Abstract
The objective of this study was to assess the efficacy of a microencapsulated probiotic as an adjunct therapy in rotavirus-positive diarrhea of neonatal calves that received supportive treatment or supportive along with microencapsulated probiotic treatment, for 5 days. We examined whether microencapsulated Lactobacillus acidophilus NCDC15 probiotic treatment in rotavirus-infected diarrhoetic calves led to faster resolution of diarrhea, amelioration of zinc-copper imbalance, improved the immunoglobulin A and immunoglobulin G, and decreased the inflammatory markers in serum. Calves with rotavirus-positive diarrhea < 4-week age and fecal scores ≥ 2 were randomly assigned into two groups. The supportive along with microencapsulated probiotic treatment significantly (p < 0.05) increased zinc and immunoglobulin A concentrations and decreased copper, tumor necrosis factor-α, and nitric oxide level in serum on days 3 and 5 from pretreatment values; the immunoglobulin G concentration was elevated (p < 0.05) on day 5. The mean resolution time of abnormal fecal score was 5.3 and 3.3 days in supportive treatment and supportive along with microencapsulated probiotic groups, respectively, in log-rank Mantel-Cox test. The calves in the supportive along with microencapsulated probiotic treatment group had faster resolution of diarrhea than supportive treatment group in Dunn’s multiple comparisons test. This study demonstrates that supportive treatment along with microencapsulated probiotic administered to naturally rotavirus-infected diarrhoetic calves at onset of diarrhea led to faster resolution of diarrhea, improved zinc and immunoglobulin levels, and decreased the inflammatory parameters in serum of rotavirus-infected diarrhoetic calves.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neonatal calf diarrhea is one of the most common causes of morbidity and mortality in young calves. It is a disease complex that is triggered by various factors and infectious pathogens [1]. Notably, rotavirus is one of the most frequently detected enteropathogen in feces from young diarrhoeic calves [2]. While dehydration and metabolic acidosis characterize the disease, it has recently been shown that rotavirus infection alters the structure of the gut microbiota and the physiological indices [2]. Acute rotavirus diarrhea causes severe loss of micronutrients such as zinc and iron, as well as an increase in copper in circulation, during the early stages of illness. Zinc, an essential micronutrient, has significant role in enhancing the enteric mucosal immunity to enteropathogens and modulating intraluminal secretion and electrolyte absorption [3]. Decreased zinc concentration in serum is observed in acute rotavirus-associated diarrhea [4]. Zinc supplementation delays diarrhea and expedites clinical recovery of young calves and human beings with rotaviral diarrhea [5, 6].
Response of innate immunity of host is vital in limiting viral infection including rotavirus [7]. Secretion of pro- and anti-inflammatory cytokines is the part of innate immune response and plays a crucial role in pathogenesis and immunity of viral diseases [8]. Rotavirus infection induces the production of various types of interferons and cytokines from immune cells [8, 9]. TNF-α, a potent inflammatory mediator, plays a key role in the antiviral immunity and inhibits rotavirus replication via classical NF-κB signaling and JAK-STAT pathways [10]. The disturbance of redox equilibrium, induced by the formation of multiple oxidants through enzymatic and non-enzymatic reactions, is another important pathophysiological aspect of viral infection [11]. The non-structural protein 4 of rotavirus stimulates the release of nitric oxide from the enterocytes, triggering intestinal motility and fluid secretion into lumen through activation of enteric nervous system [12]. The elevated concentrations of nitric oxide and TNF-α induce chloride secretion in intestinal epithelial cells leading to more diarrhea [13]. The rotavirus infection results in serum and intestinal antibody responses that protect the host from re-infection against diarrhea [14]. Among the immunoglobulins, immunoglobulin A has a multi-faceted role in mucosal immunity, and immunoglobulin G is critical to protect from systemic rotavirus infection [15]. Earlier studies have shown that patients with rotavirus gastroenteritis have substantially decreased immunoglobulin A and immunoglobulin G levels in serum than healthy one [16].
The symptomatic therapy consisting of aggressive fluid therapy, nutritional support, and antibiotic therapy to check secondary bacterial infection is the cornerstone of rotaviral diarrhea treatment in calves. Although rehydration solutions successfully prevent mortality from severe dehydration and acidosis, it often fails to shorten the duration of diarrhea and check high fluid losses [17]. In animal studies, the use of antiviral medicines such as nitazoxanide, vidarabin, cordycepin, and foscarmet for the particular treatment of rotavirus infection has shown encouraging results [18, 19]. Despite the fact that many effective compounds have been identified and a few have been successfully used to prevent infection in vivo, commercial progress is still lacking. While vaccination is another method for avoiding rotavirus infection, risks of intussusception and bowel obstruction are thought to be major issues with rotavirus vaccination [20]. Aside from some limitations, vaccines are not fully effective, and new episodes may occur, even in vaccinated children [21]. In calves, two approaches have been used to prevent rotavirus-associated diarrhea: first, stimulation of active immunity by vaccinating the newborn calf, and second, stimulation of passive lactogenic immunity by vaccinating the pregnant dam (maternal vaccination). However, currently no vaccines are commercially available for calf rotaviral diarrhea in India. While the numerous medicinal plant extracts have been reported to exhibit anti-rotavirus activity, the active ingredients have not yet been isolated and characterized fully from the promising herbs to develop anti-rotavirus drugs [22]. Anti-rotavirus activity of some Indian medicinal plants has also been reported in earlier studies [23]. Furthermore, scientific studies are required to investigate the chemical components found in herbs in large concentrations that have more anti-rotavirus activity than the crude extract [24]. Since there is no completely safe method for preventing the rotaviral diarrhea, researchers have focused their efforts on the potential usages of probiotic agents as an adjunct treatment for diarrhoetic calves. Probiotics are live microorganisms that, when consumed in sufficient amounts, provide health advantages to the recipient by limiting pathogen colonization and growth in the intestine of the host, as well as boosting host immunity. The shortening of duration of diarrhea and shedding of rotavirus by oral probiotic treatment have been reported in young animals and infants [25, 26]. Among the probiotics, Lactobacillus species have been widely researched probiotic in the treatment of viral diarrhea because of their high adherence capacity to enterocytes [27, 28]. The L. acidophilus NCDC15, a dairy origin probiotic, has shown to reduce the incidence and duration of diarrhea in crossbred calves [29]. Furthermore, the dietary supplementation of L. acidophilus NCDC15 in early weanling piglets has shown to increase the villi height and crypt depth and decrease the villi: crypt ratio and diarrhea scores after weaning. Additionally, supplementation of the probiotic in basal diet improved the growth performance, fecal microbial count, and intestinal morphology in grower-finisher pigs [30]. It is well known that the delivery of oral probiotics to the gut is often compromised because of their sensitivity to the low pH of the stomach and the high bile salt conditions of the small intestine resulting lower efficacy. The microencapsulation of probiotic is a viable strategy for the oral delivery of probiotics [31]. The viability losses can be minimized by microencapsulating the probiotic in a polymer matrix. The immobilization of probiotic into a polymer matrix helps to protect its structure in the stomach before degrading and dissolving in the intestine. The alginate is the most common bacterial encapsulation agent owing to its safety, its mild gelling conditions, and lack of toxicity [32]. Moreover, coating of microencapsulated probiotic offers additional benefit such as controlled release of probiotic in the intestine. From earlier studies, a significant protective effect was observed by coating the alginate microcapsules with chitosan [33, 34]. The unique advantages of such microencapsulated probiotic are high biocompatibility, non-toxicity, biodegradability, and cost effective over other therapeutics; therefore, it is quite suitable for use in the pharmaceutical industries [35]. In this study, the L. acidophilus NCDC 15 probiotic was encapsulated in alginate, and the encapsulated probiotic was further coated with chitosan. Until now, the therapeutic efficacy of such encapsulated probiotic in rotavirus-infected diarrhoetic calves is not investigated. The objective of this study was to evaluate efficacy of the encapsulated probiotic as an adjunct therapy in resolution of diarrhea of naturally rotavirus-infected diarrhoetic neonatal calves. In addition, we examined whether the encapsulated probiotic treatment led to amelioration of zinc-copper imbalance, improved the total immunoglobulins, and decreased the inflammatory markers of serum of rotavirus-infected diarrhoetic calves.
