Abstract
In this study, we investigated the probiotic properties and anti-obesity effects of bacterial strains isolated from homemade kimchi. Lactiplantibacillus plantarum KU15117 was isolated using lactobacilli selective medium. L. plantarum KU15117 did not produce β-glucuronidase and showed high tolerance to artificial gastric juice and bile salt, acceptable resistance to antibiotics, and high adhesion ability to HT-29 cells. The anti-adipogenic activity of L. plantarum KU15117 at 109 CFU/well was confirmed by the reduction of oil red O staining and intracellular triglyceride level. Additionally, the expression levels of fatty acid synthase, CCAAT/enhance-binding protein-α, and peroxisome proliferator-activated receptor-γ, which are associated with the early stage of adipocyte differentiation, were significantly lower in the probiotic-treated group than in the control group. These results suggest that L. plantarum KU15117 has probiotic properties and anti-obesity effects and could be used as a prophylactic probiotics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Changes in lifestyles, such as reduction in physical activities and convenient lifestyles, may induce obesity, which is not only a cosmetic problem but also a major health issue. Obesity is associated with abnormal or excessive fat accumulation leading to various diseases, including non-alcoholic fatty liver disease, cardiovascular diseases, type 2 diabetes, cancer, and hypertension [1, 2]. Particularly, obesity is caused by an imbalance between lipogenesis and lipolysis, which are complex processes regulated by various signaling molecules. Adipogenesis is characterized by changes in cell morphology, accumulation of triglycerides, and expression of related gene [3, 4]. Peroxisome activated receptor-γ (PPAR-γ) and CCAAT/enhancer-binding protein-α (C/EBP-α) are involved in the early stage of adipocyte differentiation, and some enzymes, including adipose-specific fatty acid-binding protein (aP2), fatty acid synthase (FAS), sterol regulatory element-binding protein-1c (SREBP-1c), and carnitine palmitoyltransferase-1 (CPT-1), are involved in the formation of mature adipocytes [5, 6].
Probiotics are live bacteria, mainly lactic acid bacteria, which are beneficial to humans and animals by improving intestinal microbial balance [7]. Common probiotics include representatives of Lactobacillus acidophilus, Lacticaseibacillus casei, Lactiplantibacillus plantarum, Lacticaseibacillus rhamnosus, Bifidobacterium bifidum, and Bifidobacterium longum. Some of these probiotics can be components of functional foods and may be sold as dietary supplements. Few studies have reported the pleiotropic effects of probiotics, including antimicrobial, anticancer, anti-inflammatory, antioxidant, anti-biofilm, anti-obesity, antidiabetic, and cholesterol-lowering activities [8, 9].
The gut microbiome influences human health and consists of more than 100 trillion bacterial species [10]. They include the commensal bacteria, which are involved in digestion related to metabolic disorders [11]. The cell-free extract (CFE) of Lactobacillus fermentum MG4231 and MG4244 strains showed anti-obesity effects through the inhibition of adipogenesis and lipid accumulation in 3T3-L1 preadipocytes. The anti-obesity effects of CFE also involved the downregulation of FAS, aP2, PPAR-γ, and C/EBP-α expression, as well as upregulating of AMP-activated protein kinase (AMPK) and hormone-sensitive lipase (HSL) phosphorylation [4, 12]. The adjustment of the gut microbiome has been suggested as a therapeutic approach against obesity and metabolic disorders. Therefore, in this study, we determined the probiotic properties and anti-adipogenic effects of Lactobacillus strains isolated from homemade kimchi.
Materials and Methods
Bacterial Strains and Culture Conditions
Lactiplantibacillus plantarum KU15117 (KCCM 12212P) and Latilactobacillus curvatus KU15031 were isolated using Lactobacillus Selective Medium (BD BBL, Franklin Lakes, NJ, USA) from Korean homemade diced-radish kimchi and cabbage kimchi. The commercial probiotic strain Lacticaseibacillus rhamnosus GG (KCTC 5033) was used as the reference strain. Lactobacillus strains were cultured in MRS broth at 37 °C for 24 h.
