Abstract
Oral lichen planus (OLP) is a T cell-mediated common chronic inflammatory mucosal disease, with limited therapies available for long-term use. Previous study showed that ratio of genus Streptococcus decreased significantly in OLP patients when compared with controls. Buccal cotton swab samples of 43 OLP patients and 48 healthy individuals were collected for real-time quantitative polymerase chain reaction (RT-PCR) to investigate relative abundance alteration of Streptococcus salivarius in OLP lesions. Bacterial supernatants of S. salivarius ATCC® BAA-2593™ were collected by centrifugation and added to HSC-3 cells, and quantitative analysis of expression of IL-1β, IL-6, IL-8, and TNF-α in the HSC-3 cells was determined by RT-PCR. Then, a randomized, non-blinded, controlled study was conducted. Forty patients with symptomatic OLP were randomly allocated into two groups and received topical treatment of 0.1% triamcinolone acetonide dental paste (group A) and S. salivarius K12 lozenge (group B), respectively, for 4 weeks. Sign scores, visual analogue scale (VAS), and adverse reactions were recorded. Relative abundance of S. salivarius in the OLP group was lower than that of control group (P < 0.05). After treated with 0.1% supernatants of S. salivarius ATCC® BAA-2593™, the expression level of IL-6 in the HSC-3 cells significantly reduced (P < 0.001), while IL-1β, IL-8, and TNF- α showed a decreasing tendency (P > 0.05). There was significant reduction in sign scores and VAS scores in both groups after the 4-week treatment, with no significant difference between two groups. No adverse reaction was observed. S. salivarius might maintain local immune balance by inhibiting the NF-κB pathway. Topical application of Streptococcus salivarius K12 seemed to be effective in treatment of symptomatic OLP, especially with promising potential in long-term use. More detailed clinical studies with long follow-up period and standardized usage/dosage are expected to acquire definite conclusions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral lichen planus (OLP) is a T cell-mediated common chronic inflammatory mucosal disease with unknown pathogenesis. The intraoral lesions often induce recurrent erosions and pains that affect life quality of patients, and it is difficult to achieve radical cure for OLP [1]. The WHO recognized OLP as an oral potentially malignant disorder (OPMD) in 2005 [2]. The therapies currently available for long-term use in the treatment of OLP are limited. Topical corticosteroids are recommended as the main approach for treating erosive OLP, with the effective rate of 30–75%. Triamcinolone acetonide is one of the most commonly used corticosteroids [3]. Topical application of immunosuppressive agents showed similar curative effect to corticosteroids in previous studies and has become an effective alternative to short-term treatment when patients showed no response to corticosteroids. Other treatment options still need more experimental confirmation, including laser therapy, cryotherapy, biologics that regulate cytokines, etc. [4].
With the deepening of microbiome research, it was found that microbiome and their human hosts are co-evolving, which is closely related to overall health and susceptibility of diseases [5]. Bacteria are key drivers of mucosal immune responses and can induce transformation in gene expression and function of epithelial keratinocytes [6]. Microecological imbalance can lead to activation of T cells, and thus promote immune dysfunction, which is associated with a variety of local and systemic diseases [7]. It is not clear which factors induce immune disorders in OLP, but there is growing evidence that oral symbiotic bacteria in close contact with humans may be the driving factor of the pathological process.
In our previous study, we found that microbial community diversity was increased in OLP lesions, and the microbial structure was significantly different from that of healthy control [8]. Several studies from different researchers confirmed changes in the microbial community structure of mucosal surface or saliva in OLP patients, in accordance with our findings [9,10,11]. Notably, it was suggested that ratio of genus Streptococcus decreased significantly in OLP patients when compared with controls [8, 10]. However, as is known, genus Streptococcus contains many different species. Till now, it is not clear which species are significantly decreased in bacterial community of buccal mucosa in OLP patients.
In recent years, beneficial effects of probiotics in gastrointestinal tract, vagina, urethra, skin, and oral cavity have aroused people’s attention [12, 13]. Probiotics are defined as living microorganisms that have a beneficial health effect on the host at appropriate dose. Streptococcus salivarius (S. salivarius) is a pioneer colonizer of human oral cavity and remains as predominant bacteria of native microbiota during the lifetime of its human host [14]. S. salivarius K12 became the first of S. salivarius strains to be commercially developed as a probiotic product, which has been widely used in researches and treatment of related diseases. S. salivarius K12 lozenges have showed effective in prevention and treatment of recurrent pharyngitis, tonsillitis, otitis media, and Candida albicans infection in several clinical trials with few adverse effects [15, 16]. It was demonstrated that S. salivarius K12 specifically regulated the expression of 565 host genes, particularly those involved in multiple innate defense pathways, general epithelial cell function and homeostasis, cytoskeletal remodeling, cell development and migration, and signaling pathways [17, 18].