Materials and Methods
Experimental Conditions and Animals
This was a single-blinded and restricted-randomized study in neonatal calves and conducted in the institute owned experimental cattle and buffalo farm from August 2019 to March 2020. The study was carried out in accordance with the guidelines of Institute Animal Ethics Committee, and the protocol was approved by the Purpose of Control and Supervision of Experiments on Animals, Ministry of Fisheries, Animal Husbandry and Dairying, Government of India (approval number F.25/02/2020- CPCSEA-DADF).
The experimental cattle and buffalo farm (28° 24′ N and 79° 26′ E, 179 m above sea level) of the institute was located in the northern Indian state. The institute maintains the elite herd of cattle and buffalo for research and standardization of management practices for the benefit of researchers and dairy farmers. After birth, the calves were weighed, and individual calves were given with unique identification number. The cattle calves were weaned, fed colostrum, reared in calf shed, and maintained under standard managemental conditions. Each calf was housed in separated pens that were fitted with feeding and watering troughs as required for calves. The total area per pen was 8.5 m2. The calf house was equipped with adequate ventilation, and chopped straw was used as bedding after cleaning manure daily.
Probiotic
A culture of Lactobacillus acidophilus NCDC-15 (dairy origin) was obtained from National Collection of Dairy Cultures (Division of Dairy Microbiology, National Dairy Research Institute, Karnal, India) which was used as probiotic to improve growth performance in cattle calves [36]. The probiotic was encapsulated in alginate, and encapsulated probiotic was coated with chitosan [37]. In brief, the L. acidophilus NCDC15 was revived from glycerol stock and cultured for overnight to prepare the probiotic product. The autoclaved sodium alginate (4% w/v) alone or with starch (2%w/v), and the probiotic culture in de Man, Rogosa and Sharpe medium (HiMedia Laboratories, Mumbai, India), was mixed and then sprinkled with calcium chloride (0.5 M) solution at standardized rate to form the microcapsules. The formed microcapsules were drained and kept in chitosan (0.8% w/v) solution for 30 min at refrigeration. After washing of the microcapsules with sterile normal saline, the viable cell count (colony forming unit) was assessed in 1 g of microcapsule. The product was stored at room temperature and at 4 °C, and colony forming unit was assessed till the count dropped below 106/g. The survival of microencapsulated probiotic product was better at 4 °C compared to room temperature until 14 weeks. Further, the microencapsulated probiotic product was assessed for survival with simulated gastric juice (pH 2.5) and simulated intestinal juice (pH 7.2). In simulated gastric and intestinal juices, microcapsule maintained their viability above 108 colony forming unit/g until 30 min and 120 min, respectively. The microcapsulated probiotic maintained the required colony forming unit level for more than 14 weeks. Additionally, the probiotic was observed to resist gastric acidic pH till 120 min.
Experimental Design
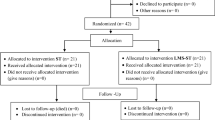
The calves under 4 weeks of age and weighing approximately 27.20 (± 0.50 standard deviation) kg were enrolled for the study. The calves were separated from their dam and kept in calf section of the farm. The diarrhoetic calves were selected on the basis of fecal consistency score and clinical dehydration score with or without elevation of rectal temperature. Calves were not enrolled if they had a fecal consistency score of 0 or 1 and clinical dehydration score of 0, whereas diarrhoetic calves included with a fecal consistency score of 2 or 3 and clinical dehydration score of 1 or 3 for the study [38]. A total of 34 diarrhoetic calves had been identified in the study period. For the diagnosis of bovine group A rotavirus, the fecal material from rectum was collected from each diarrhoetic calf. Prior to confirmatory diagnosis, irrespective of diarrhoetic calves were randomly allocated to one of the two treatment groups as supportive treatment group, fifteen diarrhoetic calves (9 female and 6 male) received supportive treatment (parenteral 0.9% isotonic saline and ringer’s lactate as per the degree of dehydration two times a day, and sulphamethoxazole + trimethoprim at the rate of 50 mg/kg, intravenous, twice in a day) and meloxicam at the rate of 0.4 mg/kg body weight, intramuscular route, once daily when indicated and supportive along with microencapsulated probiotic treatment group: nineteen diarrhoetic calves (10 female and 9 male) received encapsulated Lactobacillus acidophillus NCDC 15 as microcapsule (sodium alginate and chitosan-coated) at the rate of 1 × 109 colony forming unit /calf, oral route, twice in a day) along with above mentioned supportive treatment for 5 days. One gram of solid microencapsulated probiotic (1 × 109 colony forming unit/g) was fed orally with 10 mL sterile normal saline solution. Simultaneously six healthy calves (2 female and 4 male) of similar age group were randomly selected from normal healthy calf population and were assigned as healthy control group and they received 10 mL of normal saline by oral route, twice a day for 5 days as placebo. Each calf served as study unit. Under the supervision of veterinarian, the calf care activities, including date of treatment, diagnosis, and observation, were carried out. The treatment regimen for all the participating calves was completed except one calf in supportive treatment and two calves in supportive along with microencapsulated probiotic treatment group died during the period of study. The primary outcome of the study was to reduce the fecal consistency score ≤ 1. The secondary outcome was to reduce the TNF-α and nitric oxide levels and increase the zinc, immunoglobulin A, and immunoglobulin G level. The study flow diagram is depicted in Fig. 1.
Laboratory Investigation
Detection of Rotavirus in Calf Stool Samples
Approximately 1.0 g fecal samples from diarrhoeic calves were collected, and a suspension of 10% weight/volume was prepared with 1X phosphate buffer saline. After thorough mixing by vortex, the coarse debris was removed by centrifugation at 2370 g for 5 min. Total ribonucleic acid from supernatant was extracted using qiazol (QIAGEN, Germany), as per the protocol recommended by the manufacturer. For reverse transcription, the protocol described earlier was followed, and complementary deoxyribonucleic acid was prepared [39]. For detection of group A rotavirus infection, diagnostic polymerase chain reaction targeting the partial group-specific VP6 gene (forward primer: 5′TTTGATCACTAAYTATTCACC3′ and reverse primer: 5′GGTCACATCCTCTCACTA3′) was used, and polymerase chain reaction was performed as stated in earlier publications [39, 40]. Simultaneously, fecal samples were also taken from healthy control calves by the rectal ampulla after manual stimulation for screening rotavirus infection.