Tolerance of Bacterial Strains to Artificial Acid and Bile Salt
The tolerance of bacterial strains to artificial acid and bile salt conditions was determined as previously described by Lee et al. [9] and Son et al. [13]. To determine the tolerance of the strains to artificial acid, overnight cultures of bacterial strains were resuspended in artificial gastric acid (pH 2.5) (MRS medium containing 0.3% (w/v) of pepsin (Sigma-Aldrich, St. Louis, MO, USA)), followed by incubation at 37 °C for 3 h. To determine tolerance to bile acid, overnight cultures were resuspended in MRS medium containing 0.3% (w/v) of oxgall (BD BBL), followed by incubation at 37 °C for 24 h. Viable cells were counted after plating and incubated on MRS agar at 48 °C for 24 h. The survival rate was calculated as follows:
Enzyme Production
Enzyme production was measured using the API ZYM kit (BioMerieux, Lyon, France). Bacterial strains were centrifuged (12,000 × g, 4 °C, 10 min), and the harvested cells were resuspended in PBS at 105 CFU/mL. The resuspended cultures were inoculated in each well and incubated at 37 °C for 4 h. Next, ZYM reagents A and B were added to the cupules. The enzyme activity was determined as 0 to ≥ 40 nM based on the color change.
Adhesion of Bacterial Strains to HT-29 Cells
HT-29 (human colon adenocarcinoma, KCLB 30038) cell line was cultivated in RPMI 1640 (HyClone Laboratories, Inc., Logan, UT, USA) with 10% fetal bovine serum (FBS; HyClone Laboratories, Inc.) and 1% streptomycin/penicillin solution at 37 °C in 5% CO2 atmosphere.
The adherence of bacterial strains to HT-29 cells was performed according to the method of Son et al. [13]. HT-29 cells were seeded by 1 × 105 cells/well in a 24-well plate and cultured at 37 °C for 24 h. Bacterial strains were inoculated into each well at approximately 107 CFU and cultured at 37 °C for 2 h. Non-adhered bacteria were removed by washing thrice with PBS, followed by the addition of 1 mL Triton X-100 (1% (v/v); Sigma-Aldrich) into each well and incubation at 37 °C for 10 min. Incubated cells were harvested from each well, and adherent bacterial cells were plated on MRS plates. Adhesion activity was calculated as follows:
Antibiotic Sensitivity of Bacterial Strains
The sensitivity of the bacterial strains was measured according to the guidelines of the Clinical and Laboratory Standards Institute [15]. Each bacterial strain, at a concentration of 107 CFU/mL, was dispersed on MRS agar, and paper discs containing the antibiotics were placed on the plate after a few minutes. The antibiotics used were ampicillin (10 μg/disc), chloramphenicol (30 μg/disc), ciprofloxacin (5 μg/disc), doxycycline (30 μg/disc), gentamicin (10 μg/disc), kanamycin (30 μg/disc), streptomycin (10 μg/disc), and tetracycline (30 μg/disc). The plates were cultured at 37 °C for 24 h, and the inhibition zones were measured.
Anti-adipogenic Effect of Bacterial Strains
Preparation of Heat-Killed Bacteria
Bacterial strains were grown in MRS broth and washed twice with PBS by centrifugation at 12,000 × g at 4 °C for 10 min. The washed bacteria were resuspended in PBS at a final concentration of 108 and 109 CFU/mL, respectively, and the cells were plated to confirm the number of viable cells. Each bacterial sample was heated in a water bath at 80 °C for 30 min.
Cell Culture and Differentiation of 3T3-L1 cells
3T3-L1 preadipocytes (ATCC CL-173) were cultured in Dulbecco’s modified Eagle’s medium (DMEM; HyClone Laboratories, Inc.) supplemented with 10% bovine calf serum (HyClone Laboratories, Inc.) and 1% streptomycin/penicillin solution at 37 °C at 5% CO2. For adipocyte differentiation, the cells were seeded in 6-cm cell culture dishes at a density of 1.5 × 104 cells/dish and cultured until confluence (approximately 3 days). After confluence, the growth medium was replaced with the differentiation medium (MDI), consisting of DMEM, 10% FBS, 0.5 mM 3-isobutyl-1-methylxanthine (IBMX; Sigma-Aldrich), 10 μM dexamethasone (Sigma-Aldrich), and 5 μg/mL insulin (Sigma-Aldrich), and the cells were cultured for 2 days. Next, the medium was changed to insulin media containing DMEM, 10% FBS, and 5 μg/mL insulin, which was replaced at 2, 4, and 6 days.
