Abstract
Mastitis, common inflammation of the mammary gland, caused by various factors, is a challenge for the dairy industry. Escherichia coli (E. coli), a Gram-negative opportunistic pathogen, is one of the major pathogens causing clinical mastitis which is characterized by reduced milk production and recognizable clinical symptoms. Bacillus subtilis (B. subtilis) has been reported to have the ability to limit the colonization of pathogens and has immune-stimulatory effects on epithelial cells. The purpose of this study was to explore the preventive role of B. subtilis H28 on E. coli–induced mastitis in mice. The mastitis model was established by nipple duct injection of E. coli into mice, while B. subtilis H28 was utilized 2 h before E. coli injection. Furthermore, pathological changes in the mammary gland were evaluated by hematoxylin–eosin (H&E) staining. Enzyme-linked immunosorbent assay (ELISA) was used to detect the levels of proinflammatory cytokines (TNF-α, IL-1β, and IL-6). We also observed changes in Toll-like receptor 4 (TLR4), nuclear transcription factor kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) expression by using western blotting. The results revealed that B. subtilis H28 pretreatment reduced neutrophil infiltration in the mammary gland tissues, significantly decreased the secretion of TNF-α, IL-1β, and IL-6, and downregulated the activation of TLR4 and the phosphorylation of p65 NF-κB, IκB, p38, and ERK. In conclusion, our results indicated that B. subtilis H28 can ameliorate E. coli–induced mastitis and suggest a new method for the prevention of mastitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mastitis, a severe inflammation of the parenchyma of mammary glands generally caused by pathogens, has a huge influence on all lactating mammals and the dairy industry [1]. In cows, clinical mastitis, which is characterized by red, swollen, hot, painful discoloration at the site of the infected mammary gland, an increased number of somatic cells in the milk and reduced milk production, is commonly caused by E. coli [2]. E. coli is an opportunistic pathogen that is one of the normal host microbiota, but it can invade the mammary gland to cause inflammation under some circumstances [3, 4]. Previous studies have shown that lipopolysaccharide (LPS), a component part of the cell wall of E. coli, has the ability to induce acute mastitis by activating the NF-κB signaling pathway after recognition by TLR4 [5, 6]. Although traditional antibiotics contribute to the treatment of cow mastitis, problems such as drug residues and resistance still threaten the dairy industry.
Bacillus subtilis (B. subtilis) has the ability to survive extreme environmental conditions such as high temperature and acidity conditions [7]. Furthermore, Bacillus strains also have probiotic potential by interacting with the internal milieu of the host by generating sundry antimicrobial peptides and small active molecules [8]. Recently, B. subtilis has been reported to decrease pathogen colonization by interfering with the quorum sensing [9], producing various antibacterial and antifungal compounds [10] and, affecting the immune stimulation of epithelial cells [11]. The findings from in vivo studies claim that B. subtilis exhibits an anti-inflammatory effect by mediating the secretion of TNF-α, IL-10, and IFN-γ in mouse models [12, 13]. Interestingly, B. subtilis, as one of the normal host microbiota, also exists in the mammary gland of cows [14], which provides strong support for the use of B. subtilis for the prevention of mastitis in dairy cows. However, the effects of B. subtilis H28 on E. coli–induced mastitis and the underlying immune mechanism are poorly understood.
In this study, we used a mouse model of mastitis to investigate the preventive effect of B. subtilis H28 on E. coli–induced mastitis by injecting B. subtilis H28 at different concentrations 2 h before E. coli injection. The results demonstrated that B. subtilis H28 ameliorated the pathological damage of mammary tissues, reduced the secretion of proinflammatory cytokines, and inhibited the activation of TLR4-mediated NF-κB and MAPK signaling pathways. Therefore, potential probiotics B. subtilis H28 that target breast pathogens may be an effective strategy to protect against mastitis.
Materials and Method
Animals
A total of 90 BALB/c mice (60 female and 30 male) aged 6–8 weeks were purchased from the Liaoning Changsheng Biotechnology Co. Ltd. The animal experiments were approved by the Animal Ethics Committee of Jilin University (pzpx20201023027). Females and males were mixed at a ratio of 2 to 1 in microisolator cages where water and food were provided freely until pregnancy.