Blood Sampling and Processing
Approximately 5.0 mL blood was collected from jugular vein from each calf, in sterile vials with or without anticoagulant (dipotassium ethylenediaminetetraacetic acid) before initiation of treatment and on days 3 and 5 of treatment. 1.0 mL of whole blood was used for hematology and the remaining 4.0 mL was allowed to clot to retrieve the serum.
Hematology and Serum Biochemistry
Whole blood was used to measure hemoglobin concentration, packed cell volume, and total leukocyte counts manually using standard methods [41]. The spectrophotometric method was employed to estimate total protein (TP) and albumin concentrations in serum using commercially available kits (Coral Clinical Systems, Goa, India). Serum globulin concentration was calculated by subtracting the albumin concentration from total protein. The albumin/globulin ratio was also calculated.
Quantification Of Copper And Zinc In Serum
The copper and zinc concentrations in serum were quantified by spectrophotometric method using commercially available kits (Coral Clinical System, Goa, India) following the protocols recommended by manufacturers. The concentrations of copper and zinc were expressed as μg/dL.
Quantification of Total Immunoglobulin A and Immunoglobulin G in Serum
Total immunoglobulin A and immunoglobulin G concentrations in serum were quantified by spectrophotometric method using commercially available kits (Coral Clinical System, Goa, India) following the protocols recommended by manufacturers. The concentrations of immunoglobulin A and immunoglobulin G were expressed as mg/dL.
Quantification of TNF-α and Nitric Oxide (NO) in Serum
The commercial TNF-α enzyme-linked immunosorbent assay kit (RayBio® Bovine TNF-alpha ELISA Kit, Peachtree Corners, GA, USA) was used for the quantitative estimation of TNF- α in the serum samples following the protocol recommended by the manufacturers. Serum samples were diluted 1:2 in assay diluent A before quantification. Total serum nitrate/nitrite concentration was assayed by using acidic Griess reaction which caused color development as a result of nitrate reduction with activated copper-cadmium alloy and deproteinization [42]. Reading was performed in a Multiskan Sky Microplate Reader (Thermo Fisher Scientific).
Statistical Analysis
The data were analyzed using GraphPad Prism 6 Statistics Guide. To examine the effect of treatments on the mean values of hemogram, concentrations of serum biochemical parameters, copper, zinc, immunoglobulin A, immunoglobulin G, and TNF-α and nitric oxide repeated measures, analysis of variance was applied. The multiple comparisons between groups, days, and the interaction effects were done by using Tukey honestly significant difference test. An individual calf was considered as the experimental unit. The non-parametric data of fecal and dehydration scores were analyzed by Kruskal–Wallis test. A log-rank (Mantel-Cox) test model was used to determine the time to resolution of an abnormal fecal score between groups. Resolution was defined as fecal score ≤ 1 for 2 consecutive days. Kaplan–Meier survival functions were also created to graph time to resolution of abnormal fecal score by group. Time to resolution of diarrhea was compared between the groups with Dunn’s multiple comparisons test. The mean values and standard errors were reported. The level of significance was set at 5%.
Results
Diagnosis of Rotavirus Infection
The diarrhoetic calves exhibited the symptoms of anorexia, profuse watery diarrhea, depression, and dehydration characterized by recession of eye ball in the orbit and prolonged skin tenting time (> 2 s). Out of 15 diarrhoetic calves of supportive treatment group, six calves were found positive for group A bovine rotavirus by reverse transcription polymerase chain reaction that yielded the amplicon size of 226 bp (Supplementary file 1), and nine calves were found negative for group A bovine rotavirus. Whereas among the 17 diarrhoetic calves of supportive along with microencapsulated probiotic treatment group, six calves were found positive, and eleven calves were found negative for group A bovine rotavirus. Simultaneously, the fecal samples of healthy calves were also screened, but found negative for group A bovine rotavirus. No differences in clinical signs were observed to distinguish the diarrhoetic calves with or without rotavirus infection.
Changes In Hematology And Serum Biochemistry
Before commencement of treatment, the mean packed cell volume values were markedly increased (p < 0.05), and the total protein and albumin concentrations were significantly decreased (p < 0.05) in rotavirus-infected diarrhoetic calves as compared to healthy control group. Statistically, no significant changes of mean hemoglobin, packed cell volume, total leukocyte count, total protein, albumin, and globulin concentrations were recorded from day 0 to 5 in rotavirus-infected diarrhoetic calves that received only supportive treatment. The mean total leukocyte count, total protein, and albumin concentrations were significantly improved (p < 0.05) on day 5 from pretreatment values in rotavirus-infected diarrhoetic calves that received supportive along with microencapsulated probiotic treatment. No significant changes of mean globulin concentration and albumin: globulin ratio were observed from day 0 to 5 in any of the treatment and control groups. The interaction between treatments and day for total leukocyte count (p = 0.006) was significant, but such interaction was not present for hemoglobin, packed cell volume, total protein, albumin, globulin, and albumin: globulin ratio (Table 1).
Changes in Zinc and Copper Concentrations in Serum
The interaction between treatments and day for zinc (p = 0.02) and copper (p < 0.001) concentrations was statistically significant. The mean copper concentration was significantly (p < 0.05) higher, and zinc concentration was significantly (p < 0.05) lower in rotavirus-infected diarrhoetic calves as compared to healthy control group before initiation of therapy. The average copper and zinc concentrations did not alter significantly from day 0 to 5 in supportive treatment group, whereas the mean copper concentration was significantly (p < 0.05) decreased and mean zinc concentration was significantly (p < 0.05) increased on days 3 and 5 in supportive along with microencapsulated probiotic treatment group from pretreatment value (Table 2).
Changes In Immunoglobulin A And Immunoglobulin G Concentrations In Serum
A significant interaction was found between treatments and day for immunoglobulin A concentration (p < 0.001), but such interaction was not present for immunoglobulin G concentration (p = 0.514). A profound decrease of mean immunoglobulin A concentration (p < 0.001) was noted in serum in rotavirus-infected diarrhoetic calves as compared to healthy control group before initiation of therapy. Statistically, no significant alterations of immunoglobulin A and immunoglobulin G concentrations were observed from day 0 to 5 in rotavirus-infected diarrhoetic calves following supportive treatment. However, the mean immunoglobulin A concentration on days 3 and 5 and immunoglobulin G concentration on day 5 were significantly (p < 0.05) elevated from pretreatment value in supportive along with microencapsulated probiotic treatment group (Table 3).
Changes in TNF-α and Nitric Oxide Concentrations in Serum
A significant (p < 0.001) interaction was found between treatments and day for TNF-α and nitric oxide concentrations. The mean concentrations TNF-α and nitric oxide were significantly (p < 0.05) higher in supportive treatment and supportive along with microencapsulated probiotic treatment groups as compared to healthy control group before initiation of therapy. In supportive treatment group, the mean TNF-α concentration was significantly (p < 0.05) decreased on day 5, while nitric oxide concentrations remain unaffected from day 0 to 5, whereas the mean TNF-α and nitric oxide concentrations in serum were significantly (p < 0.05) decreased on days 3 and 5 from pretreatment value in supportive along with microencapsulated probiotic treatment group (Table 4).
