Abstract
In this study, a bacterial strain COFCAU_P1, isolated from the digestive tract of a freshwater teleost rohu (Labeo rohita), was identified as Bacillus amyloliquefaciens using 16S rRNA gene sequence analysis combined with amplification of species-specific BamHI and barnase genes. The probiotic potential of the strain was evaluated using an array of in vitro tests along with safety and genetic analyses. The isolate showed potent antimicrobial response against several fish pathogenic bacteria, survived a wide pH range (2–9), and was resistant up to 10% bile salt concentration. With regard to the in vitro adhesion properties, the strain showed significantly high in vitro adhesion to mucus, auto and co-aggregation capacity, and cell surface hydrophobicity. The strain was non-haemolytic, able to produce extracellular enzymes, viz., proteinase, amylase, lipase, and cellulase, and showed significant free radical scavenging activity. A challenge study in rohu revealed the strain COFCAU_P1 as non-pathogenic. The presence of putative probiotic marker genes including 2, 3-bisphosphoglycerate-independent phosphoglycerate mutase, arginine/ornithine antiporter ArcD, choloylglycine hydrolase, LuxS, and E1 β-subunit of the pyruvate dehydrogenase complex was confirmed by PCR, suggesting the molecular basis of the probiotic-specific functional attributes of the isolate. In conclusion, the in vitro and genetic approaches enabled the identification of a potential probiotic from autochthonous source with a potential of its utilization in the aquaculture industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aquaculture has emerged as one of the most promising food production sectors throughout the world, owing to its contribution in providing premium quality animal protein. With the ever-increasing world population, the demand for fish and fishery products has been growing continuously. To meet this increasing demand, there has been a transition from the extensive and semi-intensive to intensive aquaculture practices. However, intensive culture practices are associated with certain drawbacks, most importantly, the vulnerability of fishes to environmental stresses and numerous pathogenic organisms. As a consequence, aquaculture farms are experiencing frequent outbreaks of diseases with varying aetiology which has now been considered as a major stumbling block in the growth of the fish farming industry [1, 2]. To avoid or minimize the loss in aquaculture due to disease, use of the chemotherapeutics, antibiotics, and vaccines has become a customary practice. However, the indiscriminate use of these substances is plagued with negative consequences, and now the situation lies where the chemotherapeutics are at risk of running out of efficiency. As an answer to this very concern, the probiotics have established their significance as an effective and sustainable biocontrol strategy in global aquaculture [3].
Probiotic has achieved significant scientific and commercial attention as a healthy alternative to chemotherapeutics in aquaculture. Usually, probiotics uphold a close relationship with the host and impart their effect on the host for better physiological and immunological status [4, 5]. Potential probiotics required to possess some inherent attributes, including the natural availability, non-pathogenicity, acceptance from the host, capability to colonize, and proliferation at different target sites of the host [6, 7]. The selection of probiotic strain is crucial, and lacking desired criteria may lead to adverse side effects to either the host organism or the aquatic environment. Therefore, prior to the field trial of any probiotic, screening for the perfect beneficial strain should go through the vigorous systematic assessment by different approaches [8, 9]. It has been reported that probiotic strain from the same host and origin (autochthonous) is more likely to be much effective as probiotic [6]. The gastrointestinal tract of fish offers a conducive environment for the beneficial endosymbiotic bacteria to colonize and proliferate [10, 11]. Therefore, it is highly advisable to select gut endosymbionts rather than from other sources. The isolated gut symbiotic bacteria must possess ideal probiotic characteristics such as production of extracellular enzymes, tolerance to low pH and high bile salt concentration, production of antimicrobial compounds, and high adherence capacity to different mucosal and hydrophobic surfaces, and it should not induce any pathological condition on the host [12,13,14,15].
Additionally, genetic assessment by employing molecular markers for probiotic properties can facilitate rapid and proper identification of potential probiotics [8]. Molecular screening of the putative probiotic marker genes has strengthened the classical phenotypic microbiological typing. Several genes related to the probiotic associated properties including acid/bile tolerance, adhesion, and quorum sensing have been identified using genetic studies [8]. Thus, the present study was undertaken to screen and characterize potential probiotic bacterial species from the gastrointestinal tract of a freshwater teleost Labeo rohita using an array of in vitro and genetic approaches.
Materials and Methods
Isolation of Gut Bacteria
Fingerlings of healthy L. rohita (12.6 ± 1.54 g, 10.4 ± 1.72 cm) were collected from local aquaculture farms. The fish were starved for 48 h; digestive tracts were dissected out and pooled in aseptic condition following the methods described by Mandal and Ghosh [16], and Mukherjee and Ghosh [17] with some modification. Briefly, the homogenate of the gut was serially diluted with 0.9% normal saline solution (1:10; w/v) and kept for some time to settle down the debris. The homogenized samples were serially diluted up to 10–6 and spread over nutrient agar (NA) (HiMedia, Mumbai, India) and incubated at 30 °C for 48 h. Morphologically different bacterial colonies were picked for further analysis. In total, 67 intestinal bacteria were screened initially by the agar-overlay method to check for inhibitory activity against Aeromonas hydrophila ATCC 7966 following the method described by Spelhaug and Harlander [18]. Briefly, the isolated bacteria were allowed to grow for 48 h and subsequently killed by chloroform vapour. The killed colonies were overlaid with 5 mL of 0.8% agar, which was seeded with pathogenic A. hydrophila ATCC 7966 and reincubated at 30 °C for the next 24 h. Clear zone over the NA plate around the colonies indicated the potential beneficial properties of the isolates. Based on the results of the agar-overlay method, strain COFCAU_P1 was selected for further characterization.
Antagonism Assay
The antagonism by the strain COFCAU_P1 was analysed firstly by cross-streak technique (to determine the inhibitory activity) [19] followed by parallel streaking method (to determine the severity of inhibition) [20] against fourteen pathogenic indicator bacteria comprising 7 reference strains (ATCC) and 7 field strains isolated and identified by our group (Table 1).
In the cross-streak method, a fresh culture of the strain was streaked (width 4 mm) across the diameter of the NA plate and incubated for 24 h at 30 °C. Subsequently, the pathogenic indicator bacteria were inoculated across/ perpendicular to the strain apart by 2–3 mm, and the plates were reincubated for another 24 h at 30 °C. The inhibitory activity was observed by inhibition of growth of indicator bacteria, and reading was recorded in mm.