Cell Viability of 3T3-L1
The effect of bacterial strains on the viability of 3T3-L1 cells was evaluated using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich) assay. 3T3-L1 cells were plated at 1 × 104 cells/well in 24-well plates until confluence. Next, the Lactobacillus strains were added at 108 and 109 CFU/well and incubated at 37 °C for 48 h in an incubator (5% CO2). The supernatant was aspirated, and the cells were incubated with MTT solution (2.5 mg/mL) for 1 h. After discarding the supernatant, DMSO (Sigma-Aldrich) was added to each well to dissolve the generated formazan. The dissolved solution was measured at 570 nm using a microplate reader, and cell viability was calculated.
Oil Red O Staining and Intracellular Triglyceride Contents
The effects of bacterial strains on oil red O-stained differentiated 3T3-L1 were determined as described by Park et al. [16]. Differentiated 3T3-L1 cells were fixed with 10% formaldehyde solution for 20 min, followed by the addition of 0.5% oil red O solution (Sigma-Aldrich) to each dish and incubation at room temperature for 20 min. After staining, the cells were washed twice with PBS and isopropanol was added to each dish, and the absorbance was measured at 520 nm.
To determine the intracellular triglyceride level, a triglyceride quantification kit (BioVision, Milpitas, CA, USA) was used. Differentiated 3T3-L1 cells were harvested and centrifuged at 14,000 × g for 25 min at 4 °C. Triglyceride levels were determined according to the manufacturer’s protocol.
Semi-Quantitative RT-PCR Analysis
The 3T3-L1 cells were seeded in 6-cm cell culture dishes (1.5 × 104 cells/dish) and differentiated into mature adipocytes. This was followed by the addition of bacterial strains (108 and 109 CFU/well). RNA was isolated from the treated 3T3-L1 cells using the RNeasy Mini Kit (Qiagen, Germany), and cDNA was synthesized using the Revert Aid First Strand cDNA Synthesis Kit (ThermoFisher Scientific, MA, USA). The expression of adipogenesis-related genes was measured by RT-PCR using synthesized cDNA, primers (shown in Table 1), and SYBR Green PCR Master Mix (PikoReal 96, ThermoFisher Scientific). The RT-PCR conditions were 95 °C for 2 min, 40 cycles of 95 °C for 5 s, and 60 °C for 15 s. Gene expression was determined by relative quantification with β-actin as the house-keeping gene.
Western Blot Analysis
The expression of obesity-related proteins was investigated by western blotting. Differentiated 3T3-L1 adipocytes treated with bacterial strains (108 and 109 CFU/well) were harvested by using RIPA lysis and extraction buffer with Halt™ Protease and Phosphatase Inhibitor Cocktail (ThermoFisher Scientific), and the cell lysates were sonicated (5 AMP; pulse on, 3 s; pulse off 3 s) for a total period of 9 s and placed on ice. The sonicated cell lysate was harvested, and the supernatant was obtained by centrifugation at 14,000 × g at 4 °C for 25 min. The protein concentration of the supernatant was measured using a DC™ protein assay kit (Bio-Rad Laboratories Inc., Hercules, CA, USA). Each protein was separated using sodium dodecylsulfate–polyacrylamide gel electrophoresis gel. The separated proteins were transferred onto a polyvinylidene fluoride membrane (Millipore, Bedford, MA, USA). The membrane was blocked with 5% skim milk for 30 min and reacted with a specific primary antibody at 4 °C for 20 h. The membrane was then incubated with a horseradish peroxidase–conjugated secondary antibody for 2 h. The protein bands were visualized using a chemiluminescence detection kit (Thermo FisherScientific), and the thickness was analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical Analysis
All experiments were performed in triplicate and presented as the mean ± standard deviation using one-way analysis of variance (ANOVA) and Duncan’s multiple range test. Values were considered significant at P < 0.05. All analyses were conducted using the Statistical Package for the Social Sciences (SPSS), version 24 (IBM, Chicago, IL, USA).