Changes in Clinical Scores and Resolution of Diarrhea
The median values of fecal consistency and dehydration scores were significantly (p < 0.05) higher in rotavirus-infected diarrhoetic calves when compared with control group before initiation of treatment. The median values of fecal consistency score were significantly decreased on day 5 from day 0 and 3 in supportive treatment and supportive along with microencapsulated probiotic treatment groups. The median value of dehydration score did not vary significantly from day 0 to 5 in supportive treatment and supportive along with microencapsulated probiotic treatment groups (Fig. 2). The mean resolution time of abnormal fecal score was 5.3 and 3.3 days in supportive treatment and supportive along with microencapsulated probiotic treatment groups, respectively. The calves in the supportive along with microencapsulated probiotic treatment group had faster resolution of diarrhea than supportive treatment group in log-rank (Mantel-Cox) and Dunn’s multiple comparison tests (Fig. 3).
Results of the Kaplan–Meier failure estimates evaluating days to resolution of diarrhea to an abnormal fecal score between supportive treatment (RV-ST) and supportive treatment plus microencapsulated probiotic (ST-MP) groups by time from initiation of treatment (Chi square = 7.764; df = 1; P = 0.0053)
Discussion
In the current study, marked increase (p < 0.05) of mean packed cell volume in rotavirus-infected diarrhoetic calves than healthy control group is due to dehydration resulting in hemoconcentration [43]. The mechanisms involved in the dehydration in rotaviral diarrhea are the destruction of mature enterocytes in gut, viral enterotoxin non-structural protein 4-mediated Ca+2-dependent transepithelial chloride ion secretion, inhibition of sodium D-glucose linked transporter-mediated water reabsorption, reduced activity of brush border membrane disaccharidases, stimulation of enteric nervous system, and villous ischemia [44, 45]. Earlier studies also reported the increase of hemoglobin and packed cell volume in farm animal with rotavirus diarrhea [43, 46]. A marked decrease of total leukocyte count in packed cell volume-infected diarrhoetic calves could be due to depletion of progenitor cells of bone marrow and hematopoietic and lymphoproliferative organs [47]. In rotaviral diarrhea, leucopenia is associated with sepsis and extravasations of neutrophils at the site of inflammation and simultaneously inadequate entry of newly formed leukocyte from lymphoproliferative organs because of compromised hematopoietic system resulting in reduction of total leukocyte count [48]. In supportive along with microencapsulated probiotic treatment group, the enhancement of mean total leukocyte count on day 5 of treatment could be due to stimulation of hematopoietic system and increased innate immune reactions by probiotic treatment [49]. The increase of total leukocyte count by lactobacillus probiotic treatments has been reported in calves [50]. The hypoproteinemia and hypoalbuminemia in rotavirus-infected diarrhoetic groups are the result of protein losing enteropathy from damaged intestinal mucosa and increased intestinal mucosal permeability [51, 52]. Here, the elevation of mean total protein level on days 3 and 5 and albumin level on day 5 in supportive along with microencapsulated probiotic treatment group might be due to gut mucosa protective mechanism of probiotic, and the findings are in accordance with earlier studies in broiler chicken [53].
The early reaction of the host to rotavirus infection is the acute-phase response through the induction of cytokines, resulting in the production of acute-phase proteins. Neonatal calf diarrhea with rotavirus infection substantially increases acute-phase proteins including ceruloplasmin and transferrin in the circulation [54]. The ceruloplasmin, the major copper-binding protein, has an essential role in iron metabolism and the elimination of free iron. The copper concentrations, unlike iron and zinc, increase as part of the acute-phase response. Zinc is a negative acute-phase reactant, and around 90% of circulating zinc is bound to albumin. Therefore, hypoalbuminemia lowers the zinc level in serum [55]. In the current study, increased copper level could be a result of acute-phase response, and low zinc level may be due to decreased absorption or protein loosing enteropathy in rotavirus-infected diarrhoetic calves. The similar findings were recorded in earlier studies on pediatric patients with rotaviral gastroenteritis [4, 56]. The absorption of copper in intestine is dependent on at least two transport proteins, the high-affinity copper transporter 1 (CTR1) and ATP7A that is a copper-transporting P-type ATPase [57]. Recently, it has been reported that L. acidophilus probiotic has a negative effect related to the decrease expression of the copper-translocating P-type ATPase gene that encodes for proteins required for the transport and delivery of copper [58]. Here, the drop in serum copper concentration following probiotic therapy in rotavirus-infected diarrhoetic calves might be due to poor absorption of copper in the intestine by inhibition of copper transport proteins by probiotic [59]. Although no direct effect of probiotics on zinc and copper is evident, its effect on the iron status of the host has been reported, which indirectly modulates the copper and zinc level in serum [60]. Skrypnik et al. [61] observed that the concentrations of copper and zinc in the internal vital organs were substantially improved by probiotic supplementations in rats. The probiotic’s adsorption ability of zinc varies with probiotic strains and depends on its properties such as structure, functional groups, and surface area [62]. It is reported that lactobacilli probiotics act as good vehicle for biotransformation of inorganic minerals into more bioavailable organic form, in which zinc binds in the form of protein complexes leading to absorption into the gut [63]. The marked improvement of zinc level in serum in the present study following probiotic therapy in rotavirus-infected diarrhoetic calves might be due to increased bioavailability of zinc for absorption by probiotic. The amelioration of mineral imbalance by probiotic supplementation has been reported in laboratory animal model [64].
A profound decrease of immunoglobulin A and immunoglobulin G concentrations in serum of rotavirus-infected diarrhoetic calves than healthy control calves in this study is in accordance with our earlier research and other investigations on neonatal animals [46, 54]. In the probiotic treatment group, the average serum immunoglobulin A and immunoglobulin G levels were significantly (p < 0.05) increased on days 3 and 5 of treatment. The beneficial effects of probiotics have been explained by its immunomodulatory role [65]. It is reported that L. acidophilus potentiates both cellular and humoral immune responses following oral rotavirus vaccination and stimulates mucosal and systemic T and B cell responses to viral infection [66]. Enhancement of the immunoglobulin A and immunoglobulin G concentrations in systemic circulations by oral probiotic supplementations has been observed in neonatal calves and lambs [67, 68]
A significant increase (p < 0.05) of serum nitric oxide and TNF-α in rotavirus-positive diarrhoetic calves than healthy calves in this study could be due to increased expression of inducible nitric oxide synthase and secretion of inflammatory cytokines from intestinal epithelial cells and immune cells by the enterotoxigenic effect of NSP4 of rotavirus as reported in diarrhoetic calves and piglets [69, 70]. The anti-inflammatory and immunomodulatory potential of probiotics has been documented in gastrointestinal diseases [71]. In our study, mean nitric oxide and TNF-α concentrations were significantly (p < 0.5) decreased on days 3 and 5 from pre-treatment value in rotavirus-positive diarrhoetic calves treated with supportive along with microencapsulated probiotic treatment, and the findings are in accordance with earlier in vivo and in vitro studies [72,73,74]. The L. acidophilus has a role in decreasing serum nitric oxide and TNF-α concentrations by its antioxidant and anti-inflammatory properties as observed in piglets and in vitro studies [73, 75].