In parallel streak method, the strain was streaked (width 4 mm) across the NA plate in two separate lines apart by 3 cm and incubated for 24 h at 30 °C. After 24 h, indicator strain was streaked (width 4 mm) in the centre of producer strain followed by reincubation of plates for another 24 h at 30 °C. The severity of inhibition was rated as “###” for complete inhibition, “##” for moderate inhibition, and “#” for low inhibition.
Identification of the Strain
The strain COFCAU_P1 was identified by polymerase chain reaction (PCR) using 16S rRNA universal primers, 27F (5′ AGAGTTTGATCCTGGCTCAG 3′) and 1492R (5′ GGTTACCTTGTTACGACTT 3′) [21]. Genomic DNA of bacteria was isolated using Insta™ DNA Kit (Himedia) as per manufactures instruction and quantified using a bio-spectrophotometer (Eppendorf, Hamburg, Germany). The PCR reaction mixture was composed of 1 μL template (100 ng µl−1), 1 μL of each forward and reverse primer (10 pmol µl−1), 13 μL of Taq 2X Master Mix, and 10 μL of nuclease free water. The PCR condition was as follows: initial denaturation for 10 min at 94 °C; 35 cycles of denaturation for 1 min at 94 °C, annealing for 45 s at 55 °C and extension at 72 °C for 1 min; and final extension at 72 °C for 7 min. Purified PCR products were sequenced (Applied Biosystems, Beverly, MA, USA), and a phylogenetic tree was constructed by the neighbour-joining method using MEGA 6 software. Phylogenetic neighbours were selected based on the results of BLAST (basic local alignment search tool). Sequences which showed maximum similarity (≥ 99%) with zero E-value were selected for the evolutionary tree construction [22, 23]. The 16S rRNA sequence analysis identified the strain as Bacillus amyloliquefaciens. The presence of two signature genes of B. amyloliquefaciens were also screened for the additional confirmation of the strain namely, BamHI (F: 5′ TCATTGAACGGTGGGCAGAA 3′; R: 5′ GCCAGCAACCTCAAAAACC 3′) and barnase (F: 5′ CACACAAGCCGCTCAAAACA 3′; R: 5′ TAATCCGCAACCCCGTCAAA 3′). The PCR condition was as follows: initial denaturation at 94 °C for 10 min; 35 cycles of denaturation for 1 min at 94 °C, annealing for 45 s at 47 °C (for barnase) and 50 °C (for BamHI), and extension for 45 s at 72 °C; and final extension at 72 °C for 5 min.
pH and Bile Tolerance
The pH and bile tolerance were measured using the method described by Hoque [24] and Nikoskelainen et al. [25], respectively with some modification. For pH tolerance, an overnight culture of the strain COFCAU_P1 was adjusted to an OD of 0.25 at 600 nm to achieve a cell concentration of 107 CFU mL−1. From there, 0.1 mL broth was taken, and inoculated in nutrient broth with variable pH (2–9). The pH was adjusted with 1 N HCL and 1 N NaOH. The broth cultures were further incubated at 30 °C for the next 24 h, and growth was monitored by observing the change in optical density (OD) in a spectrophotometer (Thermo Scientific, Waltham, MA, USA) at a wavelength of 600 nm. For the determination of bile tolerance, an overnight culture of the strain was adjusted to an OD of 0.25 at 600 nm to achieve a cell concentration of 107 CFU mL−1. From there, 1 mL broth was taken, centrifuged at 10,000 g for 10 min at 4 °C, and washed with phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4, and 2 mM KH2PO4; pH 7.4). Bacterial pellet was uniformly dissolved in PBS containing bile salts (HiMedia) with variable concentration (0% (control), 2.5%, 5% and 10%), incubated at 30 °C for 1.5 h, and absorbance was recorded using spectrophotometer (Thermo Scientific) at 600 nm.
Haemolytic Assay
The assay was carried following the method of Joseph et al. [26]. Briefly, the strain was streaked on a plate containing blood agar base (HiMedia) enriched with 5% chicken blood and incubated at 30 °C for 24 h. Haemolytic zones were observed and subsequently classified as α (incomplete/partial haemolysis), β (complete haemolysis), or γ (non-haemolytic) haemolysis.
Extracellular Enzyme Production
Qualitative assessment of the extracellular enzyme-producing capabilities of the strain was done using the previously described protocols with slight modifications. Briefly, the isolated strain was inoculated in gelatine-peptone agar (HiMedia) and incubated at 30 °C for 48 h followed by flooding with 15% HgCl2; the appearance of clear zone around the colony indicated proteinase production [27]. Bacteria-inoculated starch agar (HiMedia) plates were incubated for 24 h at 30 °C and flooded with Lugol’s iodine solution; the appearance of whitish-yellow discolouration over the media indicated positive amylase test [27]. Appearance of whitish opaque colouration around the bacterial colony over tributyrin agar (HiMedia) showed lipolytic enzyme-producing ability of the strain. Bacteria-inoculated carboxymethylcellulose agar (HiMedia) was incubated for 48 h at 30 °C and then flooded with gram’s iodine solution; whitish appearance on the periphery of colony indicated cellulase production by the strain [28].
Bacterial Cell Surface Hydrophobicity
The cell surface hydrophobicity was examined, according to Lee et al. [29], with some modification. This method was based on adhesion of cells to the organic solvents. Briefly, the bacterial strain was grown for 48 h at 30 °C in nutrient broth, centrifuged at 10,000 g for 3 min, followed by washing with PBS twice. Pellet was resuspended in PBS (pH 7.4), and OD at 600 nm was measured (ODa). The bacterial suspension was mixed with equal volume of each solvent namely, xylene and chloroform separately, and vortexed for 5 min. The mixture was allowed to separate into two phases for 30 min, and the OD of the aqueous phase was measured at 600 nm (ODb). A non-probiotic bacterium, A. hydrophila ATCC 7966, was used as a control. Hydrophobicity was calculated using the following formula:
Growth on Mucus
The adherence capability of the strain and a non-probiotic bacterium (A. hydrophila ATCC 7966) on the skin mucus was determined using the method of Midhun et al. [30] with modification. Fish were anaesthetized using clove oil (50 μL L−1) [31] and kept separately for 10 min in sterile beakers containing 5 mL of 100 mM ammonium bicarbonate buffer (pH 7.8). During removal of the fish from the beakers, fish were rinsed with 5 mL of the same buffer. The buffer containing the mucus was then transferred into sterile 15 mL tubes and centrifuged at 12,000 g for 15 min at 4 °C. After centrifugation, the supernatant was sterilized through a 0.22 μm syringe filter (HiMedia). Filter-sterilized mucus samples were inoculated with the bacteria (106 CFU mL−1), incubated for 24 h at 30 °C, and the OD was measured at 600 nm wavelength.