In all, we speculated that S. salivarius may play an important role in maintaining mucosa immune balance as a beneficial bacterium in OLP. Therefore, in the present study, we aimed to explore whether the relative abundance of S. salivarius was decreased on buccal mucosa of OLP and its anti-inflammation mechanism in vitro. Based on the above results, we performed a pilot randomized controlled study to evaluate whether S. salivarius K12 could take effect in the treatment of symptomatic OLP.
Methods
Subjects Recruitment
Patients were recruited from the outpatient department of the Affiliated Stomatology Hospital of Tongji University. OLP patients were diagnosed on the basis of clinical manifestations and biopsy specimen supporting the clinical diagnosis [19]. Patients of ages above 18 years old without skin involvement were included, with willingness and ability to complete the present clinical trial. Exclusion criteria were as follows: patients with lichenoid contact lesions, suspicion of lichenoid drug reactions, graft vs. host disease-related lichenoid lesion, or other serious oral mucosal diseases; patients with uncontrolled hypertension or diabetes, immune system diseases such as tumors or Sjogren’s syndrome; pregnant or lactating women; and patients undergoing treatment with corticosteroids/immunomodulatory agents in the past 3 months or antibiotics/antifungal drugs in the past 1 month. The research protocols were approved by the ethics committee of faculty of medicine for human studies, school of medicine, Tongji University (Ethical Reference Number: 2017–45; SL2019SR21). The procedures followed were in accordance with the Helsinki Declaration of 1975, as revised in 1983. A written consent was obtained from every patient before initiating researches.
Analysis of the Relative Abundance of Streptococcus salivarius on Buccal Mucosa of OLP
Every subject received necessary oral examination and information recording before buccal cotton swab sample collection. After fasting 2 h, subjects gently rinse mouths with water for 1 min. Sterile cotton swabs were used to wipe clockwise on buccal mucosa for 20 s and then sealed and transferred to − 80 °C refrigerator within 24 h for subsequent use.
Total DNA Extraction
Total DNA from oral samples was extracted and purified with a Gentra Buccal Cell Kit (Qiagen, Duesseldorf, Germany) according to the manufacturer’s tissue protocol instructions. The DNA concentration (absorbance at 260 nm; A260) and the purity (A260/A280) were calculated using a One Drop OD_1000+ spectrophotometer (Reco Science & Technology, Shanghai, China).
Real-Time Quantitative Polymerase Chain Reaction
For real-time quantitative polymerase chain reaction (RT-PCR), primers were designed based on the glucosyltransferase (GTF) gene [20]. Forward primer MKK1-F: 5’-CAACAGAGCGAGCAGAAGTTACTG-3′ and reverse primer MKK1-R: 5′- TACTGCTGCAGCTCTATCACTAGTTGT-3′, targeting a 92-base-pair stretch. 16S rRNA gene was selected as internal reference gene.
Amplification and detection of DNA by RT-PCR was performed with the aid of the QuantStudio™ 7 Flex Real-time PCR System using optical grade 384-well plates, RNase free ddH2O as negative control. The temperature profile was as follows: denaturation 95 °C for 15 min, followed by 40 cycles of 95 °C for 10 s, and stringent annealing at 60 °C for 32 s. Data acquisition and subsequent analysis were performed using the GraphPad Prism version 7.04.
In Vitro Anti-Inflammation Assay of Streptococcus salivarius
HSC-3 cells (kindly provided by Professor Chen Qian-ming from West China school of stomatology, Sichuan University) were seeded at 2.25 × 105 cells per well into 12-well plates and incubated for 24 h in DMEM (Hyclone, Logan, UT, USA) supplemented with 2-mM glutamine and 10% fetal bovine serum in a humidified 5% CO2 atmosphere at 37 °C.
Streptococcus salivarius ATCC® BAA-2593™ was obtained from the American Type Culture Collection. Pre-activated strain (OD600 = 0.1) was grown in culture medium of 10% Brain Heart Infusion broth (BHI, BD Biosciences, San Jose, CA, USA) in a CO2 atmosphere at 37 °C for 14 h overnight. Afterwards, centrifugation was performed for 15 min at 4000 rpm at room temperature and then filtered through 0.22-μm filters to collect bacterial supernatants (Sn) as conditioned medium. BHI blank medium was used as a negative control. After 6 h stimulation at 37 °C in 5% CO2, culture supernatants were removed and TRNzol A+ total RNA extraction reagent (Tiangen Biotech, Beijing, China) was added in each well for further detection.
