). After the mice were euthanized, cecal samples were immediately collected in clean centrifuge tubes and placed in dry ice, and then stored at − 80 °C until microbiological analysis. Hippocampal tissues were weighed and subsequently frozen at − 80 °C for quantitative real-time PCR analysis (qRT-PCR).
Quantitative real-time PCR
Quantitative real-time PCR was performed to detect the RNA expression level of brain-derived neurotrophic factor (BDNF) in the hippocampus. Trizol extraction reagent (Invitrogen, Carlsbad, CA, USA) was used to extract hippocampal RNA. QRT-PCR was conducted on the 7500 Fast real-time PCR system (Applied Biosystems, Foster City, CA, USA) using SYBR Green Master Mix (Vazyme Biotech Co. Ltd., Nanjing, JS, China). Amplification reactions were run in the “no template control” mode, and all reactions were performed in triplicate, for each probe used. Cycle threshold (Ct) values were recorded and normalized to the GAPDH using the 2−ΔΔCt method. All procedures were performed based on the manufacturer’s instruction. The primers (Vazyme Biotech Co. Ltd., Nanjing, JS, China) used were as follows:
GAPDH forward: 5′-TCATACTTCGGTTGCATGAAGG-3′.
GAPDH reverse: 5′-ATGTCACGCACGATTTCC-3′.
BDNF forward: 5′-TCATACTTCGGTTGCATGAAGG-3′.
BDNF reverse: 5′-AGACCTCTCGAACCTGCCC-3′.
Determination of corticosterone, serotonin, and dopamine levels in serum
Corticosterone level was determined using commercial ELISA kits (Yi Fei Xue Biotechnology, Nanjing, JS, China) while serotonin and dopamine levels were determined using commercial ELISA kits (Jin Yibai Biological Technology Co. Ltd., Nanjing, JS, China) according to the manufacturer’s instructions.
16S rRNA gene sequence analysis
Cecal and fecal samples were collected after the mice were euthanized for 16S rRNA gene sequence analysis. Microbial DNA was extracted using HiPure Soil DNA Kits (Magen, Guangzhou, GD, China) according to the manufacturer’s protocols. The 16S rDNA targeting the V3-V4 region of the ribosomal RNA gene was amplified by PCR. Amplicons were excised from 2% agarose gels, purified using AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) and quantified using ABI StepOnePlus Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). Purified amplicons were pooled in equimolar and paired-end sequenced (PE250) on an Illumina platform (Illumina, San Diego, CA, USA). Paired-end clean reads were merged as raw tags using fast length adjustment of short reads (FLSAH) with a minimum overlap of 10 bp and mismatch error rates of 2% (Magoč and Salzberg, 2011). The effective tags were clustered into operational taxonomic units (OTUs) with ≥ 97% similarity using the UPARSE pipeline (http://drive5.com/uparse/) (Edgar 2013). Comparisons of species between groups were performed using R software (v2.15.3, http://www.R-project.org). The analyses results of OTUs based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway were inferred using Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) (Langille et al. 2013). The Functional Annotation of Prokaryotic Taxa (FAPROTAX) database and associated software were used to generate the ecological function profiles of bacteria. The raw 16S rRNA sequence data have been deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) database under accession number PRJNA707611.
Analysis of serum metabolomics using ultra high-performance liquid chromatography-Q exactive Orbitrap-mass spectrometry
Liquid chromatography-tandem mass spectrometry (LC–MS/MS) analyses were performed using a UHPLC system (1290, Agilent Technologies, Hilden, Germany) with a UPLC HSS T3 column (2.1 mm × 100 mm, 1.8 μm) coupled to a Q Exactive benchtop Orbitrap mass spectrometer (Orbitrap MS, Thermo, Waltham, MA, USA).
The mobile phase A comprised of 0.1% formic acid in water for positive ionization mode, and 5 mmol/L ammonium acetate in water for negative ionization mode, and the mobile phase B comprised of acetonitrile. The elution gradient was set as follows: 0 min, 1% B; 1 min, 1% B; 8 min, 99% B; 10 min, 99% B; 10.1 min, 1% B; and 12 min, 1% B. The flow rate was set to 0.5 mL/min and the injection volume was 2 μL. The Q Exactive mass spectrometer was used because of its ability to acquire MS/MS spectra on an information-dependent acquisition (IDA) mode during the LC–MS/MS experiment. The data acquisition software (Xcalibur 4.0.27, Thermo, Waltham, MA, USA) continuously evaluated the full scan survey MS data in the IDA-based mode because it collects and triggers acquisition of MS/MS spectra depending on the preselected criteria. Electrospray ionization (ESI) source conditions were set as follows: sheath gas flow rate was 45 Arb, Aux gas flow rate was 15Arb, capillary temperature was 320 °C, full Ms resolution was 70,000, MS/MS resolution was 17,500, collision energy was 20/40/60 eV in normalized collisional energy model, spray voltage was 3.8 kV (positive mode) or − 3.1 kV (negative mode).
Full MS raw data files including retention time alignment, peak detection, and peak matching were converted to mzML format using ProteoWizard (https://proteowizard.sourceforge.io/), and processed using R package XCMS (v3.2, http://www.R-project.org). Afterwards, the data files were filtered based on the following criterion: sample numbers with metabolites that were less than 50% of all sample numbers in a group. Subsequently, normalization to an internal standard for each sample was conducted, and missing values were replaced by half of the minimum value observed in the dataset by default. The preprocessing results generated a data matrix comprising retention times (RTs), mass-to-charge ratio (m/z) values, and peak intensity.
Data analysis
Statistical analyses were performed using IBM SPSS Statistics version 22 (IBM Corp., Armonk, NY, USA) and GraphPad Prism version 7 (GraphPad Software, Inc., La Jolla, CA, USA). All data were presented as mean ± standard error of the mean (SEM). Significant differences between the two groups were analyzed using Student’s t-test, and multiple comparisons were analyzed using one-way ANOVA followed with Tukey’s post hoc multiple comparisons test. Microbiota-related analyses were performed using Welch’s t-test. The associations of serum metabolite intensities with genera levels were subsequently analyzed using Pearson’s correlation coefficient. A P value of < 0.05 was considered statistically significant.