Auto-aggregation and Co-aggregation
The auto-aggregation and co-aggregation capacity of the strain COFCAU_P1 was determined using the method of Del Re et al. [32] and Handley et al. [33], respectively. In the auto-aggregation assay, 24-h-old bacterial broth was centrifuged for 3 min at 10,000 g and suspended in PBS (pH 7.4) to achieve an OD of 0.5 at 600 nm. The bacterial suspension (2 mL) was again centrifuged, and the cells resuspended in their culture supernatant. The culture was incubated for 2 h at 30 °C, 1 mL of the upper suspension was withdrawn, and the OD measured at 600 nm. Auto-aggregation percentage was expressed using the following formula:
In the co-aggregation test, the capacity of the co-aggregation of the strain was evaluated using the pathogenic A. hydrophila ATCC 7966. Briefly, 24-h-old bacterial broths were centrifuged (10,000 g for 3 min) and washed twice with PBS (pH 7.4). The washed pellets were suspended in PBS and OD adjusted to 1.0 at 660 nm. The test isolate (0.5 mL) and A. hydrophila ATCC 7966 (0.5 mL) were mixed and incubated for 24 h without agitation followed by measurement of OD at 660 nm. The co-aggregation capacity of isolate was calculated as
Antioxidant Activity
For the determination of radical scavenging activity, initial sample preparation was done following the method of Xing et al. [34]. Briefly, 24-h-old bacterial broth culture was centrifuged at 8000 g for 10 min at 4 °C, and the cell-free supernatant was collected in a separate tube for scavenging assays. Ascorbic acid (10%) was used as a standard antioxidant agent in all the assays.
DPPH (1, 1-Diphenyl-2-picrylhydrazyl) activity was determined following the method described by Brand-Williams et al. [35]. Briefly, 100 μL of the supernatant was mixed with 3 mL of absolute ethanol and 2 mL of 0.06 mM DPPH (HiMedia) solution. The supernatant was incubated for 30 min, and absorbance (A) was recorded at 517 nm. The mixture of ethanol and sample served as a blank, whereas DPPH solution without sample served as control. The scavenging percentage was determined using the following formula:
The ability of the supernatant to scavenge H2O2 radicals was determined according to the method of Ruch et al. [36]. Briefly, 100 μL of the supernatant was mixed with 600 μL of 40 mM H2O2 (HiMedia) dissolved in PBS (pH 7.4), followed by 10 min incubation and measurement of absorbance (A) at 230 nm against a blank (PBS without H2O2) and control (H2O2 solution without sample). The percentage scavenging activity was calculated using the equation:
For measuring alkaline DMSO scavenging capacity [36], 100 μL of the supernatant was mixed with 1 mL of DMSO (HiMedia) and 200 μL of 20 mM nitroblue tetrazolium (NBT) (HiMedia), followed by 10 min incubation and measurement of absorbance (A) at 560 nm against a control (DMSO and NBT mixture). The percentage scavenging activity was determined as
Ferric reducing antioxidant potential (FRAP) was determined using the method described by Han et al. [37] with modification. Briefly, 3 mL of freshly prepared FRAP solution [300 mM acetate buffer (pH 3.6), 10 mM tripyridyltriazine (TPTZ) (HiMedia) in 40 mM HCl, and 20 mM FeCl3. 6H2O in the proportion of 10:1:1] was mixed with 100 μL of the supernatant and 300 μL of distilled water. The mixture was placed in dark for 5 min, and absorbance (A) was measured at 593 nm against a control (FRAP solution and water). The percentage ferric reducing potential was determined as
For determination of ABTS [2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)] scavenging activity [37], 100 μL of the supernatant was mixed with 3 mL ABTS (HiMedia) solution (20 mM ABTS in sodium acetate buffer; pH 4.5) and incubated for 6 min, followed by measurement of absorbance at 734 nm against a control (ABTS solution without supernatant). The percentage scavenging activity was determined using the following formula:
Challenge Study
The safety of the isolated strain was evaluated using a challenge study [38]. The fish in triplicate tanks (6 fish per tank) were administered intraperitoneally with 0.1 mL of fresh probiotic bacterial suspension (1010 CFU mL−1). Control group fish were injected with an equal volume of sterile PBS (pH 7.2). Fish were kept under observation for 2 weeks for possible mortalities or clinical signs.
Genetic Analysis
Genetic analysis was carried out for further confirmation of the probiotic attributes of the isolated strain. Five marker genes, related to acid/ bile tolerance, and adhesion were selected based on previously published literatures [8, 39]. Complete genomic sequences of B. amyloliquefaciens, available in the NCBI (national centre for biotechnology information) database, were screened for the selected genes. The primers for the genes namely, 2, 3-bisphosphoglycerate-independent phosphoglycerate mutase, arginine/ornithine antiporter ArcD, choloylglycine hydrolase, LuxS, and E1 β-subunit of the pyruvate dehydrogenase complex were designed with the help of NCBI primer designing tool (Table 2). The PCR reaction mixture was composed of 13 μL of Taq Master Mix (2X), 1 μL of each forward and reverse primer (10 pmol μL−1), 1 μL of the template (100 ng µL−1), and 10 μL of nuclease-free water. The PCR condition was as follows: 1 cycle at 94 °C for 10 min; 35 cycles of 1 min at 94 °C, 45 s of annealing temperature for each primer (Table 2) and then 45 s at 72 °C; followed by 1 cycle of elongation for 5 min at 72 °C. The PCR amplicon of a particular gene was separated on agarose gel (1.5%) and observed for specific bands in a gel documentation (Thermo Scientific). The amplified products were purified and sequenced (Applied Biosystems). The homology of the sequences was checked with the help of BLAST, and sequences were submitted to the NCBI database.