In rotavirus infection, two important pathways are known to develop diarrhea. One is directly linked to the destruction of mature enterocytes by the lytic effect of virus, and another pathway is the production of rotaviral enterotoxin that is non-structural protein 4 [76, 77]. The NSP4 induces diarrhea by removing Ca2+ from the endoplasmic reticulum resulting in loss of chloride ions and electrolyte homeostasis [78, 79]. Longer period of diarrhea by rotavirus causes more loss of fluids, resulting in dehydration and electrolyte abnormalities [80]. Hence, shortening of duration of rotaviral diarrhea is utmost important for speedy recovery. In this study, the time to resolution of diarrhea was markedly (p < 0.05) lower in supportive along with microencapsulated probiotic treatment group than supportive treatment group when evaluated with a log-rank (Mantel-Cox) test. In supportive along with microencapsulated probiotic treatment group, duration of diarrhea was reduced by 2.0 day as compared to supportive treatment group, and it could have prevented the loss of fluid and electrolyte over that time. Probiotics of Lactobacillus strains and their metabolites such as organic acids, hydrogen peroxide, and proteinaceous molecules exhibit anti-rotaviral activities through a variety of mechanisms, including blocking virus attachment to cell surfaces, lowering rotavirus induced reactive oxygen species, and decreasing IL-6 production [81, 82]. Hemaiswarya et al. [83] observed that the lactic acid bacteria diminish rotavirus diarrhea by increased mucosal immunity through inducing immunoglobulin A-specific antibody-secreting cell. Recently, it is documented that metabolites of Lactobacillus strains show antiviral activity against rotavirus by reducing of NSP4 production and Ca2+ liberation from the intracellular site [84]. Here, in the log-rank (Mantel-Cox) test, the decreased time to resolution of diarrhea in supportive along with microencapsulated probiotic treatment group than supportive treatment group might be due to different anti-rotavirus mechanisms of L. acidophilus probiotic. Another reason might be better survival of microencapsulated probiotic during harsh abomasal acidic pH, due to encapsulation with sodium alginate and chitosan coating resulting in adequate availability at the site of action [85, 86].
In conclusion, results of the study support the advantage of microencapsulated L. acidophilus NCDC 15 probiotic as an adjunct therapy in treating rotavirus-infected neonatal calf diarrhea over supportive therapy alone. Besides resolution of diarrhea, it resulted in improving the leukocyte count in blood, total protein, albumin, zinc, immunoglobulin A, and immunoglobulin G levels and decreasing the copper and inflammatory parameters (nitric oxide and TNF-α) in serum of rotavirus-infected diarrhoetic calves. Thus, the encapsulated probiotic can be considered as a new candidate approach for additional treatment of rotavirus-infected neonatal calf diarrhea. Our findings suggest that microencapsulated L. acidophilus NCDC 15 probiotic may be useful for the treatment of acute rotaviral enteritis in neonatal calves as an adjunct therapy without adverse effects. However, to gain an improved understanding of the clinical response, additional studies involving larger number of rotavirus-associated neonatal diarrhea cases are warranted.
Supplementary file 1 Agarose gel electrophoresis of RT-PCR of mammalian group A rotavirus using primer pair RVA-D-226-FP and RVA-D-226-RP. Lane M: 100 bp DNA ladder as marker. Lane P: positive control. Lane N: negative control. Lanes 1, 4, 6–7, 8–9, 12, 14–17, 19–20: positive for group A bovine rotavirus. Lanes 2–3, 5, 10–11, 13 and 18: negative for group A bovine rotavirus.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Fernandez S, Fraga M, Castells M, Colina R, Zunino P (2020) Effect of the administration of Lactobacillus spp. strains on neonatal diarrhoea, immune parameters and pathogen abundance in pre-weaned calves. Benef Microbes 11:477–488. https://doi.org/10.3920/BM2019.0167
Jang JY, Kim S, Kwon MS, Lee J, Yu DH, Song RH, Choi HJ, Park J (2019) Rotavirus-mediated alteration of gut microbiota and its correlation with physiological characteristics in neonatal calves. J Microbiol 57:113–121. https://doi.org/10.1007/s12275-019-8549-1
Kalita A, Talukdar M, Sarma K, Kalita PC, Roychoudhury P, Kalita G, Choudhary OP, Chaudhary JK, Doley PJ, Debroy S (2020) Small intestinal mucosal cells in piglets fed with probiotic and zinc: a qualitative and quantitative microanatomical study. Folia Morphol (Warsz) 80(3):605–617. https://doi.org/10.5603/FM.a2020.0091
Afolabi OF, Saka AO, Ojuawo A, Biliaminu SA (2019) Serum zinc levels of hospitalized children with acute diarrhea differ by the isolated viruses. Int J Med Sci 13:4–10
Feldmann HR, Williams DR, Champagne JD, Lehenbauer TW, Aly SS (2019) Effectiveness of zinc supplementation on diarrhea and average daily gain in pre-weaned dairy calves: a double-blind, block-randomized, placebo-controlled clinical trial. PLoS ONE 14:e0219321. https://doi.org/10.1371/journal.pone.0219321
Florez ID, Niño-Serna LF, Beltrán-Arroyave CP (2020) Acute infectious diarrhea and gastroenteritis in children. Curr Infect Dis Rep 22:4. https://doi.org/10.1007/s11908-020-0713-6
Ortiz-Perez A, Donnelly B, Temple H, Tiao G, Bansal R, Mohanty SK (2020) Innate immunity and pathogenesis of biliary atresia. Front Immunol 11:329. https://doi.org/10.3389/fimmu.2020.00329
Zhou Y, Qiao H, Yin N, Chen L, Xie Y, Wu J, Du J, Lin X, Wang Y, Liu Y, Yi S, Zhang G, Sun M, He Z, Li H (2019) Immune and cytokine/chemokine responses of PBMCs in rotavirus-infected rhesus infants and their significance in viral pathogenesis. J Med Virol 91:1448–1469. https://doi.org/10.1002/jmv.25460
Sen A, Namsa ND, Feng N, Greenberg HB (2020) Rotavirus reprograms multiple interferon receptors and restricts their intestinal antiviral and inflammatory functions. J Virol 94(6):e01775-e1819. https://doi.org/10.1128/JVI.01775-19
Hakim MS, Ding S, Chen S, Yin Y, Su J, van der Woude CJ, Fuhler GM, Peppelenbosch MP, Pan Q, Wang W (2018) TNF-α exerts potent anti- RV effects via the activation of classical NF-kB pathway. Virus Res 253:28–37. https://doi.org/10.1016/j.virusres.2018.05.022
Patra U, Mukhopadhyay U, Mukherjee A, Sarkar R, Chawla-Sarkar M (2020) Progressive rotavirus infection downregulates redox-sensitive transcription factor Nrf2 and Nrf2-driven transcription units. Oxid Med Cell Longev 2020:7289120. https://doi.org/10.1155/2020/7289120
Rodríguez Díaz J, Banasaz M, Istrate C, Buesa J, Lundgren O, Espinoza F, Sundqvist T, Rottenberg M, Svensson L (2006) Role of nitric oxide during RV infection. J Med Virol 78:979–985. https://doi.org/10.1002/jmv.20650
Jiang B, Snipes-Magaldi L, Dennehy P, Keyserling H, Holman RC, Bresee J, Gentsch J, Glass RI (2003) Cytokines as mediators for or effectors against rotavirus disease in children. Clin Diagn Lab Immunol 10:995–1001. https://doi.org/10.1128/cdli.10.6.995-1001.2003
Carvalho MF, Gill D (2019) Rotavirus vaccine efficacy: current status and areas for improvement. Hum Vaccin Immunother 15:1237–1250. https://doi.org/10.1080/21645515.2018.1520583
Otero CE, Langel SN, Blasi M, Permar SR (2020) Maternal antibody interference contributes to reduced rotavirus vaccine efficacy in developing countries. PLoS Pathog 16:e1009010. https://doi.org/10.1371/journal.ppat.1009010