Data availability statement
The datasets generated during the current study are available from the corresponding author on reasonable request.
Results
Safety testing of AKK
We analyzed the serum levels of ALT, AST, creatinine, urea, TCHO, triglyceride, HDL-C, and LDL-C by animal biochemical analyzer. As shown in Supplemental Fig. S1a-h, compared to control group, single oral administration of AKK did not significantly affect the biochemical parameters of mice.
AKK ameliorates CRS-induced depressive-like behavior in mice
CRS increased the incidence of depressive-like behavior in mice. To investigate the ability of AKK in ameliorating depression in mice under stress conditions, AKK was orally administered to the mice for 3 weeks, then open-field test (OFT), forced swimming test (FST), and tail suspension test (TST) were performed. As shown in Fig. 1b–d, the total distance covered by the mice in the CRS group significantly reduced. However, mice treated with AKK exhibited a significant improvement in OFT. CRS results revealed that mice floated passively in water, in turn increasing immobility time of mice in FST. In contrast with the CRS group, AKK administration significantly decreased the immobility time of swimming. In TST, the immobility of the stress mice increased, while AKK significantly reduced immobility, indicating a higher activity rate than the CRS and normal groups. To verify the antidepressant effect of AKK on CRS mice again, we added two doses of Lactobacillus administration groups. As shown in Supplemental Fig. S2, the traveling distance in the CRS + AKK group, the CRS + Lactobacillus L group, and the CRS + Lactobacillus H group was significantly more than that in the CRS group (P < 0.05). In addition, administration of AKK or high dose of Lactobacillus significantly decreased the immobility times of mice in TST (P < 0.01, P < 0.05). The immobility times of swimming in the CRS + AKK group, CRS + Lactobacillus L group, and CRS + Lactobacillus H group were significantly decreased compared to those in the CRS group (P < 0.01, P < 0.05, P < 0.01). Besides, AKK increased the body weight and food intake of CRS mice (Supplemental Fig. S3).
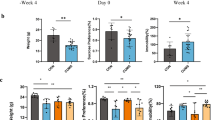
AKK regulates abnormal variations in hormone, neurotransmitter, and BDNF expression levels in CRS-induced mice
Hormone, neurotransmitters, and BDNF have been associated with the behavioral stress response. We analyzed the concentrations of hormone and neurotransmitters in the serum, as well as BDNF expression level in the hippocampus. As shown in Fig. 2a–d, CRS increased corticosterone concentration in serum, and decreased the concentration of dopamine and serotonin significantly. Moreover, BDNF expression at the mRNA level in the hippocampus of mice in the CRS group reduced significantly when compared to the control group (P < 0.05), whereas AKK inhibited CRS-induced increase in corticosterone concentration in serum, which in turn caused significant recovery in dopamine and BDNF levels when compared with the CRS group (P < 0.01). The results suggested that AKK could ameliorate depressive-like behavior in CRS-induced mice by regulating abnormal variations in the concentrations of hormone, neurotransmitters, and BDNF expression levels.
AKK regulates abnormal variations in hormone, neurotransmitter, and BDNF levels in CRS-induced mice. a Corticosterone concentration in serum. b Dopamine concentration in serum. c Serotonin concentration in serum. d BDNF mRNA level in mice hippocampus. Data are presented as means ± SEM; * and ** indicate P < 0.05 and P < 0.01
AKK regulates gut microbiota at the phylum and genus levels in CRS-induced mice by 16SrRNA gene sequencing
We evaluated the effects of AKK on the gut microbiota composition by sequencing bacterial 16S rRNA V3 + V4 region. A high-throughput pyrosequencing of the samples generated 1,149,582 raw tags, which were processed by QIIME (Caporaso et al. 2010). The tags were clustered into OTUs with ≥ 97% similarity using UPARSE pipeline (Kryukov et al. 2020). The analyses results revealed that the microbiota composition was altered significantly by CRS, and details are illustrated in Fig. 3a and b. At the phylum level, AKK upregulated Verrucomicrobia, and downregulated Epsilonbacteraeota, Patescibacteria, Chloroflexi, and Acidobacteria when compared with the CRS group (Fig. 3c). At the genus level, AKK increased the relative abundance of Akkermansia, and decreased the relative abundance of Helicobacter, Candidatus_Saccharimonas, Eubacterium_brachy_group, and Lachnoclostridium (Fig. 3d).
AKK regulates gut microbiota in CRS-induced mice. a, b Relative abundance distribution of the most abundant microbial taxa at the phylum (a) and genus (b) levels. c, d The heat map representing the key taxa that were significantly altered in the control group vs the CRS group and the CRS group vs the CRS + AKK group at the phylum (c) and genus (d) levels through indicator species analysis. Values are presented as means ± SEM (n = 5). Differences were analyzed using t-test, *P < 0.05 and **P < 0.01 vs the CRS group
We examined the structure of gut microbiota using principal component analysis (PCA) and beta diversity using analysis of similarity (ANOSIM) (Lee et al. 2018; Ali et al. 2021). No significant difference was observed between the CRS group and the CRS + AKK group (Supplemental Fig. S4).
Predicted function of gut microbiota regulated by AKK
To evaluate variations in the functional capacities of intestinal bacterial community between the CRS and CRS + AKK groups, PICRUSt analysis was performed to determine KEGG pathways that are associated with intestinal microbiota. The KEGG pathway analyses revealed that pathways associated with neurodegenerative diseases were inhibited in the CRS + AKK group when compared to the CRS group (Fig. 4a). The CRS + AKK group exhibited significantly lower relative abundances of genes involved in tryptophan metabolism, geraniol degradation, caprolactam degradation, fluorobenzoate degradation, and Parkinson’s disease than in the CRS group, which was consistent with the KEGG pathway analysis results (P < 0.05) (Fig. 4b).
AKK regulates abnormal variations in serum metabolites in CRS-induced mice
UHPLC-QE Orbitrap/MS was used to analyze serum samples from the control, CRS, and CRS + AKK groups in the positive ion mode, which represented physiological status, pathological conditions, and intervening effects.