Results
Gastric Acid and Bile Tolerance, Enzyme-Production Ability, Antibiotic Susceptibility, and Adhesion Ability of Bacterial Strains
The tolerance of L. rhamnosus GG, L. curvatus KU15031, and L. plantarum KU15117 to gastric acids and bile salts is shown in Table 2. All bacterial strains exhibited high resistance with over 96% of survival rate in gastric conditions (0.3% pepsin, pH 2.5). L. rhamnosus GG (102.88%) exhibited higher resistance than L. curvatus KU15031 (92.33%) and L. plantarum KU15117 (84.70%) in bile conditions (0.3% oxgall).
The enzymes produced by the different bacterial strains are shown in Table 3. The tested bacterial strains did not produce α-galactosidase (diabetes-related enzyme) or β-glucuronidase. However, L. rhamnosus GG and L. plantarum KU15117 produced β-galactosidase.
The antibiotic sensitivity of bacterial strains is presented in Table 4. L. curvatus KU15031 had a similar sensitivity to L. rhamnosus GG, except for ampicillin (10 μg). L. plantarum KU15117 was resistant to ampicillin (10 μg) and doxycycline (30 μg).
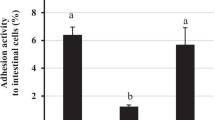
The bacterial strains showed a high adhesion rate to HT-29 cells (Fig. 1). L. rhamnosus GG exhibited a higher adhesion (6.37%) than L. curvatus KU15031 (1.33%) and L. plantarum KU15117 (2.34%).
Adhesion activity of bacterial strains to HT-29 cells. LGG, L. rhamnosus GG; KU15031, L. curvartus KU15031; KU15117, L. plantarum KU15117. Error bars indicate standard deviation of three independent experiments. All values are expressed as mean ± standard deviation. Letters denote statistical significance (P < 0.05) as determined by Duncan’s multiple range test
Effects of Bacterial Strains on Lipid Accumulation during Adipogenesis
The viability of 3T3-L1 cells treated with L. plantarum KU15117 and L. rhamnosus GG at 108 and 109 CFU/well was over 96% (Fig. 2). However, the viability of the cells treated with L. curvatus KU15031 was 34.58% and 26.79% at 108 and 109 CFU/well, respectively. Therefore, L. plantarum KU15117 and L. rhamnosus GG were used to determine lipid accumulation.
Effects of bacterial strains on the viability of 3T3-L1 adipocytes. LGG, L. rhamnosus GG; KU15031, L. curvartus KU15031; KU15117, L. plantarum KU15117. Filled square, 108 CFU/well; empty square, 109 CFU/well. All values are expressed as mean ± standard deviation. Letters denote significance (P < 0.05) as determined by Duncan’s multiple range test
L. plantarum KU15117 and L. rhamnosus GG inhibited lipid accumulation, as shown by oil red O staining (Fig. 3A). In comparison with that of the positive control (100.81%), the oil red stain contents was as follows: L. plantarum KU15117 (31.88%) at 109 CFU/well < L. rhamnosus GG (45.02%) at 109 CFU/well < L. plantarum KU15117 (86.95%) at 108 CFU/well < L. rhamnosus GG (96.78%) at 108 CFU/well (Fig. 3B). In addition, triglyceride contents of positive control were 5.661 mM, while L. plantarum KU15117 and L. rhamnosus GG showed 0.399 mM and 1.684 mM at 109 CFU/well, respectively (Fig. 3C).
Anti-obesity effects of bacterial strains on 3T3-L1 adipocytes. A Photograph of oil red O staining, B related absorbance of oil red O staining, and C triglyceride content. A, NC (negative control, non-treated with MDI in adipocytes); B, PC (positive control, treated with MDI in adipocytes); C, L. rhamnosus GG (108 CFU/well); D, L. rhamnosus GG (109 CFU/well); E, L. plantarum KU15117 (108 CFU/well); F, L. plantarum KU15117 (109 CFU/well); gray square, 108 CFU/well; white square, 109 CFU/well, LGG, L. rhamnosus GG; KU15117, L. plantarum KU15117. All values are expressed as mean ± standard deviation. Letters denote significance (P < 0.05) as determined by Duncan’s multiple range test
Effects of Bacterial Strains on the mRNA and Protein Levels of Adipogenesis-Related Genes in Differentiated 3T3-L1 Adipocytes
Figure 4 shows the regulation of FAS, C/EBP-α, and PPAR-γ mRNA levels in differentiated 3T3-L1 adipocytes. L. rhamnosus GG and L. plantarum KU15117 at 109 CFU/well significantly decreased the mRNA levels of the genes in 3T3-L1 cells. Particularly, L. plantarum KU15117 at 109 CFU/well decreased the expression of FAS (92.96%), C/EBP-α (99.41%), and PPAR-γ (95.26%) (Fig. 4A–C). L. rhamnosus GG at 108 CFU increased the expression of FAS, C/EBP-α, and PPAR-γ mRNA.