Materials
ELISA kits of TNF-α, IL-6, and IL-1β were purchased from Biolegend, CA, USA. All the monoclonal antibodies, including β-actin, p65, p38, and the phosphorylation of p65 and p38, were purchased from Cell Signaling Technology, Beverly, MA, USA. Luria–Bertani (LB) medium, Bacillus medium, and MacConkey agar were purchased from Qingdao Haibo Biotechnology Co., Ltd, Qingdao, China.
Bacteria and the Culture Conditions
B. subtilis H28 was isolated from healthy cow’s milk samples and identified by 16S rDNA sequencing and PCR. E. coli CVCC 1418 was purchased from China Veterinary Culture Collection Center (CVCC). In our study, E. coli was inoculated into a fresh LB medium at a ratio of 1:100 for approximately 6 h after E. coli was cultured overnight in LB medium at 37 °C and 120 r/min, and then the OD600 was approximately 0.5 (the concentration was approximately 108 CFU/mL). B. subtilis H28 was inoculated into Bacillus medium by the same inoculation method, and then, the OD600 was approximately 0.6 (the concentration was approximately 109 CFU/mL) after 6 h of growth.
Mastitis Model and Experiment Design
The model of mouse mastitis was established according to the previous studies [15, 16]. In brief, pregnant BALB/c mice were administered mammary gland injections at the fourth pair of nipples 5–7 days after delivery. The mice were randomly divided into 6 groups: a control group, E. coli group (107 CFU/100 μL), B. subtilis H28 (107, 108, and 109 CFU/100 μL) + E. coli and B. subtilis H28 group (109 CFU/100 μL). The B. subtilis H28 or PBS (control and E. coli group) was injected into each side of the nipple 2 h prior to E. coli (107 CFU/100 μL) treatment, and then 100 μL E. coli was administered in the same way. Then, 24 h after the last injection, the mice were euthanized, and mammary gland tissue was collected and stored at −20 °C until analysis.
Histological Evaluation
Mammary gland tissues were fixed in 4% formalin for at least 48 h and sliced by paraffin embedding. Then the slices were stained by hematoxylin and eosin after xylene dewaxing and rehydrating with graded alcohols. Finally, the pathological changes of the mammary gland were evaluated under a light microscope (×100).
Cytokine Assay
The proinflammatory cytokines were measured by using an ELISA assay kit according to the manufacturer’s instructions. Mammary gland tissue (0.05 g) was weighed and ground into homogenate by adding 500 μL PBS. Then the supernatant was collected after centrifugation at 12,000 r/min for 10 min at 4 °C.
MPO Activity Determination
To homogenize the mammary gland tissues, HEPES was added, and then the precipitate was collected. Furthermore, the precipitate was ground and centrifuged after adding cetyltrimethylammonium chloride (CTAC), and the supernatant was collected for the examination of MPO, which was detected by calculating the absorbance (OD) at 450 nm.
Western Blot Analysis
To extract the total proteins of the mammary tissues, tissue protein extract was used. Then, the supernatant was collected, and the protein concentration was detected with a BCA protein assay kit. SDS-PAGE (10%) was used to separate the protein samples; subsequently, the protein samples were transferred to PVDF membranes and blocked in 5% skim milk for 2 h at room temperature. The PVDF membranes were incubated with specific primary antibodies against phospho-p38 (1:1000), phospho-ERK (1:1000), p38 (1:1000), ERK (1:1000), β-actin (1:1000), phospho-IκB (1:1000), IκB (1:1000), NF-κB p65 (1:1000), and phospho-NF-κB p65 (1:1000) at 4 °C overnight. Then, the membranes were washed 3 times with tris-buffered saline-Tween (TBST) for 10 min each time and incubated with goat anti-rabbit secondary antibodies for 2 h at room temperature. The membranes were detected by the ECL Plus Western blotting Detection System.
Statistical Analysis
These data were analyzed applying one-way ANOVA and Tukey’s multiple comparisons test by using GraphPad Prism 5 (Manufacturer, La Jolla, CA, USA). All data are expressed as mean ± SEM.
Results
Effects of B. subtilis H28 on Mammary Gland Tissues Histopathological Changes in E. coli–Induced Mastitis
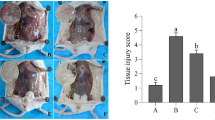
As shown in Fig. 1, the E. coli group had mammary gland structure destruction and inflammatory cell infiltration, while B. subtilis H28 pretreatment recovered the structure of the mammary gland and decreased the inflammatory infiltration. Notably, there was no significant pathological change in the B. subtilis H28-treated (without E. coli) group compared with the control group.