The metabolic profile of the CRS group was distinct from that of the control group (Fig. 5a). Furthermore, the CRS + AKK group differed from the CRS group, although it was closely similar to the control group. The results revealed that CRS induced considerable metabolic variations in the serum and that the variations could be modulated through administration of AKK.
AKK regulates abnormal variations in serum metabolites in CRS-induced mice. aPartial least squares-discriminant analysis score plot of serum samples collected from different groups in positive mode. b Significant variations in serum metabolites in the control and CRS groups are presented in the histogram. c Top 20 of the KEGG pathway enrichment analysis of differential metabolites between the CRS and CRS + AKK groups. d A correlation matrix heat map representing statistically significant correlation values between perturbed gut microbiota genera and altered serum metabolites in mice. Red squares represent significant positive correlations; green squares represent significant negative correlations. POS05423 and POS07196 represent 2″,3″,6″-Tris-O-(3,4,5-trihydroxybenzoyl)-3′-glucosyl-2′,4′,6′-trihydroxyacetophenone and {4-[2,3-dioxo-3-(2,4,6-trihydroxy-3-methoxyphenyl)propyl]-2-hydroxy-6-methoxyphenyl}oxidanesulfonic acid. e β-Alanyl-3-methyl-l-histidine expression in serum. f Edaravone expression in serum. * and ** indicate P < 0.05 and P < 0.01
Serum metabolites that were altered substantially were selected using t-test and variable importance in projection (VIP) values. We considered the variables that were far from the origin based on the t-test (P < 0.05) with a VIP ≥ 1 potential biomarkers associated with depression. A total of 95 variables, which contributed to the clustering of the t-test and VIP values based on the variations in serum metabolic profiles between the control and CRS groups, were treated as potential biomarkers.
After data processing using an in-house MS/MS database, 23 metabolites were identified (Fig. 5b). Based on the KEGG pathway enrichment analyses, differential metabolites between CRS and CRS + AKK groups were predominantly enriched in cholinergic synapse, fat digestion and absorption, degradation of aromatic compounds, fatty acid degradation, vitamin digestion and absorption, butanoate metabolism, carbon metabolism, pantothenate and CoA biosynthesis, metabolic pathways, and digestion and absorption (Fig. 5c). The analysis results revealed that AKK ameliorated depression by regulating the pathways.
A heat map of correlation was constructed to identify the potential link between altered gut microbiota and potential biomarkers in serum that are associated with depression (P < 0.05). The results revealed multiple significant associations between the perturbed gut microbiota and altered metabolites in mice with CRS-induced depression (Fig. 5d). The correlation analysis results revealed that AKK was positively correlated with 3 metabolites (β-alanyl-3-methyl-l-histidine, edaravone, and 2″,3″,6″-Tris-O-(3,4,5-trihydroxybenzoyl)-3′-glucosyl-2′,4′,6′-trihydroxyacetophenone), and negatively correlated with 2 metabolites (2-pyrrolidineacetic acid and aminopyrrolnitrin). β-Alanyl-3-methyl-l-histidine and edaravone were upregulated when compared with the CRS group after administration of AKK (Fig. 5e, f).
Administration of potential metabolites ameliorate CRS-induced depressive-like behavior, and hormone, neurotransmitter, and BDNF levels
To investigate the effect of specific metabolites regulated by AKK on depressive behavior phenotype, CRS-induced mice were treated with β-alanyl-3-methyl-l-histidine or edaravone. OFT results revealed that the total distance covered by mice treated with β-alanyl-3-methyl-l-histidine or edaravone significantly increased when compared with the CRS group (Fig. 6a). In addition, administration of β-alanyl-3-methyl-l-histidine or edaravone decreased the immobility time of mice in TST (Fig. 6b). However, both metabolites did not reduce the immobility time of mice in FST (Fig. 6c). We subsequently determined corticosterone, neurotransmitters, and BDNF levels, and the results revealed that β-alanyl-3-methyl-l-histidine and edaravone decreased corticosterone concentration significantly (Fig. 6d). Edaravone increased serotonin concentration considerably, whereas β-alanyl-3-methyl-l-histidine increased dopamine concentration (Fig. 6e, f). Both metabolites tended to restore BDNF expression level in the hippocampus, although the effect was not statistically significant (Fig. 6g).
Administration of potential metabolites ameliorate CRS-induced depressive-like behavior, and hormone, neurotransmitters, and BDNF levels. a Total distance covered by mice in open-field testing in 5 min. b Measurement of immobility time over the last 4 min during a 6-min testing time in tail suspension test. c Measurement of immobility time over the last 4 min during a 6-min testing time in forced swimming test. d Corticosterone concentration in serum. e Dopamine concentration in serum. f Serotonin concentration in serum. g BDNF mRNA level in mice hippocampus. Data are displayed as means ± SEM and n = 5/group; * and ** indicate P < 0.05 and P < 0.01
Discussion
Depression is a psychiatric illness with elusive pathogenesis. The current clinical first-line antidepressants primarily include selective serotonin reuptake inhibitors (SSRI), and serotonin and norepinephrine reuptake inhibitors (SNRI), as well as norepinephrine and specific serotonin energy antidepressants (NaSSA). While alleviating the symptoms of depression, the antidepressants have side effects including nausea, vomiting, and sexual dysfunction (Rothmore 2020). Furthermore, a few antidepressants double the risk of suicidality and aggression in children and adolescents (Sharma et al. 2016). Thus, the development of safe and non-toxic alternatives to antidepressants is critical for the treatment of depression.
Probiotics are well-known for their regulatory activity on body weight, lipid metabolism, and immune response of the host. Nevertheless, increasing research on the gut-brain axis over the last few years has led to several studies on the effects of probiotics on mental state and cognitive functions. As a consequence, several animal experiments and clinical trials suggested that oral probiotics exhibited antidepressant effect to a certain extent (Pinto-Sanchez et al. 2017; Meyer and Vassar 2018; Liu et al. 2020; Tian et al. 2020).