Anti-adipogenic effects of bacterial strains in MDI-induced differentiation of 3T3-L1 preadipocytes. A FAS, B C/EBPα, C PPAR-γ gene expression of lipid metabolism-related genes, and D adipogenic protein expression. NC (Negative control), not treated with MDI in adipocytes; PC (Positive control), treated with MDI in adipocytes; LGG, L. rhamnosus GG; KU15117, L. plantarum KU15117. Gray square, 108 CFU/well; white square, 109 CFU/well; β-actin, loading control; LGG-8, 108 CFU/well of L. rhamnosus GG; LGG-9, 109 CFU/well of L. rhamnosus GG; 117–8, 108 CFU/well of L. plantarum KU15117; 117–9, 109 CFU/well of L. plantarum KU15117. All values are expressed as the mean ± standard deviation and standardized against the β-actin housekeeping gene. Values with different letters in the same row indicate significant differences for each characteristic (P < 0.05)
The protein expression of adipogenic transcription factors and enzymes, including FAS, C/EBP-α, and PPAR-γ (Fig. 4D) was confirmed by western blot analysis. In the positive control, the expression levels of FAS, C/EBP-α, and PPAR-γ proteins were 3.28, 6.29, and 3.67, respectively. L. rhamnosus GG at 109 CFU/well decreased the protein expression by 0.96, 1.33, and 0.24, respectively. L. plantarum KU15117 at 108 CFU/well and L. plantarum KU15117 at 109 CFU/well significantly decreased the expression levels of FAS (3.08 and 0.91, respectively), C/EBP-α (2.74 and 1.15, respectively), and PPAR-γ (1.97 and 0, respectively). The expression of FAS, C/EBP-α, and PPAR-γ proteins following treatment with LGG-8 was 3.32, 6.36, and 2.64, respectively. These results display a similar trend to those of mRNA expression levels.
Discussion
Probiotics are used as food formulations for prophylactic therapy against metabolic syndrome. In this study, the anti-obesity effects of lactic acid bacteria isolated for probiotic use were investigated. Tolerance to gastric conditions is an essential characteristic, which influences the probiotic properties of bacterial strains in the intestine [14]. Under strongly acidic and bile salt conditions, the survival rate of Levilactobacillus brevis KU15153 reduced by 70.79% and increased by > 104.47%, respectively [17]. L. curvatus KU15031 and L. plantarum possessed probiotic properties, as indicated by the resistance to gastric conditions.
Some probiotic bacteria produce useful β-galactosidase, which decreases lactose intolerance [18]. However, some probiotic bacteria also produce deleterious enzymes, such as β-glucuronidase, which have been associated with the induction of carcinogenesis, mutagenesis, and toxicity [19]. Son et al. [13] indicated that probiotic L. plantarum FI10604 and L. brevis FI10700 do not produce β-glucuronidase. Lactococcus lactis KC24 produces various enzymes, including acid phosphatase, β-galactosidase, and naphthol-AS-BI-phosphohydrolase, but not β-glucuronidase [14]. Similarly, we showed that L. curvatus KU15031 and L. plantarum KU15117 do not produce α-galactosidase and β-glucuronidase.
The sensitivity of probiotic bacteria to antibiotics is a fundamental factor because antibiotic-resistant strains may not be easily eliminated if required, and the antibiotic resistance may be transmitted to pathogenic or potentially pathogenic bacteria [20]. L. plantarum Ln4 is sensitive to commercial antibiotics, such as chloramphenicol, doxycycline, ampicillin, and tetracycline [13]. Lactobacillus spp. have intrinsic resistance to aminoglycosides (kanamycin and streptomycin) or quinolones (ciprofloxacin) [21]. Therefore, these results confirm that L. curvatus KU15031 and L. plantarum KU15117 are safe in accordance with the CLSI guidelines [15].