Statistical Analysis
The assays were performed in triplicates, and the results were analysed using SPSS-16.0 for windows software (SPSS Inc., Chicago, IL, USA). Results are represented as a mean ± standard error. The comparison of mean values was determined using one-way ANOVA and LSD post hoc test. A probability level of 0.05 was used to find out the significance in all the cases.
Results
Antagonistic Activity
The isolated strain COFCAU_P1 inhibited the growth of thirteen opportunistic pathogenic bacteria with different intensity, out of the fourteen bacteria tested (Table 1). The mean zone of inhibition ranged from 5.34 to 15.34 mm.
Identification of the Strain
The strain COFCAU_P1 was identified as B. amyloliquefaciens. The 16S rRNA gene sequence of the strain was submitted to the NCBI database and GenBank accession number obtained; thus, the strain was designated as Bacillus amyloliquefaciens COFCAU_P1 (MN880150). The PCR amplicons of the genes BamHI and barnase were separated on agarose gel with the approximate product length of 211 and 395 base pairs, respectively. The presence of these signature genes further confirmed the strain as B. amyloliquefaciens. Phylogenetic analysis (Fig. 1) revealed that the strain was closely related to other Bacillus spp.
pH Tolerance and Bile Tolerance Assay
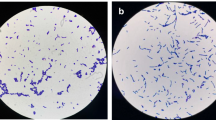
The isolated strain exhibited tolerance against highly acidic to alkaline pH (2 to 9). However, the maximum growth was observed in the pH range of 7–8 (Fig. 2a). In the bile tolerance test, the strain showed continuous and significant (P < 0.05) decline in the proliferation with the increasing concentration of bile (Fig. 2b).
Haemolytic Activity
The strain COFCAU_P1 was non-haemolytic (γ-haemolysis), as no haemolytic zone was observed on the blood agar.
Extracellular Enzyme Production
Result demonstrated that the strain was positive for the production of several extracellular enzymes, including proteinase, amylase, lipase, and cellulase.
Bacterial Cell Surface Hydrophobicity
The cell surface hydrophobicity of the strain COFCAU_P1 to both the surfactants chloroform (66.7%) and xylene (76.3%) was significantly (P < 0.05) higher compared to the non-probiotic control (Fig. 3a).
Growth on Mucus
In vitro adhesion of the strain, COFCAU_P1 to mucus was significantly (P < 0.05) higher compared to the control (Fig. 3b).
Auto-aggregation and Co-aggregation
Auto-aggregation capacity (65.1%) of the isolate COFCAU_P1 was significantly higher (P < 0.05) compared to the control (32.2%) (Fig. 3c). The co-aggregation percentage of the isolate with A. hydrophila ATCC 7966 was 43.94%.
Antioxidant Activity
The strain exhibited the potential to scavenge free radicals at variable levels (Fig. 4). The maximum and the minimum scavenging activity were observed in DPPH (63.2%) and H2O2 (34.53%), respectively.
Challenge Study
The strain was found to be non-pathogenic as no mortality or morbidity was observed in the challenged fish.
Genetic Assessment
The presence of the targeted probiotic associated marker genes was confirmed by PCR. The product size and other details are presented in Table 2. The BLAST analysis confirmed that the selected genes belonged to B. amyloliquefaciens. The gene sequences were submitted to the NCBI database, and GenBank accession numbers were obtained (Table 2).
Discussion
In vitro tests are useful for initial screening of the potential probiotics and understanding on the mechanism of probiotic effect [12]. Additionally, the molecular mechanisms that underpin probiotic attributes can be a rapid and novel way to screen and study probiotic microorganisms [8]. In this study, one antagonistic bacterium, isolated from the intestine of L. rohita, was assessed for its probiotic potential using in vitro and molecular approaches.
After the preliminary screening of 67 isolates by agar-overlay method, eight strains were selected, and further subjected to antagonism assay, by the cross and parallel streaking methods. The antagonism activity is considered as one of the major decisive factors for the initial selection of potential probiotic bacteria among the number of endosymbionts [6, 40]. The strain COFCAU_P1 showed potent antimicrobial response against several fish pathogenic bacteria, including some field strains available in our laboratory. Based on the results of the antagonism assay, strain COFCAU_P1 was selected as a potential probiotic for further characterization.
The isolated strain COFCAU_P1 was identified as B. amyloliquefaciens using the 16S rRNA gene sequence. Phylogenetic analysis of the 16S rRNA revealed that the strain was positioned in the same group as other B. amyloliquefaciens strains (JN411425, JN366747, and KM588313.1). The identification was further reinforced by confirming the presence of two B. amyloliquefaciens-related signature genes, BamHI and barnase. These two genes, responsible for the production of endonuclease (BamHI) and extracellular ribonuclease (barnase), are associated with B. amyloliquefaciens [41, 42].
Resistance to gastric (low pH) and high bile concentration in the intestine are important prerequisites for probiotic bacteria to survive and colonize the gut to exert beneficial effects [43, 44]. The intestinal pH of agastric L. rohita ranges from 6.8 to 7.1 in the intestinal bulb and decreases to 6.2–6.5 in the hind gut [45]. In the present study, the tested strain survived a wide pH range (2–9), and substantial growth was observed in the pH range of 6–8, indicating tolerance to intestinal acidic condition. In this study, the strain COFCAU_P1 showed tolerance up to 10% bile concentration, indicating the strain’s ability to survive in the intestinal milieu of the fish. The survival of probiotic candidates in the presence of bile salts can be explained by the activity of the bile salt hydrolase [46]. Tolerance to low pH and high bile concentration by different strains of B. amyloliquefaciens has also been reported from other studies [29, 47,48,49].
The adherence to the epithelial and mucosal layer is an important consideration for a potential probiotic as it ensures their ability to resist the fluctuations of the intestinal contents and to inhibit pathogenic bacteria by occupying available intestinal space [50,51,52]. Probiotic adhesion is a complex process involving contact between the bacterial cell membrane and composition of the adhering surface [53]. The strain COFCAU_P1 showed significantly higher in vitro adhesion to the mucus of L. rohita compared to a non-probiotic bacterium. Several mechanisms, including involvement of passive forces (e.g., electrostatic interaction, hydrophobic, steric forces) [54], lipoteichoic acids [55], and adhesion-promoting proteins [56] have been suggested to play significant role in adhesion.