Premkumar P, Lopman B, Ramani S, Paul A, Gladstone B, Muliyil J, Mukhopadhya I, Parashar U, Kang G (2014) Association of serum antibodies with protection against rotavirus infection and disease in South Indian children. Vaccine 32(Suppl 1):A55-61. https://doi.org/10.1016/j.vaccine.2014.04.077
Canani RB, Cirillo P, Terrin G, Cesarano L, Spagnuolo MI, De Vincenzo A, Albano F, Passariello A, De Marco G, Manguso F, Guarino A (2007) Probiotics for treatment of acute diarrhoea in children: randomised clinical trial of five different preparations. Br Med J 335:340. https://doi.org/10.1136/bmj.39272.581736.55
Rios M, Munoz M, Spencer E (1995) Antiviral activity of phosphonoformate and rotavirus transcription and replication. Antiviral Res 27:71–83. https://doi.org/10.1016/0166-3542(94)00085-m
Crawford SE, Ramani S, Tate JE, Parashar UD, Svensson L, Hagbom M, Franco MA, Greenberg HB, O’Ryan M, Kang G, Desselberger U, Estes MK (2017) Rotavirus infection Nat Rev Dis Primers 3:17083. https://doi.org/10.1038/nrdp.2017.83
Chen YN, Liu N (2020) A systematic review of the risk of oral rotavirus vaccine and intussusception. Zhonghua Yu Fang Yi Xue Za Zhi 54:793–797. https://doi.org/10.3760/cma.j.cn112150-20191115-00860
Gandhi GR, Barreto PG, Lima BD, Quintans JS, Araújo AA, Narain N, Quintans-Júnior LJ, Gurgel RQ (2016) Medicinal plants and natural molecules with in vitro and in vivo activity against rotavirus: a systematic review. Phytomedicine 23:1830–1842. https://doi.org/10.1016/j.phymed.2016.11.005
Kim JH, Kim K, Kim W (2020) Genipin inhibits rotavirus-induced diarrhea by suppressing viral replication and regulating inflammatory responses. Sci Rep 10:15836. https://doi.org/10.1038/s41598-020-72968-7
Dhawan BN (2012) Anti-viral activity of Indian plants. Proc Natl Acad Sci India Sect B Biol Sci 82:209–224. https://doi.org/10.1007/s40011-011-0016-7
Ali SI, Sheikh WM, Rather MA, Venkatesalu V, Muzamil Bashir S, Nabi SU (2021) Medicinal plants: treasure for antiviral drug discovery. Phytother Res 35(7):3447–3483. https://doi.org/10.1002/ptr.7039
Renaud DL, Kelton DF, Weese JS, Noble C, Duffield TF (2019) Evaluation of a multispecies probiotic as a supportive treatment for diarrhea in dairy calves: a randomized clinical trial. J Dairy Sci 102:4498–4505. https://doi.org/10.3168/jds.2018-15793
Azagra-Boronat I, Massot-Cladera M, Knipping K, Garssen J, Ben Amor K, Knol J, Franch À, Castell M, Rodríguez-Lagunas MJ, Pérez-Cano FJ (2020) Strain-specific probiotic properties of bifidobacteria and lactobacilli for the prevention of diarrhea caused by rotavirus in a preclinical model. Nutrients 12:498. https://doi.org/10.3390/nu12020498
Jiang Y, Ye L, Cui Y, Yang G, Yang W, Wang J, Hu J, Gu W, Shi C, Huang H, Wang C (2017) Effects of Lactobacillus rhamnosus GG on the maturation and differentiation of dendritic cells in rotavirus-infected mice. Benef Microbes 8:645–656. https://doi.org/10.3920/BM2016.0157
Monteagudo-Mera A, Rastall RA, Gibson GR, Charalampopoulos D, Chatzifragkou A (2019) Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl Microbiol Biotechnol 103:6463–6472. https://doi.org/10.1007/s00253-019-09978-7
Agarwal N, Kamra DN, Chaudhary LC, Agarwal I, Sahoo A, Pathak NN (2002) Microbial status and rumen enzyme profile of crossbred calves fed on different microbial feed additives. Lett Appl Microbiol 34:329–336. https://doi.org/10.1046/j.1472-765x.2002.01092.x
Dowarah R, Verma AK, Agarwal N, Singh P, Patel BHM (2017) Effect of swine based probiotic on performance, diarrhoea scores, intestinal microbiota and gut health of grower-finisher crossbred pigs. Livest Sci 195:74–79. https://doi.org/10.1016/j.livsci.2016.11.006
Yao M, Xie J, Du H, McClements DJ, Xiao H, Li L (2020) Progress in microencapsulation of probiotics: a review. Compr Rev Food Sci Food Saf 19:857–874. https://doi.org/10.1111/1541-4337.12532
Qi W, Liang X, Yun T, Weiqun G (2019) Growth and survival of microencapsulated probiotics prepared by emulsion and internal gelation. J Food Sci Technol 56:1398–1404. https://doi.org/10.1007/s13197-019-03616-w
Albadran HA, Monteagudo-Mera A, Khutoryanskiy VV, Charalampopoulos D (2020) Development of chitosan-coated agar-gelatin particles for probiotic delivery and targeted release in the gastrointestinal tract. Appl Microbiol Biotechnol 104:5749–5757. https://doi.org/10.1007/s00253-020-10632-w
Qi X, Simsek S, Ohm JB, Chen B, Rao J (2020) Viability of Lactobacillus rhamnosus GG microencapsulated in alginate/chitosan hydrogel particles during storage and simulated gastrointestinal digestion: role of chitosan molecular weight. Soft Matter 16:1877–1887. https://doi.org/10.1039/c9sm02387a
Calinoiu LF, Stefanescu BE, Pop ID, Muntean L, Vodnar DC (2019) Chitosan coating applications in probiotic microencapsulation. Coatings 9:194. https://doi.org/10.3390/coatings9030194
Ramaswami N, Chaudhary LC, Agarwal N, Kamra DN (2005) Effect of lactic acid producing bacteria on the performance of male crossbred calves fed roughage based diet. Asian-Austral J Anim Sci 18:1110–1115. https://doi.org/10.5713/ajas.2005.1110
Kala A, Mahesh Kumar GR, Chaudhary LC, Agarwal N (2020) Development of microencapsulated and lyophilized probiotic and its comparative evaluation. National seminar on ‘feed additives for improving the efficiency and sustainability of milk production in dairy animals’, organized by Department of Animal Nutrition, College of Veterinary Science and Animal Husbandry, SDAU, Sardarkrushinagar, Gujarat, July 20–21, 2020, https://www.researchgate.net/publication/344100341_Development_of_microencapsulated_and_lyophilized_probiotic_and_its_comparative_evaluation
Walker PG, Constable PD, Morin DE, Drackley JK, Foreman JH, Thurmon JC (1998) A reliable, practical, and economical protocol for inducing diarrhea and severe dehydration in the neonatal calf. Can J Vet Res 62:205–213
Malik YPS, Sharma K, Vaid N, Chakravarti S, Chandrashekar KM, Basera SS, Singh R, Prasad G, Gulati BR, Bhilegaonkar KN, Pandey AB (2012) Frequency of group A rotavirus with mixed G and P genotypes in bovines: predominance of G3 genotype and its emergence in combination with G8/G10 types. J Vet Sci 13:271–278. https://doi.org/10.4142/jvs.2012.13.3.271
Mondal A, Sharma K, Malik YPS, Joardar SN (2013) Detection of group A rotavirus in faeces of diarrhoeic bovine porcine and human population from eastern India by reverse transcriptase–polymerase chain reaction. Adv Anim Vet Sci 1:18–19
Schalm OW, Jain NC (1986) Schalm’s Veterinary Hematology. Lea and Febiger, Philadelphia
Sastry KVH, Moudgal RP, Mohan J, Tyagi JS, Rao G (2002) Spectrophotometric determination of serum nitrite and nitrate by copper–cadmium alloy. Anal Biochem 306:79–82. https://doi.org/10.1006/abio.2002.5676
Barua SR, Md Rakib T, Das S, Masuduzzaman M, Hossain MA, Chowdhury S (2018) Hematological and serological changes in neonatal diarrhoeic calves infected with bovine rotavirus. Multidisciplinary Adv Vet Sci 2:356–366
Foster DM, Smith GW (2009) Pathophysiology of diarrhea in calves. Vet Clin North Am Food Anim Pract 25:13–36. https://doi.org/10.1016/j.cvfa.2008.10.013
Chang-Graham AL, Perry JL, Engevik MA, Engevik KA, Scribano FJ, Gebert JT, Danhof HA, Nelson JC, Kellen JS, Strtak AC, Sastri NP, Estes MK, Britton RA, Versalovic J, Hyser JM (2020) Rotavirus induces intercellular calcium waves through ADP signaling. Science 370:eabc3621. https://doi.org/10.1126/science.abc3621.