AKK, a symbiotic bacterium of the mucus layer, is considered a potential probiotic. The probiotic effects of AKK, including metabolic modulation, immune regulation, and gut health protection, have been investigated extensively. Reports indicate that various neurological disorders including ALS, AD, and autism disrupt the abundance of AKK (Blacher et al. 2019; Li et al. 2019; Wang et al. 2011). Symptoms could be relieved by simply administering AD mice with AKK (Ou et al. 2020).
We demonstrated for the first time that orally administered AKK could ameliorate depressive-like behavior caused by exposure to chronic stress using a validated chronic restraint stress model of depression (Fig. 1).
AKK alleviates depression-like behaviors by affecting the levels of the monoamine neurotransmitter and BDNF
Several hypotheses have been posited in relation to the pathogenesis of depression; the monoamine neurotransmitter hypothesis has been supported by numerous studies (Haase and Brown 2015). Decreased levels of monoamine neurotransmitters in the central nervous system could cause depression (Castrén 2005). The classic monoamine neurotransmitters associated with the pathophysiology of depression include serotonin and dopamine (Hasler 2010). Our data corroborate with findings of previous studies, which demonstrated AKK increased serum concentrations of serotonin and dopamine in CRS-induced mice. Chronic stress could result in excessive activation of the HPA (hypothalamic–pituitary–adrenal) axis, causing an increase in circulating glucocorticoids, including increasing the corticosterone levels in rodents (Spencer and Deak 2017). We established that supplementing CRS-induced mice with AKK could ameliorate stress-induced serum corticosterone levels.
BDNF is a neurotrophin that modulates neuroplasticity in the brain, which regulates the pathogenesis of depression. It is widely distributed in the hippocampus and closely associated with the regeneration and repair of neurons. In addition, the BDNF activation pathway is associated with neurotrophic plasticity and synaptic plasticity in depression (Rakhit et al. 2005). mRNA levels of BDNF were decreased in the hippocampus of chronically stressed rats and depressed subjects (Bai et al. 2016; Duman and Monteggia, 2006). Generally, low BDNF protein and mRNA levels were observed in patients with MDD. Antidepressant treatment has been reported to increase the BDNF levels (Martinotti et al. 2016). CRS resulted in a decrease of BDNF levels in the hippocampus of mice, whereas the administration of AKK increased BDNF mRNA expression levels in the hippocampus (Fig. 2d), suggesting that AKK could enhance the synaptic signaling pathway and neuronal connections.
AKK improves depression by regulating the dysbiosis of the gut microbiota
Several strains have been demonstrated to possess antidepressant effects, which could be one of the mechanisms of modulating intestinal microecology (Moya-Pérez et al. 2017; Pinto-Sanchez et al. 2017; Tian et al. 2020). Therefore, we performed a comprehensive analysis of gut microbiota by sequencing the 16S rRNA gene. PCA and ANOSIM analysis results revealed that chronic stress exerted considerable effect on the overall gut microbiota composition. Nevertheless, AKK did not significantly influence variations in gut microbiota induced by chronic stress. AKK did not alter the stress-induced structure of gut microbiota, although prebiotics could decrease the diversity of gut microbiota due to selective proliferation of beneficial bacteria, and inhibit the growth of conditionally pathogenic bacteria (Slavin 2013). We further selected certain key taxa based on Welch’s t-test results to analyze the effect of chronic stress on gut microbiota. Notably, not all the results were consistent with the findings of previous studies. For example, previous studies reported a decrease in the abundance of Firmicutes, whereas Bacteroidetes, Proteobacteria, and Actinobacteria increased considerably in patients with MDD (Jiang et al. 2015). In the present study, the tendency of the depressed mice was converse. The abundance of Desulfovibrio decreased in the Aβ-induced AD-like mice, although treatment with macro-molecular yeast β-glucan could reverse the occurrence and confer benefit for the enhancement of Aβ-induced cognitive decline (Xu et al. 2020). The present study revealed that chronic stress could reduce the abundance of Desulfovibrio. However, the alteration of bacterial abundance was irreversible after administration of AKK. Our results revealed that AKK increased the abundance of Verrucomicrobia and Akkermansia substantially in CRS-induced mice, which could be attributed to the fact that AKK belongs to the phylum Verrucomicrobia, and genus Akkermansia.
Strikingly, the relative abundance of Acidobacteria “normalized” after administration of AKK. Notably, an increase in the abundance of Acidobacteria is rarely reported in patients with depression. However, a significant difference was observed in the results of our study. The observation could be a consequence of disease or temporary stress feedback. A previous study suggested that persistent pathogens such as Helicobacter could differentially influence mood disorders among women and men (Simanek et al. 2018). Here, the relative abundance of Helicobacter was decreased after AKK administration in CRS-induced mice.
We used PICRUSt to predict the functional capabilities of the microbial community. Differential gene expression and KEGG pathways between CRS and CRS + AKK groups were largely associated with neurodegenerative diseases, metabolism, and degradation (Fig. 4). Remarkably, pathways involved in neurodegenerative diseases, tryptophan metabolism, and geraniol degradation were downregulated considerably after AKK treatment. Tryptophan metabolism has been a therapeutic target in neurodegeneration (Platten et al. 2019). During infection, tryptophan metabolism is affected and gut microbiota activate indoleamine 2,3-dioxygenase (IDO), which degrades tryptophan via the kynurenine pathway and depleted tryptophan, thereby causing depression. After the production of kynurenine through tryptophan metabolism, quinolinic acid was produced under the mediation of enzymatic reaction, causing neurodegenerative changes. The maternal separation model of depression in rats fed with Bifidobacterium infants revealed reduced tryptophan metabolism, and the rats exhibited antidepressant ability (Desbonnet et al. 2008). Geraniol, with neuroprotection and anti-inflammation activities, has been demonstrated to exhibit antidepressant-like effect (Deng et al. 2015). Administration of AKK potentially reduced the degradation of geraniol to relieve depression.