The adhesion of bacterial strains to intestinal cells is the most important factor associated with their probiotic properties [22]. Song et al. [23] showed that L. brevis KCCM 12203P (6.84%) and L. rhamnosus GG (6.21%) have similar adhesion rates. Zhang et al. [24] and Jeon et al. [25] showed that L. plantarum strains (< 2%) and B. subtilis P223 (1.33%) have diminished adhesion to intestinal epithelial cells, respectively. Therefore, L. curvatus KU15031 (1.33%) and L. plantarum KU15117 (2.34%) have acceptable adhesion rates to HT-29 cells.
Obesity is related to the differentiation, expansion, and lipid accumulation of adipocytes [4]. L. brevis B151, L. fermentum KCCM 200060, and L. plantarum Ln4 exhibited reduced lipid accumulation in both heat-killed cells and freeze-dried broth [11]. During the differentiation period, L. plantarum Q180 dose-dependently inhibited 3T3-L1 adipogenesis in terms of lipid accumulation by 14.63% compared with that by control cells [26]. Park et al. [27] reported that the addition of L. brevis OPK-3 (40 μg/mL) showed 40% reduction in triglyceride accumulation. In our data, L. plantarum KU15117 (92.95%) and L. rhamnosus GG (68.93%) inhibited lipid accumulation by triglyceride accumulation.
In the early stage of adipocyte differentiation, adipocyte-specific FAS, C/EBP-α, and PPAR-γ are regulated [28]. Weissella koreensis OK1-6 significantly reduced the mRNA expression levels of SREBP1, aP2, FAS, and C/EBP-α [29]. L. brevis OPK-3 significantly downregulated the mRNA expression of C/EBP-α and PPAR-γ in differentiating 3T3-L1 adipocytes [27]. Similarly, L. plantarum KY1032 decreased the expression of PPAR-γ, C/EBP-α, FAS, and A-FABP proteins [30]. L. acidophilus and cocktail of Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus were reduced adipogenesis genes (PPAR-γ, CD36, and aP2) expression [31]. These results showed that the mRNA and protein expression levels of heat-killed L. plantarum KU15117 at 108 CFU/well and 109 CFU/well might have been downregulated during adipocyte differentiation. Therefore, L. plantarum KU15117 could be influenced in the early stage of adipocyte differentiation in animal models.
In conclusion, we demonstrated the probiotic properties and anti-obesity effects of L. plantarum KU15117. L. plantarum KU15117 showed high tolerance to gastric conditions, safe enzyme activity, high adhesion rate to intestinal cells, and safe antibiotic sensitivity. Additionally, the anti-adipogenic activity of L. plantarum KU15117 was demonstrated by reduced lipid accumulation, low level of intercellular triglyceride, and suppressed expression of adipocyte-specific genes and proteins that are associated with the early stage of adipocyte differentiation. Therefore, L. plantarum KU15117 is a probiotic strain with anti-obesity effects. In addition, this study should be confirmed in animal model for further study.
Data Availability Statement
All data generated or analyzed during this study are included in this article.