Effects of B. subtilis H28 on mammary gland tissues histopathological changes. Histopathologic sections of mammary tissues (H&E, ×100). A The control group, B B. subtilis H28 group, C E. coli group, D B. subtilis H28 (107 CFU/mL) + E. coli, E B. subtilis H28 (108 CFU/mL) + E. coli, and F B. subtilis H28 (109 CFU/mL) + E. coli groups. The values presented are the means ± SEM of three independent experiments, * P < 0.05
Effects of B. subtilis H28 on MPO Activity
MPO is a biomarker of neutrophil infiltration and can indirectly reflect the degree of inflammation [17]. As shown in Fig. 2, the results revealed that MPO activity in the E. coli group was significantly increased relative to that in the control group. In contrast, MPO activity was reduced in the B. subtilis H28-treated group compared with the E. coli group. The MPO activity was not increased markedly in the group treated with B. subtilis H28 alone.
Effects of B. subtilis H28 on Proinflammatory Cytokine Levels in E. coli-Induced Mastitis
To explore the anti-inflammatory effects of B. subtilis H28 on E. coli–induced mastitis, TNF-α, IL-1β, and IL-6 levels were detected by ELISA assays. As shown in Fig. 3, the expression of TNF-α, IL-1β, and IL-6 increased significantly compared with that in the control group after E. coli stimulation. However, B. subtilis H28 dramatically reduced the increases in their levels in a dose-dependent manner. No significant inflammatory factor release was observed in the B. subtilis H28-treated group compared with the control group.
Effect of B. subtilis H28 administration on pro-inflammatory cytokine production in mammary glands. The levels of pro-inflammatory cytokines in mammary gland tissues were detected at 24 h after E. coli treatment by using ELISA kits. The values presented are the means ± SEM of three independent experiments, * P < 0.05
Effects of B. subtilis H28 on the Activity of the NF-κB and MAPK Signaling Pathways
To elucidate the mechanisms of B. subtilis H28 on mastitis, we examined the effect of B. subtilis H28 on the activity of the NF-κB and MAPK signaling pathways. The western blot results demonstrated that TLR4, phosphorylation of NF-κB p65, phosphorylation of IκB, phosphorylation of p38, and phosphorylation of ERK were upregulated in the E. coli group, but all of them were significantly decreased after B. subtilis H28 treatment compared with the E. coli group. However, there were no significant changes in these signaling pathways in the B. subtilis H28 group compared with the control group (Figs. 4 and 5).
Effects of B. subtilis H28 on the activity of the TLR4-mediated NF-κB signaling pathway. Effects of B. subtilis H28 on the expression of the TLR4-mediated NF-κB pathways were induced by E. coli. All protein samples were analyzed by western blot with specific antibodies, and β-actin was used as a control. The values presented are the means ± SEM of three independent experiments. * P < 0.05
Effects of B. subtilis H28 on the activity of the MAPK signaling pathways. Effects of B. subtilis H28 on the expression of the MAPK pathways were induced by E. coli. All protein samples were analyzed by western blot with specific antibodies, and β-actin was used as a control. The values presented are the means ± SEM of three independent experiments, * P < 0.05
Discussion
Mastitis is characterized by the destruction of the structure of the breast acinus and a large amount of neutrophil infiltration, accompanied by the secretion of inflammatory factors. Recently, an increasing number of studies have shown that probiotics have the potential to treat a variety of diseases, such as inflammatory bowel disease, obesity, and diabetes [14, 18, 19]. Unlike other probiotics such as lactobacilli and Lacticaseibacillus rhamnosus, which have clear probiotic effects and extensive research [20, 21]. However, the use of Bacillus has been controversial because certain strains of Bacillus, such as Bacillus cereus, can cause diarrhea by producing some toxins [22]. However, although some Bacillus may be harmful, B. subtilis has long been considered a safe and effective probiotic that is widely used in industry [8]. Recently, B. subtilis has been reported to reduce pathogen colonization by interfering with quorum sensing [9], to produce various antibacterial and antifungal compounds [10] and to affect immune stimulation of epithelial cells, which provides B. subtilis the possibility to protect against inflammation [23]. Hence, we have used a mouse model of mastitis to investigate the protective role of B. subtilis H28 against local inflammation of the mammary gland.