AKK ameliorates depression by regulating metabolic disorders related to gut microbiota
Dysbiosis of the gut microbiota in mice with CRS was coupled with the alteration in serum metabolome. We used untargeted metabolomics to detect metabolites in the serum of mice to elucidate the underlying molecular mechanisms of depression in the CRS model by regulating gut microbiota. The results of metabolomics analyses revealed the various metabolic profiles of the control, CRS, and CRS + AKK groups, indicating that AKK could regulate the abnormal metabolic profiles of mice with CRS-induced gut microbiota dysbiosis. A total of 23 serum metabolites were identified as potential biomarkers implicated in gut microbiota dysbiosis between control and CRS groups based on the t-test and VIP values in the positive mode. Penicillamine is a thiol drug predominantly used in the treatment of Wilson’s disease and rheumatoid arthritis, and adverse effects, which may include neuropathy, frequently occur during normal use of the drug (Pool et al. 1981). Mesalazine is a primary treatment for IBD, and a multicenter trial revealed that it caused adverse effects of depression, although only in 5.4% of patients (Reinisch et al. 2010). β-Alanyl-3-methyl-l-histidine is also called anserine. Long-term consumption of fish stock reduces anxiety and modifies central amino acid levels including anserine in rats (Funatsu et al. 2015). A previous study revealed that anserine ameliorated neurovascular-unit dysfunction and spatial memory in an aged mouse model of Alzheimer’s disease (Kaneko et al. 2017). A clinical research conducted in Japan investigated the association between serum concentrations of β-alanine, which is a metabolite of anserine, and the risk of dementia. The results of the study revealed that a high intake of anserine could be beneficial in the prevention of dementia (Hata et al. 2019). Edaravone, which exhibits a neuroprotective effect attributed to the potent-free radical scavenging activity, is largely used during the clinical acute phase of cerebral infarction adjuvant therapy (Lee and Xiang 2018). The present study has demonstrated that edaravone presents antidepressant-like activity. Studies using animal models have demonstrated that the antidepressant mechanism of edaravone is influenced by the expression of neurotransmitter turnover (Herbet et al. 2019), which could be coupled with inhibition of oxido-nitrosative stress, neuroinflammation, and the endoplasmic reticulum stress cascade (Jangra et al. 2017). Furthermore, clinical trials have revealed that edaravone decreases depression severity in patients with symptomatic intracranial stenosis and has been associated with serum expression of sex hormones (Kong et al. 2020).
KEGG ontology enrichment analysis of the CRS and CRS + AKK groups (Fig. 5c) revealed that it was predominantly enriched in 9 pathways, which included cholinergic synapse, fat digestion and absorption, degradation of aromatic compounds, fatty acid degradation, vitamin digestion and absorption, butanoate metabolism, carbon metabolism, pantothenate, and CoA biosynthesis.
Accumulating evidence suggests that the cholinergic system plays a vital role in major depression and bipolar disorders. Specifically, previous studies established that scopolamine exerted rapid and sustained antidepressant effects on depressed humans (Dulawa and Janowsky, 2019). Furthermore, fluoxetine decreased cholinergic synaptic transmission and plasticity in established synapses to anti-depression (Getz et al. 2011). Fatty acid degradation is closely associated with depression; ω-3 polyunsaturated fatty acids (n-3 PUFAs) have been used as antidepressants in the treatment of MDD (Guu et al. 2019). Vitamins are essential nutrients; for instance, vitamin B or vitamin D deficiency triggers various disorders including depression symptoms (Milaneschi et al. 2014; Ghaleiha et al. 2016). Supplementing the diets of patients with MDD with vitamins confers beneficial effects on the symptoms (Sepehrmanesh et al. 2016). Pathway enrichment analysis results using non-targeted metabolomics to evaluate differential metabolites in a mouse model of depression induced by maternal separation (MS) demonstrated the significance of pantothenate and CoA biosynthesis. Therefore, the MS model increased susceptibility of rats to depression, which could regulate pantothenate and CoA biosynthesis (Cui et al. 2020). The pathway associated with pantothenate and CoA biosynthesis was enriched considerably in the KEGG pathways of MDD and bipolar depression (Ren et al. 2017). The results suggested that pantothenate and CoA biosynthesis could be key factors influencing depression. Based on the results of the metabolic pathway analyses, we hypothesized that the intervening effects of AKK against depression primarily occurred via regulation of the pathways.
Correlation analyses revealed that AKK exhibited a significant positive correlation with β-alanyl-3-methyl-l-histidine and edaravone, and administration of AKK restored the level of serum β-alanyl-3-methyl-l-histidine and edaravone in mice subjected to CRS (Fig. 5d–f). We therefore hypothesized that AKK ameliorates depression by increasing levels of β-alanyl-3-methyl-l-histidine and edaravone in serum. We conducted animal experiments to validate the antidepressant effects of β-alanyl-3-methyl-l-histidine and edaravone. The results revealed that β-alanyl-3-methyl-l-histidine and edaravone effectively alleviated CRS-induced depressive-like behavior (Fig. 6). In addition, administration of β-alanyl-3-methyl-l-histidine and edaravone increased dopamine, serotonin levels in serum, and BDNF expression in the hippocampus, particularly, significantly decreasing serum corticosterone levels in CRS-induced mice.
In conclusion, we demonstrated that AKK reduces CRS-induced depressive-like behavior in mice. The potential mechanisms involved influence hormone, neurotransmitter, and BDNF levels, as well as resulting in modifications in gut microbiota and serum metabolism. The findings of the present study could provide potential interventions of coping with stress-induced depression disorder with regard to gut microbiome and metabolomics. Besides, we provided a novel psychobiotic as a therapeutic strategy for depression. Nonetheless, further studies should be conducted to elucidate the association between depression and administration of AKK.