Abbreviations
- FAS:
-
Fatty acid synthase
- C/EBP-α:
-
CCAAT/enhance-binding protein-α
- PPAR-γ:
-
Peroxisome proliferator-activated receptor-γ
References
Cui M, Kim HY, Lee KH, Jeong JK, Hwang JH, Yeo KY et al (2015) Antiobesity effects of kimchi in diet-induced obese mice. J Ethn Foods 2:137–144. https://doi.org/10.1016/j.jef.2015.08.001
Li JJ, Huang CJ, Xie D (2008) Anti-obesity effects of conjugated linoleic acid, docosahexaenoic acid, and eicosapentaenoic acid. Mol Nutr Food Res 52:631–645. https://doi.org/10.1002/mnfr.200700399
Jang M, Choi HY, Kim GH (2019) Inhibitory effects of Orostachys malacophllus var. iwarenege extracts on reactive oxygen species production and lipid accumulation during 3T3-L1 adipocyte differentiation. Food Sci Biotechnol 28:227–236. https://doi.org/10.1007/s10068-018-0426-x
Kim SJ, Choi SI, Jang M, Jeong Y, Kang CH, Kim GH (2020) Anti-adipogenic effect of Lactobacillus fermentum MG4231 and MG4244 through AMPK pathway in 3T3-L1 preadipocytes. Food Sci Biotechnol 29:1541–1551. https://doi.org/10.1007/s10068-020-00819-2
Park JA, Tirupathi Pichiah PB, Yu JJ, Oh SH, Daily JWIII, Cha YS (2012) Antiobesity effect of kimchi fermented with Weissella koreensis OK1-6 as starter in high-fat diet-induced obese C57BL/6J mice. J Appl Microbiol 113:1507–1516. https://doi.org/10.1111/jam.12017
Yu HS, Kim WJ, Bae WY, Lee NK, Paik HD (2020) Inula britannica inhibits adipogenesis of 3T3-L1 preadipocytes via modulation of mitotic clonal expansion involving ERK 1/2 and Akt signaling pathways. Nutrients 12:3037. https://doi.org/10.3390/nu12103037
Reid G, Jass J, Sebulsky MT, McCormick JK (2003) Potential uses of probiotics in clinical practice. Clin Microbiol Rev 16:658–672. https://doi.org/10.1128/CMR.16.4.658-672.2003
Cheon S, Lee KW, Kim KE, Park JK, Park S, Kim CH et al (2011) Heat-killed Lactobacillus acidophilus La205 enhances NK cell cytotoxicity through increased granule exocytosis. Immunol Lett 136:171–176. https://doi.org/10.1016/j.imlet.2011.01.007
Lee NK, Son SH, Jeon EB, Jung GH, Lee JY, Paik HD (2015) The prophylactic effect of probiotic Bacillus polyfermenticus KU3 against cancer cells. J Funct Foods 14:513–518. https://doi.org/10.1016/j.jff.2015.02.019
Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A et al (2019) What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 7:14. https://doi.org/10.3390/microorganisms7010014
Lee EJ, Jung SR, Lee SY, Lee NK, Paik HD, Lim SI (2018) Lactobacillus plantarum strain Ln4 attenuates diet-induced obesity, insulin resistance, and changes in hepatic mRNA levels associated with glucose and lipid metabolism. Nutrients 10:643. https://doi.org/10.3390/nu10050643
Won SM, Chen S, Park KW, Yoon JH (2020) Isolation of lactic acid bacteria from kimchi and screening of Lactobacillus sakei ADM14 with anti-adipogenic effect and potential probiotic properties. LWT-Food Sci Technol 126:109296. https://doi.org/10.1016/j.lwt.2020.109296
Son SH, Jeon HL, Jeon EB, Lee NK, Park YS, Paik HD (2017) Potential probiotic Lactobacillus plantarum Ln4 from kimchi: evaluation of β-galactosidase and antioxidant activities. LWT-Food Sci Technol 85:181–186. https://doi.org/10.1016/j.lwt.2017.07.018
Lee NK, Han KJ, Son SH, Eom SJ, Lee SK, Paik HD (2015) Multifunctional effect of probiotic Lactococcus lactis KC24 isolated from kimchi. LWT-Food Sci Technol 64:1036–1041. https://doi.org/10.1016/j.lwt.2015.07.019
CLSI (2012) Performance standards for antimicrobial susceptibility testing; twenty-second informational supplement. Clinical and Laboratory Standards Institute 32:44–49
Park SY, Cho SA, Lee MK, Lim SD (2015) Effect of Lactobacillus plantarum FH185 on the reduction of adipocyte size and gut microbial changes in mice with diet-induced obesity. Food Sci Anim Resour 35:171–178. https://doi.org/10.5851/kosfa.2015.35.2.171