MPO is a functional marker and activation marker of neutrophils, and its level and activity changes represent the function and activity status of the PMN [17]. PMN can kill microorganisms in phagocytic cells and can also be secreted extracellularly to target multiple cells, but high concentrations of MPO can cause oxidative stress and tissue damage [24]. As shown in our study, MPO activity in the E. coli group was significantly increased relative to that in the control group, but MPO activity was reduced after B. subtilis H28 administration, while B. subtilis H28 administration without E. coli group did not show obvious amplification compared with that in the control group. Cytokines are reactive indicator of inflammation, including proinflammatory and anti-inflammatory cytokines. TNF-α, IL-1β, and IL-6 are the main proinflammatory cytokines present in mastitic inflammation [25]. In our study, the results showed that B. subtilis H28 markedly reduced the production of TNF-α, IL-1β, and IL-6 in a dose-dependent manner, which illustrated that the degree of E. coli–induced inflammation was ameliorated by B. subtilis H28.
To further clarify the mechanism by which B. subtilis H28 improves inflammation, we detected the NF-κB and MAPK signaling pathways. It has been reported that the Gram-negative bacteria, such as E. coli, are recognized by TLR4 [26], a pattern recognition receptor (PRR) on the cell surface. The downstream signaling pathways NF-κB and activator protein 1 (AP-1) are then activated in a myeloid differentiation primary response 88 (MyD88)-dependent or MyD88-non-dependent manner [27], ultimately promoting cytokine secretion. NF-κB, a fast-acting nuclear transcription factor involved in multiple proteins which mainly include RelA (p65), RelB, c-Rel, p50, and p52 in mammals [28], plays an important role in the inflammatory and immune response. Generally, IκB kinase (IKK) is activated to degrade IκB, which is normally bound to NF-κB in the cytoplasm of unstimulated cells. Then, the free NF-κB enters the nucleus and binds to genes with NF-κB binding sites to initiate transcriptional processes. In fact, TNF-α and pro-IL-1β are secreted during the initial transcriptional process following activation of NF-κB, while IL-6 is released during secondary transcription which is related to inhibition of IκB [27]. In addition, the MAPK signaling pathways also have important regulatory effects on inflammation via the phosphorylation of p38 and ERK [29]. Our results showed that B. subtilis H28 downregulated the activation of TLR4 and the phosphorylation of p65 NF-κB, IκB, ERK, and p38, which suggests that the inhibitory effect of B. subtilis H28 on the secretion of pro-inflammatory cytokines may be due to the suppression of the NF-κB and MAPK pathways.
In summary, our study indicated that the preventive effect of B. subtilis H28 during E. coli–induced mastitis may be due to its ability to inhibit the TLR4-mediated NF-κB and MAPK signaling pathways (Fig. 6). Therefore, our study provides a support for the potential probiotic effect of B. subtilis H28, and B. subtilis H28 may be a promising candidate for the prevention of mastitis.
Availability of Data and Material
All data and materials are available from the corresponding authors on reasonable request.
References
Seegers H, Fourichon C, Beaudeau F (2003) Production effects related to mastitis and mastitis economics in dairy cattle herds. Vet Res 34(5):475–491. https://doi.org/10.1051/vetres:2003027
Kandasamy S, Green BB, Benjamin AL, Kerr DE (2011) Between-cow variation in dermal fibroblast response to lipopolysaccharide reflected in resolution of inflammation during Escherichia coli mastitis. J Dairy Sci 94(12):5963–5975. https://doi.org/10.3168/jds.2011-4288
Goldstone RJ, Harris S, Smith DG (2016) Genomic content typifying a prevalent clade of bovine mastitis-associated Escherichia coli. Sci Rep 6:30115. https://doi.org/10.1038/srep30115