Chethan GE, Garkhal J, Sircar S, Malik YPS, Mukherjee R, Sahoo NR, Agarwal RK, De UK (2017) Immunomodulatory potential of β-glucan as supportive treatment in porcine RV enteritis. Vet Immunol Immunopathol 191:36–43. https://doi.org/10.1016/j.vetimm.2017.07.012
Prittie J (2004) Canine parvoviral enteritis: a review of diagnosis, management, and prevention. J Vet Emerg Crit Care 14:167–176. https://doi.org/10.1111/j.1534-6935.2004.04020.x
Shim JO, Son DW, Shim SY, Ryoo E, Kim W, Jung YC (2012) Clinical characteristics and genotypes of rotaviruses in a neonatal intensive care unit. Pediatr Neonat 53:18–23. https://doi.org/10.1016/j.pedneo.2011.11.005
Mohammadian T, Dezfuly ZT, Motlagh RG, Jangaran-Nejad A, Hosseini SS, Khaj H, Alijani N (2020) Effect of encapsulated Lactobacillus bulgaricus on innate immune system and hematological parameters in rainbow trout (Oncorhynchus mykiss), post-administration of Pb. Probiotics Antimicrob Proteins 12:375–388. https://doi.org/10.1007/s12602-019-09544-7
Dar A, Singh SK, Palod J, al Ain K, Kumar N, Khadda B, Farooq F, (2017) Effect of probiotic, prebiotic and synbiotic on hematological parameters of crossbred calves. Int J Livestock Res 7:127–136. https://doi.org/10.5455/ijlr.20170312053224
Parisi A, Cafarotti A, Salvatore R, Pelliccia P, Breda L, Chiarelli F (2018) Protein losing enteropathy in an infant with rotavirus infection. Paediatr Int Child Health 8:154–157. https://doi.org/10.1080/20469047.2017.1295011
Trivedi M, Jain A, Shah D, Gupta P (2019) Rotavirus gastroenteritis associated with encephalopathy, myositis, transaminitis and hypoalbuminemia. Indian J Pediatr 86:642–644. https://doi.org/10.1007/s12098-019-02959-8
Younis TM, El-Shafei AA, Al-Gamal MA, El-Sayed AL (2013) Effects of commercial probiotics on productive and physiological performance of broiler chickens. J Appl Sci Res 9:6643–6654
Rocha TG, Silva FDF, Bortoletto C, Silva DG, Buzinaro MG, Zafalon LF, Fagliari JJ (2016) Serum concentrations of acute phase proteins and immunoglobulins of calves with RV diarrhea. Arq Bras Med Vet Zootec 68:865–872. https://doi.org/10.1590/1678-4162-7965
Galloway P, McMillan DC, Sattar N (2000) Effect of the inflammatory response on trace element and vitamin status. Ann Clin Biochem 37:289–297. https://doi.org/10.1258/0004563001899429
Percy S, Hijam D, Dubey D, Devi NO, Devi OP, Singh KIM, Singh MA (2015) Role of serum zinc and copper in children with gastroenteritis. J Dental Med Sci 14:6–10. https://doi.org/10.9790/0853-141150610
van den Berghe PVE, Klomp LWJ (2009) New developments in the regulation of intestinal copper absorption. Nutr Rev 67:658–672. https://doi.org/10.1111/j.1753-4887.2009.00250.x
De Cesare A, Sala C, Castellani G, Astolfi A, Indio V, Giardini A, Manfreda G (2020) Effect of Lactobacillus acidophilus D2/CSL (CECT 4529) supplementation in drinking water on chicken crop and caeca microbiome. PLoS ONE 15:e0228338. https://doi.org/10.1371/journal.pone.0228338
Mrvcic J, Stanzer D, Bacvun-druzina V, Stehlik-tomas V (2009) Copper binding by lactic acid bacteria (LAB). Biosci Microflora 28:1–6. https://doi.org/10.12938/bifidus.28.1.