Jang HJ, Lee NK, Paik HD (2019) Probiotic characterization of Lactobacillus brevis KU15153 showing antimicrobial and antioxidant effect isolated from kimchi. Food Sci Biotechnol 28:1521–1528. https://doi.org/10.1007/s10068-019-00576-x
Vasiljevic T, Jelen P (2001) Production of β-galactosidase for lactose hydrolysis in milk and dairy products using thermophilic lactic acid bacteria. Innov Food Sci Emerg Technol 2:75–85
Dabek M, McCrae SI, Stevens VJ, Duncan SH, Louis P (2008) Distribution of β-glucosidase and β-glucuronidase activity and of β-glucuronidase gene gus in human colonic bacteria. FEMS Microbiol Ecol 66:487–495. https://doi.org/10.1111/j.1574-6941.2008.00520.x
Coppola R, Succi M, Tremonte P, Reale A, Salzano G, Sorrentino E (2005) Antibiotic susceptibility of Lactobacillus rhamnosus strains isolated from parmigiano reggiano cheese. Lait 85:193–204. https://doi.org/10.1051/lait:2005007
Álvarez-Cisneros YM, Ponce-Alquicira E (2018) Antibiotic resistance in lactic acid bacteria. In: Kumar Y (ed) Antimicrobial resistance: a global threat. IntechOpen, London, pp 53–73
Monteagudo-Mera A, Robert A, Rastall RA, Gibson GR, Charalampopoulos D, Chatzifragkou A (2019) Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl Microbiol Biotechnol 103:6463–6472. https://doi.org/10.1007/s00253-019-09978-7
Song MW, Jang HJ, Kim KT, Paik HD (2019) Probiotic and antioxidant properties of novel Lactobacillus brevis KCCM 12203P isolated from kimchi and evaluation of immune-stimulating activities of its heat-killed cells in RAW 264.7 cells. J Microbiol Biotechnol 29:1894–1903
Zhang J, Zhang X, Zhang L, Zhao Y, Niu C, Yang Z et al (2014) Potential probiotic characterization of Lactobacillus plantarum strains isolated from inner mongolia “Hurood” cheese. J Microbiol Biotechnol 24:225–235. https://doi.org/10.4014/jmb.1308.08075
Jeon HL, Lee NK, Yang SJ, Kim WS, Paik HD (2017) Probiotic characterization of Bacillus subtilis P223 isolated from kimchi. Food Sci Biotechnol 26:1641–1648. https://doi.org/10.1007/s10068-017-0148-5
Park SY, Cho SA, Kim SH, Lim SD (2014) Physiological characteristics and anti-obesity effect of Lactobacillus plantarum Q180 isolated from feces. Food Sci Anim Resour 34:647–655. https://doi.org/10.5851/kosfa.2014.34.5.647
Park JE, Oh SH, Cha YS (2014) Lactobacillus brevis OPK-3 isolated from kimchi inhibits adipogenesis and exerts anti-inflammation in 3T3-L1 adipocyte. J Sci Food Agric 94:2514–2520. https://doi.org/10.1002/jsfa.6588
Rosen E, Eguchi J, Xu Z (2009) Transcriptional targets in adipocyte biology. Expert Opin Ther Targets 13:975–986. https://doi.org/10.1517/14728220903039706
Moon YJ, Soh JR, Yu JJ, Sohn HS, Cha YS, Oh SH (2012) Intracellular lipid accumulation inhibitory effect of Weissella koreensis OK1-6 isolated from Kimchi on differentiating adipocyte. J Appl Microbiol 113:652–658
Park DY, Ahn YT, Huh CS, Jeon SM, Choi MS (2011) The inhibitory effect of Lactobacillus plantarum KY1032 cell extract on the adipogenesis of 3T3-L1 cells. J Med Food 14:670–675. https://doi.org/10.1089/jmf.2010.1355
Guha D, Mukherjee R, Aich P (2021) Effects of two potential probiotic Lactobacillus bacteria on adipogenesis in vitro. Life Sci 278:119538. https://doi.org/10.1016/j.fs.2021.119538
Author information
Authors and Affiliations
Contributions
Kyoung Jun Han: investigation, methodology, writing—original draft, validation. Na-Kyoung Lee: conceptualization, investigation, methodology, validation, writing—review and editing. Hyung-Seok Yu: investigation, methodology. Hoon Park: writing—review and editing, validation. Hyun-Dong Paik: conceptualization, supervision, writing—review and editing, validation.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Han, K.J., Lee, NK., Yu, HS. et al. Anti-adipogenic Effects of the Probiotic Lactiplantibacillus plantarum KU15117 on 3T3-L1 Adipocytes. Probiotics & Antimicro. Prot. 14, 501–509 (2022). https://doi.org/10.1007/s12602-021-09818-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-021-09818-z