Auto-aggregation is an indirect way of determining the adherence capacity of bacteria [57]. Bacteria responsible for aggregation form a precipitate and hence produce a clear upper solution compared to the non-aggregating strains which produce turbid suspension [53]. In the present study, the auto-aggregation capacity of COFCAU_P1 was significantly higher than the non-probiotic strain. High auto-aggregation capacity was also exhibited by B. amyloliquefaciens, in previous studies [49, 58]. Co-aggregation assay is used to determine the level of inter-bacterial adherence between the probiotic and pathogenic strain. Probiotic bacteria use this mechanism to exclude the harmful bacteria from colonization in the host [53, 59, 60]. The B. amyloliquefaciens of this study showed higher co-aggregation capability with A. hydrophila ATCC 7966 than co-aggregation of B. amyloliquefaciens with Salmonella and Listeria monocytogens, as reported in a previous study [58]. The co-aggregation percentages differ depending on the strain specificity and interaction between the probiotic and indicator bacteria [53, 57].
The cell surface hydrophobicity helps in assessing the adherence capacity of the bacteria to gut epithelial lining [51]. In the present study, the hydrophobicity of the selected isolate was significantly higher than the non-probiotic strain. However, the hydrophobicity percentage was lower than the observation from previous study with B. amyloliquefaciens [49]. The high level of hydrophobicity shown by the isolated strain might be due to the higher capacity of electron donation and acceptance and hence significant adhesion capacity [49, 61].
The absence of haemolytic action is one of the important safety prerequisites while selecting a potential probiotic strain. Haemolytic assay conducted in this study confirmed the strain as non-haemolytic (γ-haemolysis) and hence harmless. In a previous study, B. amyloliquefaciens was also found to be non-haemolytic [48]. Besides in vitro haemolytic activity, we also conducted biosafety evaluation of the strain, using an in vivo challenge model. The challenge study revealed the strain COFCAU_P1 as non-pathogenic and hence safe. The non-pathogenicity of B. amyloliquefaciens has also previously been reported in fishes [15, 38, 48].
It has been suggested that the production of extracellular enzymes is an important attribute of an ideal probiotic strain as these enzymes can positively contribute to the digestive process of biomolecules [14, 50]. In the present study, the secretion of extracellular enzymes viz. proteinase, amylase, lipase, and cellulase by the strain COFCAU_P1 was confirmed qualitatively. Therefore, it can be assumed that the strain may contribute towards better digestion of nutrients in the host, as an added beneficial effect.
There are reports which suggest that probiotics can reduce the unwanted oxidation of biomolecules [62, 63]. The antioxidant activity of probiotics is possible due to the production of several bacteriocin compounds [63, 64]. In this study, the COFCAU_P1 showed a significant level of scavenging activity compared to a non-probiotic control. Significant reduction in the free radical production by potential probiotics has also been reported from other studies as well [65, 66].
The advent of genomic approaches has facilitated the exploration of efficient and rapid means of screening and studying putative probiotics. Identifying molecular markers of important probiotic attributes can facilitate rapid screening of potential strains and can be an effective strategy in the follow-up analysis of candidate probiotic initially established from in vitro and/or in vivo screening with conventional approaches [8, 67]. In this study, genetic screening was used to determine the presence of five marker genes associated with functional attributes of the isolated strain. The present study detected the presence of 2, 3-bisphosphoglycerate-independent phosphoglycerate mutase gene which is considered to be involved in acid tolerance [68]. The presence of arginine/ornithine antiporter ArcD gene implies the strain’s capability to survive at low pH and high bile salt concentration of the gastric environment [39]. Amplification of choloylglycine hydrolase gene, commonly known as bile salt hydrolase (bsh), is known for providing tolerance to elevated bile concentration [39]. LuxS gene plays an important role in cell-to-cell communication (quorum sensing), which facilitates adhesion and simultaneously exclusion of the pathogens [69]. Additionally, this gene is also involved in imparting tolerance to acid and high bile salt concentration [8]. Finally, E1 β-subunit of the pyruvate dehydrogenase complex gene, which encodes fibronectin binding protein (fbp), was also confirmed in this strain. Fibronectin binding protein is an extracellular matrix glycoprotein responsible for adhesion to the extracellular matrix of epithelial cells [8, 70]. Thus, the results of some of the important in vitro tests were well correlated with the amplification of putative probiotic marker genes.
In conclusion, the autochthonous isolate B. amyloliquefaciens COFCAU_P1 exhibited potent in vitro beneficial properties. The presence of probiotic marker genes reinforced the beneficial attributes of the strain. The presence of these molecular markers could be correlated with some of the important in vitro beneficial properties, including the acid/bile tolerance and adhesion properties. Overall, the potential probiotic attributes observed in the strain hold great potential to be used in aquaculture. However, in vivo assessment and efficacy studies are required prior to field application of the strain.
Data Availability
The authors declare that all data generated or analysed during this study are included in this published article.