Barbosa-Cesnik C, Schwartz K, Foxman B (2003) Lactation mastitis. JAMA 289(13):1609–1612. https://doi.org/10.1001/jama.289.13.1609
He X, Wei Z, Zhou E, Chen L, Kou J, Wang J, Yang Z (2015) Baicalein attenuates inflammatory responses by suppressing TLR4 mediated NF-kappaB and MAPK signaling pathways in LPS-induced mastitis in mice. Int Immunopharmacol 28(1):470–476. https://doi.org/10.1016/j.intimp.2015.07.012
Liu C, Tang X, Zhang W, Li G, Chen Y, Guo A, Hu C (2019) 6-Bromoindirubin-3’-oxime suppresses LPS-induced inflammation via inhibition of the TLR4/NF-kappaB and TLR4/MAPK signaling pathways. Inflammation 42(6):2192–2204. https://doi.org/10.1007/s10753-019-01083-1
Jia H, Lee FS, Farinas ET (2014) Bacillus subtilis spore display of laccase for evolution under extreme conditions of high concentrations of organic solvent. ACS Comb Sci 16(12):665–669. https://doi.org/10.1021/co500113t
Elshaghabee FMF, Rokana N, Gulhane RD, Sharma C, Panwar H (2017) Bacillus as potential probiotics: status, concerns, and future perspectives. Front Microbiol 8:1490. https://doi.org/10.3389/fmicb.2017.01490
Piewngam P, Zheng Y, Nguyen TH, Dickey SW, Joo HS, Villaruz AE, Glose KA, Fisher EL, Hunt RL, Li B et al (2018) Pathogen elimination by probiotic Bacillus via signalling interference. Nature 562(7728):532–537. https://doi.org/10.1038/s41586-018-0616-y
Stein T (2005) Bacillus subtilis antibiotics: structures, syntheses and specific functions. Mol Microbiol 56(4):845–857. https://doi.org/10.1111/j.1365-2958.2005.04587.x
Fujiya M, Musch MW, Nakagawa Y, Hu S, Alverdy J, Kohgo Y, Schneewind O, Jabri B, Chang EB (2007) The Bacillus subtilis quorum-sensing molecule CSF contributes to intestinal homeostasis via OCTN2, a host cell membrane transporter. Cell Host Microbe 1(4):299–308. https://doi.org/10.1016/j.chom.2007.05.004
Foligné B, Peys E, Hemel JVJV, Dewulf J, Breton J, Pot B (2012) Spores from two distinct colony types of the strain Bacillus subtilis PB6 substantiate anti-inflammatory probiotic effects in mice. Clin Nutr 31(6):987–994. https://doi.org/10.1016/j.clnu.2012.05.016
Yin Y, Zhang P, Yue X, Du X, Li W, Yin Y, Yi C, Li Y (2018) Effect of sub-chronic exposure to lead (Pb) and Bacillus subtilis on Carassius auratus gibelio: bioaccumulation, antioxidant responses and immune responses. Ecotoxicol Environ Saf 161:755–762. https://doi.org/10.1016/j.ecoenv.2018.06.056
Duanis-Assaf D, Kenan E, Sionov R, Steinberg D, Shemesh M (2020) Proteolytic activity of Bacillus subtilis upon κ-casein undermines its “caries-safe” effect. Microorganisms 8(2):221. https://doi.org/10.3390/microorganisms8020221
Gutierrez-Chavez AJ, Martinez-Ortega EA, Valencia-Posadas M, Leon-Galvan MF, de la Fuente-Salcido NM, Bideshi DK, Barboza-Corona JE (2016) Potential use of Bacillus thuringiensis bacteriocins to control antibiotic-resistant bacteria associated with mastitis in dairy goats. Folia Microbiol (Praha) 61(1):11–19. https://doi.org/10.1007/s12223-015-0404-0
Mignacca SA, Dore S, Spuria L, Zanghi P, Amato B, Dupre I, Armas F, Biasibetti E, Camperio C, Lollai SA et al (2017) Intramammary infusion of a live culture of Lactococcus lactis in ewes to treat staphylococcal mastitis. J Med Microbiol 66(12):1798–1810. https://doi.org/10.1099/jmm.0.000641
Hu G, Hong D, Zhang T, Duan H, Wei P, Guo X, Mu X (2018) Cynatratoside-C from Cynanchum atratum displays anti-inflammatory effect via suppressing TLR4 mediated NF-kappaB and MAPK signaling pathways in LPS-induced mastitis in mice. Chem Biol Interact 279:187–195. https://doi.org/10.1016/j.cbi.2017.10.017
Wang X, Ba T, Cheng Y, Zhang P, Chang X (2021) Probiotics alleviate adipose inflammation in high-fat diet-induced obesity by restoring adipose invariant natural killer T cells. Nutrition 89:111285. https://doi.org/10.1016/j.nut.2021.111285
Hsieh PS, Ho HH, Tsao SP, Hsieh SH, Lin WY, Chen JF, Kuo YW, Tsai SY, Huang HY (2021) Multi-strain probiotic supplement attenuates streptozotocin-induced type-2 diabetes by reducing inflammation and beta-cell death in rats. PLoS One 16(6):e0251646. https://doi.org/10.1371/journal.pone.0251646