References
Ali A, Margetts BM, Zainuddin AA (2021) Exploration of the principal component analysis (PCA) approach in synthesizing the diet quality of the Malaysian population. Nutrients 13(1):70
Bai YY, Ruan CS, Yang CR, Li JY, Kang ZL, Zhou L, Liu D, Zeng YQ, Wang TH, Tian CF, Liao H, Bobrovskaya L, Zhou XF (2016) ProBDNF signaling regulates depression-like behaviors in rodents under chronic stress. Neuropsychopharmacology 41:2882–2892
Bárcena C, Valdés-Mas R, Mayoral P, Garabaya C, Durand S, Rodríguez F, Fernández-García MT, Salazar N, Nogacka AM, Garatachea N, Bossut N, Aprahamian F, Lucia A, Kroemer G, Freije JMP, Quirós PM, López-Otín C (2019) Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice. Nat Med 25:1234–1242
Blacher E, Bashiardes S, Shapiro H, Rothschild D, Mor U, Dori-Bachash M, Kleimeyer C, Moresi C, Harnik Y, Zur M, Zabari M, Brik RB-Z, Kviatcovsky D, Zmora N, Cohen Y, Bar N, Levi I, Amar N, Mehlman T, Brandis A, Biton I, Kuperman Y, Tsoory M, Alfahel L, Harmelin A, Schwartz M, Israelson A, Arike L, Johansson MEV, Hansson GC, Gotkine M, Segal E, Elinav E (2019) Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature 572:474–480
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pẽa AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7(5):335–336
Castrén E (2005) Is mood chemistry? Nat Rev Neurosci 6:241–246
Cryan JF, Dinan TG (2012) Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 13:701–712
Cui Y, Cao K, Lin H, Cui S, Shen C, Wen W, Mo H, Dong Z, Bai S, Yang L, Shi Y, Zhang R (2020) Early-life stress induces depression-like behavior and synaptic-plasticity changes in a maternal separation rat model: gender difference and metabolomics study. Front Pharmacol 11:102
Deng XY, Xue JS, Li HY, Ma ZQ, Fu Q, Qu R, Ma SP (2015) Geraniol produces antidepressant-like effects in a chronic unpredictable mild stress mice model. Physiol Behav 152:264–271
Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, Falony G, Raes J, Maiter D, Delzenne NM, de Barsy M, Loumaye A, Hermans MP, Thissen J-P, de Vos WM, Cani PD (2019) Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med 25:1096–1103
Derrien M, Collado MC, Ben-Amor K, Salminen S, De Vos WM (2008) The mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl Environ Microbiol 74:1646–1648
Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG (2008) The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res 43:164–174
Dulawa SC, Janowsky DS (2019) Cholinergic regulation of mood: from basic and clinical studies to emerging therapeutics. Mol Psychiatry 24:694–709
Duman RS, Monteggia LM (2006) A neurotrophic model for stress-related mood disorders. Biol Psychiatry 59:1116–1127
Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10:996–998
Franzosa EA, Huang K, Meadow JF, Gevers D, Lemon KP, Bohannan BJM, Huttenhower C (2015) Identifying personal microbiomes using metagenomic codes. Proc Natl Acad Sci U S A 112:E2930-2938
Funatsu S, Kondoh T, Kawase T, Ikeda H, Nagasawa M, Michael Denbow D, Furuse M (2015) Long-term consumption of dried bonito dashi (a traditional Japanese fish stock) reduces anxiety and modifies central amino acid levels in rats. Nutr Neurosci 18:256–264
Fung TC, Olson CA, Hsiao EY (2017) Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci 20:145–155
GBD 2016 Disease and Injury Incidence and Prevalence Collaborators (2017) Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390 (10100): 1211–1259
Getz A, Xu F, Zaidi W, Syed NI (2011) The antidepressant fluoxetine but not citalopram suppresses synapse formation and synaptic transmission between Lymnaea neurons by perturbing presynaptic and postsynaptic machinery. Eur J Neurosci 34:221–234
Ghaleiha A, Davari H, Jahangard L, Haghighi M, Ahmadpanah M, Seifrabie MA, Bajoghli H, Holsboer-Trachsler E, Brand S (2016) Adjuvant thiamine improved standard treatment in patients with major depressive disorder: results from a randomized, double-blind, and placebo-controlled clinical trial. Eur Arch Psychiatry Clin Neurosci 266:695–702
Guu TW, Mischoulon D, Sarris J, Hibbeln J, McNamara RK, Hamazaki K, Freeman MP, Maes M, Matsuoka YJ, Belmaker RH, Jacka F, Pariante C, Berk M, Marx W, Su KP (2019) International society for nutritional psychiatry research practice guidelines for omega-3 fatty acids in the treatment of major depressive disorder. Psychother Psychosom 88:263–273
Haase J, Brown E (2015) Integrating the monoamine, neurotrophin and cytokine hypotheses of depression - a central role for the serotonin transporter? Pharmacol. Ther 147: 1-11
Hagi T, Belzer C (2021) The interaction of Akkermansia muciniphila with host-derived substances, bacteria and diets. Appl Microbiol Biotechnol 105:4833–4841
Hasler G (2010) Pathophysiology of depression: do we have any solid evidence of interest to clinicians? World Psychiatry 9:155–161
Hata J, Ohara T, Katakura Y, Shimizu K, Yamashita S, Yoshida D, Honda T, Hirakawa Y, Shibata M, Sakata S, Kitazono T, Kuhara S, Ninomiya T (2019) Association between serum β-alanine and risk of dementia. Am J Epidemiol 188:1637–1645
Herbet M, Natorska-Chomicka D, Ostrowska M, Gawrońska-Grzywacz M, Izdebska M, Piątkowska-Chmiel I, Korga A, Wróbel A, Dudka J (2019) Edaravone presents antidepressant-like activity in corticosterone model of depression in mice with possible role of Fkbp5, Comt, Adora1 and Slc6a15 genes. Toxicol Appl Pharmacol 380: 114689
Jangra A, Sriram CS, Dwivedi S, Gurjar SS, Hussain MI, Borah P, Lahkar M (2017) Sodium phenylbutyrate and edaravone abrogate chronic restraint stress-induced behavioral deficits: implication of oxido-nitrosative, endoplasmic reticulum stress cascade, and neuroinflammation. Cell Mol Neurobiol 37:65–81
Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, Wang W, Tang W, Tan Z, Shi J, Li L, Ruan B (2015) Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun 48:186–194
Kaneko J, Enya A, Enomoto K, Ding Q, Hisatsune T (2017) Anserine (beta-alanyl-3-methyl-L-histidine) improves neurovascular-unit dysfunction and spatial memory in aged AβPPswe/PSEN1dE9 Alzheimer’s-model mice. Sci Rep 7:12571
Kong Z, Jiang J, Deng M, Zhang Z, Wang G, Manchia M (2020) Edaravone reduces depression severity in patients with symptomatic intracranial stenosis and is associated with the serum expression of sex hormones. Medicine (Baltimore) 99 (8): e19316
Kryukov AA, Gorbunova AO, Machs EM, Mikhaylova YV, Rodionov AV, Zhurbenko PM, Yurkov AP (2020) Perspectives of using Illumina MiSeq for identification of arbuscular mycorrhizal fungi. Vavilovskii Zhurnal Genet Selektsii 24(2):158–167
Langille MGI, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R, Beiko RG, Huttenhower C (2013) Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 31:814–821
Lee MK, Carnes MU, Butz N, Azcarate-Peril MA, Richards M, Umbach DM, Thorne PS, Freeman LEB, Peddada SD, London SJ (2018) Exposures related to house dust microbiota in a U.S. farming population. Environ Health Perspect 126(6): 067001
Lee XR, Xiang GL (2018) Effects of edaravone, the free radical scavenger, on outcomes in acute cerebral infarction patients treated with ultra-early thrombolysis of recombinant tissue plasminogen activator. Clin Neurol Neurosurg 167:157–161
Li B, He Y, Ma J, Huang P, Du J, Cao L, Wang Y, Xiao Q, Tang H, Chen S (2019) Mild cognitive impairment has similar alterations as Alzheimer’s disease in gut microbiota. Alzheimer’s Dement 15:1357–1366
Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, Wu S, Liu W, Cui Q, Geng B, Zhang W, Weldon R, Auguste K, Yang L, Liu X, Chen L, Yang X, Zhu B, Cai J (2017) Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 5:14
Liu Y, Mian MF, McVey Neufeld KA, Forsythe P (2020) CD4+CD25+ T cells are essential for behavioral effects of Lactobacillus rhamnosus JB-1 in male Balb/c mice. Brain Behav Immun 88:451–460
Luo B, Xiang D, Nieman DC, Chen P (2014) The effects of moderate exercise on chronic stress-induced intestinal barrier dysfunction and antimicrobial defense. Brain Behav Immun 39:99–106
Magoč T, Salzberg SL (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963
Martinotti G, Pettorruso M, De Berardis D, Varasano PA, Pressanti GL, De Remigis V, Valchera A, Ricci V, Di Nicola M, Janiri L, Biggio G, Di Giannantonio M (2016) Agomelatine increases BDNF serum levels in depressed patients in correlation with the improvement of depressive symptoms. Int J Neuropsychopharmacol 19(5):pyw003
McGaughey KD, Yilmaz-Swenson T, Elsayed NM, Cruz DA, Rodriguiz RM, Kritzer MD, Peterchev A V., Roach J, Wetsel WC, Williamson DE (2019) Relative abundance of Akkermansia spp. and other bacterial phylotypes correlates with anxiety- and depressive-like behavior following social defeat in mice. Sci Rep 9: 3281
Meyer C, Vassar M (2018) The fragility of probiotic Bifidobacterium longum NCC3001 use for depression in patients with irritable bowel syndrome. Gastroenterology 154:764
Milaneschi Y, Hoogendijk W, Lips P, Heijboer AC, Schoevers R, Van Hemert AM, Beekman ATF, Smit JH, Penninx BWJH (2014) The association between low vitamin D and depressive disorders. Mol Psychiatry 19:444–451
Moya-Pérez A, Perez-Villalba A, Benítez-Páez A, Campillo I, Sanz Y (2017) Bifidobacterium CECT 7765 modulates early stress-induced immune, neuroendocrine and behavioral alterations in mice. Brain Behav Immun 65:43–56
Murray CJL, Abraham J, Ali MK, Alvarado M, Atkinson C, Baddour LM, Bartels DH, Benjamin EJ, Bhalla K, Birbeck G, Bolliger I, Burstein R, Carnahan E, Chen H, Chou D, Chugh SS, Cohen A, Colson KE, Cooper LT, Couser W, Criqui MH, Dabhadkar KC, Dahodwala N, Danaei G, Dellavalle RP, Des Jarlais DC, Dicker D, Ding EL, Dorsey ER, Duber H, Ebel BE, Engell RE, Ezzati M, Felson DT, Finucane MM, Flaxman S, Flaxman AD, Fleming T, Forouzanfar MH, Freedman G, Freeman MK, Gabriel SE, Gakidou E, Gillum RF, Gonzalez-Medina D, Gosselin R, Grant B, Gutierrez HR, Hagan H, Havmoeller R, Hoffman H, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Kassebaum N, Khatibzadeh S, Knowlton LM, Lan Q, Leasher JL, Lim S, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, MacIntyre MF, Mallinger L, McDermott MM, Meltzer M, Mensah GA, Micha R, Michaud C, Miller TR, Mock C, Moffitt TE, Mokdad AA, Mokdad AH, Moran AE, Mozaffarian D, Murphy T, Naghavi M, Venkat Narayan KM, Nelson RG, Olives C, Omer SB, Ortblad K, Ostro B, Pelizzari PM, Phillips D, Pope CA, Raju M, Ranganathan D, Razavi H, Ritz B, Rivara FP, Roberts T, Sacco RL, Salomon JA, Sampson U, Sanman E, Sapkota A, Schwebel DC, Shahraz S, Shibuya K, Shivakoti R, Silberberg D, Singh GM, Singh D, Singh JA, Sleet DA, Steenland K, Tavakkoli M, Taylor JA, Thurston GD, Towbin JA, Vavilala MS, Vos T, Wagner GR, Weinstock MA, Weisskopf MG, Wilkinson JD, Lopez AD, Zabetian A (2013) The State of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA - J Am Med Assoc 310:591–608
Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, Hsiao EY (2018) The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell 173:1728–1741
Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, Mohr DC, Schatzberg AF (2016) Major depressive disorder. Nat Rev Dis Primers 2:16065
Ou Z, Deng L, Lu Z, Wu F, Liu W, Huang D, Peng Y (2020) Protective effects of Akkermansia muciniphila on cognitive deficits and amyloid pathology in a mouse model of Alzheimer’s disease. Nutr Diabetes 10:12