References
Austin B, Austin DA (1999) Bacterial fish pathogens- disease of farmed and wild fish. Chapter 13, Ellis Harwood Ltd., England, p 263–287. https://doi.org/10.1007/978-1-4020-6069-4
Bondad-Reantaso MG, Subasinghe RP, Arthur JR, Ogawa K, Chinabut S, Adlard R, Tan Z, Shariff M (2005) Disease and health management in Asian aquaculture. Vet Parasitol 132:249–272. https://doi.org/10.1016/j.vetpar.2005.07.005
Hai NV, Fotedar R, Buller N (2007) Selection of probiotics by various inhibition test methods for use in the culture of western king prawns, Penaeus latisulcatus (Kishinouye). Aquaculture 272:231–239. https://doi.org/10.1016/j.aquaculture.2007.07.223
Banerjee G, Ray AK (2017) Bacterial symbiosis in the fish gut and its role in health and metabolism. Symbiosis 72:1–11. https://doi.org/10.1007/s13199-016-0441-8
Elsabagh M, Mohamed R, Moustafa EM, Hamza A, Farrag F, Decamp O, Dawood MAO, Eltholth M (2018) Assessing the impact of Bacillus strains mixture probiotic on water quality, growth performance, blood profile and intestinal morphology of Nile tilapia, Oreochromis niloticus. Aquac Nutr 24:1–10. https://doi.org/10.1111/anu.12797
Verschuere L, Rombaut G, Sorgeloos P, Verstraete W (2000) Probiotic bacteria as biological control agents in aquaculture. Microbiol Mol Biol Rev 64:655–671. https://doi.org/10.1128/MMBR.64.4.655-671.2000
Kesarcodi-Watson A, Kaspar H, Lategan MJ, Gibson L (2008) Probiotics in aquaculture: the need, principles and mechanisms of action and screening processes. Aquaculture 274:1–14. https://doi.org/10.1016/j.aquaculture.2007.11.019
Papadimitriou K, Zoumpopoulou G, Foligne B, Alexandraki V, Kazou M, Pot B, Tsakalidou E (2015) Discovering probiotic microorganisms: in vitro, in vivo, genetic and omics approaches. Front Microbiol 6:58. https://doi.org/10.3389/fmicb.2015.00058
Mukherjee A, Dutta D, Banerjee S, Ringo E, Breines EM, Hareide E, Chandra G, Ghosh K (2017) Culturable autochthonous gut bacteria in rohu, Labeo rohita. In vitro growth inhibition against pathogenic Aeromonas spp., stability in gut, bio-safety and identification by 16S rRNA gene sequencing. Symbiosis 73:165–177. https://doi.org/10.1007/s13199-017-0474-7
Kar N, Ghosh K (2008) Enzyme producing bacteria in the gastrointestinal tracts of Labeo rohita (Hamilton) and Channa punctatus (Bloch). Turkish J Fish Aquat Sci 1:115–120
Ray AK, Roy T, Mondal S, Ringo E (2010) Identification of gut associated amylase, cellulase and protease-producing bacteria in three species of Indian major carps. Aquac Res 4:1462–1469. https://doi.org/10.1111/j.1365-2109.2009.02437.x
FAO/WHO (2001) Evaluation of health and nutritional properties of powder milk and live lactic acid bacteria. Report from FAO/WHO Expert Consultation, p 1–4
Balcazar JL, Vendrell D, de Blas I, Ruiz-Zarzuela I, Muzquiz JL, Girones O (2008) Characterization of probiotic properties of lactic acid bacteria isolated from intestinal microbiota of fish. Aquaculture 278:188–191. https://doi.org/10.1016/j.aquaculture.2008.03.014
Ray AK, Ghosh K, Ringo E (2012) Enzyme-producing bacteria isolated from fish gut: a review. Aquac Nutr 18:465–492. https://doi.org/10.1111/j.1365-2095.2012.00943.x
Nandi A, Dan SK, Banerjee G, Ghosh P, Ghosh K, Ringo E, Ray AK (2017) Probiotic potential of autochthonous bacteria isolated from the gastrointestinal tract of four freshwater teleosts. Probiotics Antimicrob Proteins 9:12–21. https://doi.org/10.1007/s12602-016-9228-8
Mandal S, Ghosh K (2013) Isolation of tannase-producing microbiota from the gastrointestinal tracts of some freshwater fish. J Appl Ichthyol 29:145–153. https://doi.org/10.1111/j.1439-0426.2012.02054.x
Mukherjee A, Ghosh K (2016) Antagonism against fish pathogens by cellular components and verification of probiotic properties in autochthonous bacteria isolated from the gut of an Indian major carp, Catla catla (Hamilton). Aquac Res 47:2243–2255. https://doi.org/10.1111/are.12676
Spelhaug SR, Harlander S (1989) Inhibition of food-borne bacterial pathogens by bacteriocins from Lactococcus lactis and Pediococcus pentosaceus. J Food Prot 52:856–862. https://doi.org/10.4315/0362-028X-52.12.856
Lemos ML, Toranzo AE, Barja JL (1985) Antibiotic activity of epiphytic bacteria isolated from intertidal seaweeds. Microb Ecol 11:149–163. https://doi.org/10.1007/BF02010487
Nakamura A, Takahashi KG, Mori K (1999) Vibrio static bacteria isolated from rearing seawater of oyster brood stock: potentiality as biocontrol agents for Vibriosis in oyster larvae. Fish Pathol 34:139–144. https://doi.org/10.3147/jsfp.34.139
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703. https://doi.org/10.1128/jb.173.2.697-703.1991
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791. https://doi.org/10.1111/j.1558-5646.1985.tb00420.x
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120. https://doi.org/10.1007/BF01731581
Hoque F (2015) Screening and characterisation of antagonistic Pseudomonas aeruginosa FARP72 as a potential probiotic agent. Indian J Fish 62:80–90
Nikoskelainen S, Salminen S, Bylund G, Ouwehand A (2001) Characterization of the properties of human and dairy-derived probiotics for prevention of infectious diseases in fish. Appl Environ Microbiol 67:2430–2435. https://doi.org/10.1128/AEM.67.6.2430-2435.2001
Joseph SW, Colwell RR, Kaper JB (1982) Vibrio parahaemolyticus and related hallophilic vibrios. Crit Rev Microbiol 10:73–124. https://doi.org/10.3109/10408418209113506
Jacob MB, Gerstein MJ (1960). Handbook of Microbiology. D. Van Nostrand Co. Inc., New York, p 139–202. /https://doi.org/10.1002/jps.2600500123