Suliburska J, Bogdañski P, Pupek-Musialik D, Krejpcio Z (2011) Dietary intake and serum and hair concentrations of minerals and their relationship with serum lipids and glucose levels in hypertensive and obese patients with insulin resistance. Biol Trace Elem Res 139:137–150. https://doi.org/10.1007/s12011-010-8650-0
Skrypnik K, Bogdanski P, Schmidt M, Suliburska J (2019) The effect of multispecies probiotic supplementation on iron status in rats. Biol Trace Elem Res 192:234–243. https://doi.org/10.1007/s12011-019-1658-1
Mudronova D, Gancarcíkova S, Nemcova R (2019) Influence of zinc sulphate on the probiotic properties of Lactobacillus plantarum CCM 7102. Folia Vet 63:45–54. https://doi.org/10.2478/fv-2019-0018
Mohan J, Ali SA, Suvartan RR, Kapila S, Sharma R, Tomar SK, Behare P, Yadav H (2018) Bioavailability of biotransformed zinc enriched dahi in wistar rats. Int J Probiotics Prebiotics 13:45–54
Kvan OV, Gavrish IA, Lebedev SV, Korotkova AM, Miroshnikova EP, Serdaeva VA, Bykov AV, Davydova NO (2018) Effect of probiotics on the basis of Bacillus subtilis and Bifidobacterium longum on the biochemical parameters of the animal organism. Environ Sci Pollution Res 25:2175–2183. https://doi.org/10.1007/s11356-017-0534-9
Zhao H, Zhang F, Chai J, Wang J (2020) Lactobacillus acidophilus reduces Listeria monocytogenes infection by inhibiting mitogen-activated protein kinase genes in growing rabbits. Rev Bras Zootec 49:e20200054. https://doi.org/10.37496/rbz4920200054
Hussein AF (2018) Effect of probiotics on growth, some plasma biochemical parameters and immunoglobulins of growing Najdi lambs. World Vet J 8: 80–89. pii:S232245681800009–8
Al-Saiady MY (2010) Effect of probiotic bacteria on immunoglobulin G concentration and other blood components of newborn calves. J Anim Vet Adv 9:604–609. https://doi.org/10.3923/javaa.2010.604.609
Karamzadeh-Dehaghani A, Towhidi A, Zhandi M, Mojgani N, Fouladi-Nashta A (2021) Combined effect of probiotics and specific immunoglobulin Y directed against Escherichia coli on growth performance, diarrhea incidence, and immune system in calves. Animal 15:100124. https://doi.org/10.1016/j.animal.2020.100124
Alfajaro MM, Kim HJ, Park JG, Ryu EH, Kim JY, Jeong YJ, Kim DS, Hosmillo M, Son KY, Lee JH, Kwon HJ (2012) Anti-rotaviral effects of Glycyrrhiza uralensis extract in piglets with rotavirus diarrhea. J Virol 9:310. https://doi.org/10.1186/1743-422X-9-310
Chethan GE, De UK, Garkhal J, Sircar S, Malik YPS, Sahoo NR, Abhishek VMR (2019) Immunomodulating dose of levamisole stimulates innate immune response and prevents intestinal damage in porcine rotavirus diarrhea: a restricted-randomized, single-blinded, and placebo-controlled clinical trial. Trop Anim Health Prod 51:1455–1465. https://doi.org/10.1007/s11250-019-01833-1
Oh NS, Joung JY, Lee JY, Kim Y (2018) Probiotic and anti-inflammatory potential of Lactobacillus rhamnosus 4B15 and Lactobacillus gasseri 4M13 isolated from infant feces. PLoS ONE 13:e0192021. https://doi.org/10.1371/journal.pone.0192021
Baillon MLA, Marshall-Jones ZV, Butterwick RF (2004) Effects of probiotic Lactobacillus acidophilus strain DSM13241 in healthy adult dogs. Am J Vet Res 65:338–343. https://doi.org/10.2460/ajvr.2004.65.338
Chen K, Liang NL, Luo XG, Zhang TC (2013) Lactobacillus acidophilus strain suppresses the transcription of proinflammatory-related factors in human HT-29 cells. J Microbiol Biotechnol 23:64–68. https://doi.org/10.4014/jmb.1208.04067
Angurana SK, Bansal A, Singhi S, Aggarwal R, Jayashree M, Salaria M, Mangat NK (2018) Evaluation of effect of probiotics on cytokine levels in critically ill children with severe sepsis: a double-blind, placebo-controlled trial. Crit Care Med 46:1656–1664. https://doi.org/10.1097/CCM.0000000000003279
Azevedo MSP, Zhang W, Wen K, Gonzalez AM, Saif LJ, Yousef AE, Yuan L (2012) Lactobacillus acidophilus and L. reuteri modulate cytokine responses in gnotobiotic pigs infected with human rotavirus. Benef Microbes 3(1): 33–42. https://doi.org/10.3920/BM2011.0041
Ball JM, Mitchell DM, Gibbons TF, Parr RD (2005) Review rotavirus NSP4: a multifunctional viral enterotoxin. Viral Immunol 18:27–40. https://doi.org/10.1089/vim.2005.18.27
Greenberg HB, Estes MK (2009) Rotavirus: from pathogenesis to vaccination. Gastroenterol 136:1939–1951. https://doi.org/10.1053/j.gastro.2009.02.076
Pham T, Perry JL, Dosey TL, Delcour AH, Hyser JM (2017) The rotavirus NSP4 viroporin domain is a calcium-conducting ion channel. Sci Rep 7:43487. https://doi.org/10.1038/srep43487
Yu B, Jiang Y, Zhang B, Yang H, Ma T (2018) Resveratrol dimer trans-ε-viniferin prevents rotaviral diarrhea in mice by inhibition of the intestinal calcium-activated chloride channel. Pharmacol Res 129:453–461. https://doi.org/10.1016/j.phrs.2017.11.016
Posovszky C, Buderus S, Classen M, Lawrenz B, Keller KM, Koletzko S (2020) Acute infectious gastroenteritis in infancy and childhood. Dtsch Arztebl Int 117:615–624. https://doi.org/10.3238/arztebl.2020.0615
Lievin-Le Moal V, Servin AL (2014) Anti-infective activities of Lactobacillus strains in the human intestinal microbiota: from probiotics to gastrointestinal antiinfectious biotherapeutic agents. Clin Microbiol Rev 27:167–199. https://doi.org/10.1128/CMR.00080-13
Al Kassaa I (2017) The antiviral activity of probiotic metabolites. In: Al Kassaa I (ed) New insights on antiviral probiotics, 1st edn. Springer, Cham, pp 83–97
Hemaiswarya S, Raja R, Ravikumar R, Carvalho IS (2015) Mechanism of action of probiotics. Braz Arch Biol Techy 56:113–119. https://doi.org/10.1590/S1516-89132013000100015
Olaya Galán NN, Ulloa Rubiano JC, Velez Reyes FA, Fernandez Duarte KP, Salas Cárdenas SP, Gutierrez Fernandez MF (2016) In vitro antiviral activity of Lactobacillus casei and Bifidobacterium adolescentis against rotavirus infection monitored by NSP4 protein production. J Appl Microbiol 120:1041–1051. https://doi.org/10.1111/jam.13069
Whorwell PJ, Altringer L, Morel J, Bond Y, Charbonneau D, O’Mahony L, Kiely B, Shanahan F, Quigley EM (2006) Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol 101:1581–1590. https://doi.org/10.1111/j.1572-0241.2006.00734.x
Arora S, Kaur IP, Chopra K, Rishi P (2014) Efficiency of double layered microencapsulated probiotic to modulate proinflammatory molecular markers for the management of alcoholic liver disease. Mediators Inflamm 2014:715130. https://doi.org/10.1155/2014/715130
Acknowledgements
This work was supported by the All India Network Programme on Neonatal Mortality project of Indian Council of Agriculture Research. First author (JSG) thanks Indian Council of Agriculture Research-Indian Veterinary Research Institute for granting research fellowship for his research program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
All procedures used during the research were approved by Institute Animal Ethics Committee and the protocol was approved by the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Fisheries, Animal Husbandry and Dairying, Government of India (approval number F.25/02/2020-CPCSEA-DADF). All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. The research protocol was approved by the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Fisheries, Animal Husbandry and Dairying, Government of India (approval number F.25/02/2020-CPCSEA-DADF).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gandhar, J.S., De, U.K., Kala, A. et al. Efficacy of Microencapsulated Probiotic as Adjunct Therapy on Resolution of Diarrhea, Copper-Zinc Homeostasis, Immunoglobulins, and Inflammatory Markers in Serum of Spontaneous Rotavirus-Infected Diarrhoetic Calves. Probiotics & Antimicro. Prot. 14, 1054–1066 (2022). https://doi.org/10.1007/s12602-021-09862-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-021-09862-9