Tomusiak-Plebanek A, Heczko P, Skowron B, Baranowska A, Okon K, Thor PJ, Strus M (2018) Lactobacilli with superoxide dismutase-like or catalase activity are more effective in alleviating inflammation in an inflammatory bowel disease mouse model. Drug Des Devel Ther 12:3221–3233. https://doi.org/10.2147/DDDT.S164559
Zhang J, Ma JY, Li QH, Su H, Sun X (2018) Lactobacillus rhamnosus GG induced protective effect on allergic airway inflammation is associated with gut microbiota. Cell Immunol 332:77–84. https://doi.org/10.1016/j.cellimm.2018.08.002
Al-Khatib MS, Khyami-Horani H, Badran E, Shehabi AA (2007) Incidence and characterization of diarrheal enterotoxins of fecal Bacillus cereus isolates associated with diarrhea. Diagn Microbiol Infect Dis 59(4):383–387. https://doi.org/10.1016/j.diagmicrobio.2007.06.014
Kawarizadeh A, Pourmontaseri M, Farzaneh M, Hosseinzadeh S, Ghaemi M, Tabatabaei M, Pourmontaseri Z, Pirnia MM (2021) Interleukin-8 gene expression and apoptosis induced by Salmonella Typhimurium in the presence of Bacillus probiotics in the epithelial cell. J Appl Microbiol 131(1):449–459. https://doi.org/10.1111/jam.14898
Ahn J, Ambrosone CB, Kanetsky PA, Tian C, Lehman TA, Kropp S, Helmbold I, von Fournier D, Haase W, Sautter-Bihl ML et al (2006) Polymorphisms in genes related to oxidative stress (CAT, MnSOD, MPO, and eNOS) and acute toxicities from radiation therapy following lumpectomy for breast cancer. Clin Cancer Res 12(23):7063–7070. https://doi.org/10.1158/1078-0432.CCR-06-0039
Shah KN, Valand P, Nauriyal DS, Joshi CG (2018) Immunomodulation of IL-1, IL-6 and IL-8 cytokines by Prosopis juliflora alkaloids during bovine sub-clinical mastitis. 3 Biotech 8(10):409. https://doi.org/10.1007/s13205-018-1438-1
Suhs KA, Marthaler BR, Welch RA, Hopkins WJ (2011) Lack of association between the Tlr4 (Lpsd/Lpsd) genotype and increased susceptibility to Escherichia coli bladder infections in female C3H/HeJ mice. MBio 2(3):e00094-e111. https://doi.org/10.1128/mBio.00094-11
Takeuchi O, Akira S (2010) Pattern recognition receptors and inflammation. Cell 140(6):805–820. https://doi.org/10.1016/j.cell.2010.01.022
Kumar H, Kawai T, Akira S (2009) Toll-like receptors and innate immunity. Biochem Biophys Res Commun 388(4):621–625. https://doi.org/10.1016/j.bbrc.2009.08.062
Chen J, Xu J, Li J, Du L, Chen T, Liu P, Peng S, Wang M, Song H (2015) Epigallocatechin-3-gallate attenuates lipopolysaccharide-induced mastitis in rats via suppressing MAPK mediated inflammatory responses and oxidative stress. Int Immunopharmacol 26(1):147–152. https://doi.org/10.1016/j.intimp.2015.03.025
Funding
This work was supported by a grant from the China Postdoctoral Science Foundation (2020TQ0120, 2020M681045) and the National Natural Science Foundation of China (Nos. 31972749, 31772812).
Author information
Authors and Affiliations
Contributions
X. H. and C. J. contributed to article writing, literature search, result evaluation. R. T. and R. M. contributed to sample collection. Y. W. and Y. G. contributed to literature search and result evaluation. R. T. and Y. F. contributed to the manuscript revised. N. Z. and Y. F. contributed to the study design and the final revision of the article and expert opinions.
Corresponding authors
Ethics declarations
Ethical Approval
The animal experiments were approved by Jilin University Animal Ethics Committee, and the present study was performed according to the care and use of Laboratory Animals published manual by the US National Institutes of Health.
Consent for Publication
All authors consent with the publication of this article.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hu, X., Tang, R., Zhao, C. et al. The Prevention Effect of Bacillus subtilis on Escherichia coli–Induced Mastitis in Mice by Suppressing the NF-κB and MAPK Signaling Pathways. Probiotics & Antimicro. Prot. 15, 74–81 (2023). https://doi.org/10.1007/s12602-021-09854-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-021-09854-9