Teather RM, Wood PJ (1982) Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl Environ Microbiol 43:777–780. https://doi.org/10.1128/AEM.43.4.777-780.1982
Lee S, Lee J, Jin YI, Jeong JC, Chang YH, Lee Y, Jeong Y, Kim M (2017) Probiotic characteristics of Bacillus strains isolated from Korean traditional soy sauce. LWT-Food Sci Technol 79:518–524. https://doi.org/10.1016/j.lwt.2016.08.040
Midhun SJ, Neethu S, Vysakh A, Sunil MA, Radhakrishnan EK, Jyothis M (2017) Antibacterial activity of autochthonous bacteria isolated from Anabas testudineus (Bloch, 1792) and it’s in vitro probiotic characterization. Microb Pathog 113:312–320. https://doi.org/10.1016/j.micpath.2017.10.058
Devi AA, Kamilya D (2019) Efficacy and effects of clove oil and MS-222 on the immune-biochemical responses of juvenile rohu Labeo rohita. Aquac Res 50:957–963. https://doi.org/10.1111/are.13980
Del Re B, Sgorbati B, Miglioli M, Palenzona D (2000) Adhesion, autoaggregation and hydrophobicity of 13 strains of Bifidobacterium longum. Lett Appl Microbiol 31:438–442. https://doi.org/10.1046/j.1365-2672.2000.00845.x
Handley PS, Harty DW, Wyatt JE, Brown CR, Doran JP, Gibbs AC (1987) A comparison of the adhesion, coaggregation and cell-surface hydrophobicity properties of fibrillar and fimbriate strains of Streptococcus salivarius. J Gen Microbiol 133:3207–3217. https://doi.org/10.1099/00221287-133-11-3207
Xing J, Wang G, Zhang Q, Liu X, Gu Z, Zhang H, Chen YQ, Chen W (2015) Determining antioxidant activities of Lactobacilli cell-free supernatants by cellular antioxidant assay: a comparison with traditional methods. PLoS One 10:e0119058. https://doi.org/10.1371/journal.pone.0119058
Brand W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. Lebenson Wiss Technol 28:25–30. https://doi.org/10.1016/S0023-6438(95)80008-5
Ruch RJ, Cheng SJ, Klaunig JE (1989) Prevention of cytotoxicity and inhibition of intracellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 10:1003–1008. https://doi.org/10.1093/carcin/10.6.1003
Han Q, Kong B, Chen Q, Sun F, Zhang H (2017) In vitro comparison of probiotic properties of lactic acid bacteria isolated from Harbin dry sausages and selected probiotics. J Funct Foods 32:391–400. https://doi.org/10.1016/j.jff.2017.03.020
Das A, Nakhro K, Chowdhury S, Kamilya K (2013) Effects of potential probiotic Bacillus amyloliquifaciens FPTB16 on systemic and cutaneous mucosal immune responses and disease resistance of catla (Catla catla). Fish Shellfish Immunol 35:1547–1553. https://doi.org/10.1016/j.fsi.2013.08.022
Kapsea NG, Engineera AS, Gowdamana V, Waghb S, Dhakephalkara PK (2019) Functional annotation of the genome unravels probiotic potential of Bacillus coagulans HS243. Genomics 111:921–929. https://doi.org/10.1016/j.ygeno.2018.05.022
Vine NG, Leukes WD, Kaiser H (2006) Probiotics in marine larviculture. FEMS Microbiol Rev 30:404–427. https://doi.org/10.1111/j.1574-6976.2006.00017.x
Hartley RW, Barker EA (1972) Amino-acid sequence of extracellular ribonuclease (barnase) of Bacillus amyloliquefaciens. Nature New Biol 235:15–16. https://doi.org/10.1038/newbio235015a0
Roberts R, Wilson G, Young F (1977) Recognition sequence of specific endonuclease BamHI from Bacillus amyloliquefaciens H. Nature 265:82–84. https://doi.org/10.1038/265082a0
Cartman ST, La Ragione RM, Woodward MJ (2008) Bacillus subtilis spores germinate in the chicken gastrointestinal tract. Appl Environ Microb 74:5254–5258. https://doi.org/10.1128/AEM.00580-08
Ramesh D, Vinothkanna A, Rai AK, Vignesh VS (2015) Isolation of potential probiotic Bacillus spp. and assessment of their subcellular components to induce immune responses in Labeo rohita against Aeromonas hydrophila. Fish Shellfish Immunol 45:268–276. https://doi.org/10.1016/j.fsi.2015.04.018
FAO (2020). Aquaculture Feed and Fertilizer Resources Information System. Retrieved from http://www.fao.org/fishery/affris/species-profiles/roho-labeo/rohu-home/en/. Accessed Feb 2020
De Smet I, Hoorde LV, Woestyne MV, Christiaens H, Verstraete W (1995) Significance of bile salt hydrolytic activities of lactobacilli. J Appl Bacteriol 79:292–301. https://doi.org/10.1111/j.1365-2672.1995.tb03140.x
Giri SS, Sen SS, Sukumaran V (2012) Effects of dietary supplementation of potential probiotic Pseudomonas aeruginosa VSG-2 on the innate immunity and disease resistance of tropical freshwater fish, Labeo rohita. Fish Shellfish Immunol 32:1135–1140. https://doi.org/10.1016/j.fsi.2012.03.019
Kavitha M, Raja M, Perumal P (2018) Evaluation of probiotic potential of Bacillus spp. isolated from the digestive tract of freshwater fish Labeo calbasu (Hamilton, 1822). Aquac Rep 11:59–69. https://doi.org/10.1016/j.aqrep.2018.07.001
Kuebutornye FK, Lu Y, Abarike ED, Wang Z, Li Y, Sakyi ME (2019) In vitro assessment of the probiotic characteristics of three Bacillus species from the gut of Nile tilapia, Oreochromis niloticus. Probiotics Antimicrob Proteins 17:1–13. https://doi.org/10.1007/s12602-019-09562-5
Tuomola E, Crittenden R, Playne M, Isolauri E, Salminen S (2001) Quality assurance criteria for probiotic bacteria. J Clin Nutr 73:393S-398S. https://doi.org/10.1093/ajcn/73.2.393s
Kos B, Suskovic J, Vukovic S, Simpraga M, Frece J, Matosic S (2003) Adhesion and aggregation ability of probiotic strain Lactobacillus acidophilus M92. J Appl Microbiol 94:981–987. https://doi.org/10.1046/j.1365-2672.2003.01915.x
Guo XH, Kim JM, Nam HM, Park SY, Kim JM (2010) Screening lactic acid bacteria from swine origins for multistrain probiotics based on in vitro functional properties. Anaerobe 16:321–326. https://doi.org/10.1016/j.anaerobe.2010.03.006