Pinto-Sanchez MI, Hall GB, Ghajar K, Nardelli A, Bolino C, Lau JT, Martin FP, Cominetti O, Welsh C, Rieder A, Traynor J, Gregory C, De Palma G, Pigrau M, Ford AC, Macri J, Berger B, Bergonzelli G, Surette MG, Collins SM, Moayyedi P, Bercik P (2017) Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology 153:448–459
Platten M, Nollen EAA, Röhrig UF, Fallarino F, Opitz CA (2019) Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov 18:379–401
Png CW, Lindén SK, Gilshenan KS, Zoetendal EG, McSweeney CS, Sly LI, McGuckin MA, Florin THJ (2010) Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am J Gastroenterol 105:2420–2428
Pool KD, Feit H, Kirkpatrick J (1981) Penicillamine-induced neuropathy in rheumatoid arthritis. Ann Intern Med 95:457–458
Rakhit S, Clark CJ, O’Shaughnessy CT, Morris BJ (2005) N-Methyl-D-aspartate and brain-derived neurotrophic factor induce distinct profiles of extracellular signal-regulated kinase, mitogen- and stress-activated kinase, and ribosomal S6 kinase phosphorylation in cortical neurons. Mol Pharmacol 67:1158–1165
Reinisch W, Angelberger S, Petritsch W, Shonova O, Lukas M, Bar-Meir S, Teml A, Schaeffeler E, Schwab M, Dilger K, Greinwald R, Mueller R, Stange EF, Herrlinger KR (2010) Azathioprine versus mesalazine for prevention of postoperative clinical recurrence in patients with Crohn’s disease with endoscopic recurrence: Efficacy and safety results of a randomised, double-blind, double-dummy, multicentre trial. Gut 59:752–759
Ren J, Zhao G, Sun X, Liu H, Jiang P, Chen J, Wu Z, Peng D, Fang Y, Zhang C (2017) Identification of plasma biomarkers for distinguishing bipolar depression from major depressive disorder by iTRAQ-coupled LC–MS/MS and bioinformatics analysis. Psychoneuroendocrinology 86:17–24
Rothmore J (2020) Antidepressant-induced sexual dysfunction. Med J Aust 212:329–334
Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, Fidelle M, Flament C, Poirier-Colame V, Opolon P, Klein C, Iribarren K, Mondragón L, Jacquelot N, Qu B, Ferrere G, Clémenson C, Mezquita L, Masip JR, Naltet C, Brosseau S, Kaderbhai C, Richard C, Rizvi H, Levenez F, Galleron N, Quinquis B, Pons N, Ryffel B, Minard-Colin V, Gonin P, Soria JC, Deutsch E, Loriot Y, Ghiringhelli F, Zalcman G, Goldwasser F, Escudier B, Hellmann MD, Eggermont A, Raoult D, Albiges L, Kroemer G, Zitvogel L (2018) Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359(6371):91–97
Sepehrmanesh Z, Kolahdooz F, Abedi F, Mazroii N, Assarian A, Asemi Z, Esmaillzadeh A (2016) Vitamin D supplementation affects the beck depression inventory, insulin resistance, and biomarkers of oxidative stress in patients with major depressive disorder: a randomized, controlled clinical trial. J Nutr 146:243–248
Sharma A, Guski LS, Freund N, Gøtzsche PC (2016) Suicidality and aggression during antidepressant treatment: systematic review and meta-analyses based on clinical study reports. BMJ 352:i65
Simanek AM, Parry A, Dowd JB (2018) Differences in the association between persistent pathogens and mood disorders among young- to middle-aged women and men in the U.S. Brain Behav Immun 68: 56-65
Slavin J (2013) Fiber and prebiotics: mechanisms and health benefits. Nutrients 5:1417–1435
Smith K (2014) Mental health: a world of depression. Nature 515(7526):181
Spencer RL, Deak T (2017) A users guide to HPA axis research. Physiol Behav 178:43–65
Tian P, O’Riordan KJ, Lee Y kun, Wang G, Zhao J, Zhang H, Cryan JF, Chen W (2020b) Towards a psychobiotic therapy for depression: Bifidobacterium breve CCFM1025 reverses chronic stress-induced depressive symptoms and gut microbial abnormalities in mice. Neurobiol Stress 12: 100216
Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA (2011) Low relative abundances of the mucolytic bacterium Akkermansia muciniphila and Bifidobacterium spp. in feces of children with autism. Appl Environ Microbiol 77:6718–6721
Wang L, Tang L, Feng Y, Zhao S, Han M, Zhang C, Yuan G, Zhu J, Cao S, Wu Q, Li L, Zhang Z (2020) A purified membrane protein from Akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of CD8 + T cells in mice. Gut 69:1988–1997
Xu M, Mo X, Huang H, Chen X, Liu H, Peng Z, Chen L, Rong S, Yang W, Xu S, Liu L (2020) Yeast β-glucan alleviates cognitive deficit by regulating gut microbiota and metabolites in Aβ1–42-induced AD-like mice. Int J Biol Macromol 161:258–270
Zhai Q, Feng S, Arjan N, Chen W (2019) A next generation probiotic, Akkermansia muciniphila. Crit Rev Food Sci Nutr 59:3227–3236
Zhang T, Ji X, Lu G, Zhang F (2021) The potential of Akkermansia muciniphila in inflammatory bowel disease. Appl Microbiol Biotechnol 105:5785–5794
Funding
This work was financially supported by the Natural Science Foundation of Jiangsu Province (No. 81873309).
Author information
Authors and Affiliations
Contributions
Y. D. designed the study, performed the experiments, analyzed the data, and wrote the manuscript. F. B., T. C., G. P. S., Z. Y. F., Z. L. D., and R. W. helped with performed experiments and analyzed data. Y. G. C. and X. M. Y. helped with design and review of the manuscript. S. M. Z., Q. W., and J. Y. Z contributed analytical tools. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ding, Y., Bu, F., Chen, T. et al. A next-generation probiotic: Akkermansia muciniphila ameliorates chronic stress–induced depressive-like behavior in mice by regulating gut microbiota and metabolites. Appl Microbiol Biotechnol 105, 8411–8426 (2021). https://doi.org/10.1007/s00253-021-11622-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-021-11622-2