Balakrishna A, Kumar NA (2012) Preliminary studies on siderophore production and probiotic effect of bacteria associated with the Guppy, Poecilia reticulata Peters, 1859. Asian Fish Sci 25:193–205. https://doi.org/10.33997/j.afs.2012.25.2.008
Servin AL, Coconnier MH (2003) Adhesion of probiotic strains to the intestinal mucosa and interaction with pathogens. Best Pract Res Clin Gastroenterol 17:741–754. https://doi.org/10.1016/S1521-6918(03)00052-0
Granato D, Perotti F, Masserey I, Rouvet M, Golliard M, Servin A, Brassart D (1999) Cell surface-associated lipoteichoic acid acts as an adhesion factor for attachment of Lactobacillus johnsonii LaI to human enterocyte-like Caco-2 cells. Appl Environ Microbiol 65:1071–1077. https://doi.org/10.1128/AEM.65.3.1071-1077.1999
Rojas M, Ascencio F, Conway PL (2002) Purification and characterization of a surface protein from Lactobacillus fermentum 104R that binds to porcine small intestinal mucus and gastric mucin. Appl Environ Microbiol 68:2330–2336. https://doi.org/10.1128/aem.68.5.2330-2336.2002
Collado MC, Meriluoto J, Salminen S (2008) Adhesion and aggregation properties of probiotic and pathogen strains. Eur Food Res Technol 226:1065–1073. https://doi.org/10.1007/s00217-007-0632-x
Manhar AK, Saikia D, Bashir Y, Mech RK, Nath D, Konwar BK, Mandal M (2015) In vitro evaluation of cellulolytic Bacillus amyloliquefaciens AMS1 isolated from traditional fermented soybean (Churpi) as an animal probiotic. Res Vet Sci 99:149–156. https://doi.org/10.1016/j.rvsc.2015.01.008
Spencer RJ, Chesson A (1994) The effect of Lactobacillus spp. on the attachment of enterotoxigenic Escherichia coli to isolated porcine enterocytes. J Appl Bacteriol 77:215–220. https://doi.org/10.1111/j.1365-2672.1994.tb03066.x
Boris S, Suarez JE, Barbes C (1997) Characterization of the aggregation promoting factor from Lactobacillus gasseria vaginal isolate. J Appl Microbiol 83:413–420. https://doi.org/10.1046/j.1365-2672.1997.00250.x
Bellon-Fontaine MN, Rault J, Van Oss CJ (1996) Microbial adhesion to solvents: a novel method to determine the electron-donor/ electron-acceptor or Lewis acid-base properties of microbial cells. Colloids Surf B Biointerfaces 7:47–53. https://doi.org/10.1016/0927-7765(96)01272-6
Lin MY, Yen CL (1999) Antioxidative ability of lactic acid bacteria. J Agri Food Chem 47:1460–1466. https://doi.org/10.1021/jf981149l
Wang Y, Wu Y, Wang Y, Xu H, Mei X, Yu D, Wang Y, Li W (2017) Antioxidant properties of probiotic bacteria. Nutrients 9:521. https://doi.org/10.3390/nu9050521
Li Y, Hugenholtz J, Abee T, Molenaar D (2003) Glutathione Protects Lactococcus lactis against oxidative stress. Appl Environ Microbiol 69:5739–5745. https://doi.org/10.1128/AEM.69.10.5739-5745.2003
Pieniz S, Andreazza R, Anghinoni T, Camargo F, Brandelli A (2014) Probiotic potential, antimicrobial and antioxidant activities of Enterococcus durans strain LAB18s. Food Control 37:251–256. https://doi.org/10.1016/j.foodcont.2013.09.055
Giri SS, Jun JW, Yun S, Kim HJ, Kim SG, Kang JW, Kim SW, Han SJ, Park SC, Sukumaran V (2019) Characterisation of lactic acid bacteria isolated from the gut of Cyprinus carpio that may be effective against lead toxicity. Probiotics Antimicrob Proteins 11:65–73. https://doi.org/10.1007/s12602-017-9367-6
Turpin W, Humblot C, Guyot JP (2011) Genetic screening of functional properties of lactic acid bacteria in a fermented pearl millet slurry and in the metagenome of fermented starchy foods. Appl Environ Microbiol 77:8722–8734. https://doi.org/10.1128/AEM.05988-11
Oliveira LC, Saraiva TD, Silva Pereira UP, Campos BC, Benevides LJ, Rocha FS, Figueiredo HC, Azevedo V, Soares SC (2017) Analyses of the probiotic property and stress resistance-related genes of Lactococcus lactis subsp. lactis NCDO 2118 through comparative genomics and in vitro assays. PLoS One 12:e0175116. https://doi.org/10.1371/journal.pone.0175116
Jia FF, Zheng, HQ, Sun SR, Pang XH, Liang Y, Shang JC, Zhu ZT, Meng XC (2018) Role of luxS in stress tolerance and adhesion ability in Lactobacillus plantarum KLDS1.0391. Biomed Res Int 2018:4506829. https://doi.org/10.1155/2018/4506829
Azcarate-Peril MA, Altermann E, Goh YJ, Tallon R, Sanozky-Dawes RB, Pfeiler EA, O’Flaherty S, Buck BL, Dobson A, Duong MMJ (2008) Analysis of the genome sequence of Lactobacillus gasseri ATCC 33323 reveals the molecular basis of an autochthonous intestinal organism. Appl Environ Microbiol 74:4610–4625. https://doi.org/10.1128/AEM.00054-08
Acknowledgements
The study was financially supported by a research grant from Department of Biotechnology, New Delhi, India [BT/PR25008/NER/95/953/2017]. The authors thank Dr. Janmejay Parhi, Mr. Shongsir Joy Monsang, and Ms. Mithila Debbarma for their technical advice and help. First author would also like to thank University Grant Commission, New Delhi, India for financial assistance [F.No.61-1/2019 (SA-III)] during the research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Statement
All experimental procedures involving fish were performed in accordance with the guidelines and policies of the ethical committee of the institute (No. CAU-CF/48/IAEC/2018/09 dated 06/01/2020).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khan, M.R., Kamilya, D., Choudhury, T.G. et al. Deciphering the Probiotic Potential of Bacillus amyloliquefaciens COFCAU_P1 Isolated from the Intestine of Labeo rohita Through In Vitro and Genetic Assessment. Probiotics & Antimicro. Prot. 13, 1572–1584 (2021). https://doi.org/10.1007/s12602-021-09788-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-021-09788-2