Total RNA of each well was extracted and then reversely transcribed with the FastQuant RT Kit (With gDNase) (Tiangen Biotech). Quantitative analysis of expression of IL-1β, IL-6, IL-8, and TNF-α was determined using SuperReal fluorescent quantitative reagent enhanced version, SYBR Green (Tiangen Biotech) (primer sequence shown in the supplementary table). The amplification and detection were performed with the Applied Biosystems® QuantStudio™ 7 Flex Real-Time PCR System. Levels of cytokines were calculated utilizing the 2–ΔΔCT method with β-actin as the internal reference.
Clinical Trial
Study Design and Interventions
The patients were randomly allocated into two groups. The randomization sequence for patients was sourced using computer-generated numbers. Group A: patients received 0.1% triamcinolone acetonide dental paste (Bright Future Pharmaceutical Laboratories Ltd., Hong Kong, China) thrice daily for 4 weeks. Group B: patients received S. salivarius K12 lozenge (Now Foods Ltd., Bloomingdale, IL, USA) 1 tablet twice daily, which contained no less than 1 billion CFU/tablet of Streptococcus salivarius K12 for 4 weeks.
Assessment of Treatment Efficacy
All patients had five visits for the study; they were reviewed on the 0 (baseline), 1st, 2nd, 3rd, and 4th week. Sign scores were assessed using the evaluation criterion in Table 1 [21, 22] at each visit. The lesion size was measured by two observers, including extent of white striae and area of atrophic/hyperemia and erosion. Site of OLP lesion (buccal mucosa, vestibule, keratinized gingiva, palate, or tongue) and adverse effects were recorded.
Subjective assessment was done by means of a visual analogue scale (VAS) for symptoms of pain and burning sensation (0 = no pain, 10 = unbearable pain). The difference between VAS scores (baseline VAS score V0 and VAS score of each visit VX) was expressed in an equation (V0-VX) × 100/V0 to calculate the percentage of pain improvements. All clinical data was recorded at baseline and at each subsequent visit after the administration of medical therapy, and subjected to appropriate statistical analysis.
Adverse Reactions
In the event of adverse reactions, they were noted and the patient was put under observation. In case of serious reactions, treatment was discontinued and the subject was sent for treatment to doctors not involved in the research.
Alternation of S. salivarius Relative Abundance after Treatment
Buccal cotton swab samples of the subjects were collected at baseline and after 4-week treatment for RT-PCR to investigate relative abundance alteration of S. salivarius. Methods of sample collection, DNA extraction, and RT-PCR were the same as above.
Statistical Methods
The statistical analysis was performed using IBM SPSS Statistics version 22 for Windows (IBM Corp, Armonk, NY, USA). The statistical significance of changes in the sign scores and VAS values in the different treatment arms over time was examined with Wilcoxon signed-rank tests, and intergroup comparison was examined with Mann-Whitney U tests. Probability values of < 0.05 were considered statistically significant. Line charts and histograms were performed using the GraphPad Prism version 5.01.
Results
Decrease of Streptococcus salivarius on Buccal Mucosa of OLP
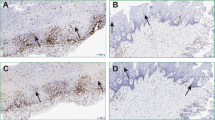
Forty-three patients with OLP (20 erosive OLP and 23 non-erosive OLP, with lesions on buccal mucosa) and 48 healthy individuals as control were studied. Relative abundance of S. salivarius in the OLP group is lower than that of the control group by RT-PCR (P = 0.041) (Fig. 1).
Streptococcus salivarius Could Inhibit NF-κB Pathway Activation
After treated with 0.1% supernatants (Sn) of S. salivarius ATCC® BAA-2593™, the expression level of IL-6 in HSC-3 cells significantly reduced (P < 0.001), while IL-1β, IL-8, and TNF- α showed a decreasing tendency with no statistical significance (Fig. 2).
Clinical Parameter
Finally, forty patients with symptomatic and histopathologically confirmed OLP were recruited from the outpatient department of the Affiliated Stomatology Hospital of Tongji University from November 2017 to October 2019 (Table 2). There were 31 females and 9 males, and the age of the patients ranged from 22 to 79 years (mean age 53.12 ± 14.2 years). Duration of the symptoms ranged from 3 months to 5 years. There was no statistic difference in clinical parameters between two groups.
Treatment Efficacy
At baseline, there was no difference between two groups in sign scores (P = 0.341) and VAS scores (P = 0.383). There was significant reduction in sign scores in both groups with treatment. No statistical difference was observed between two groups after 4-week treatment (Table 3 and Fig. 3).
Statistically significant decrease in VAS scores was observed in both groups and it showed no significant difference between two groups after a 4-week therapy (Table 4 and Fig. 4).
Adverse Reactions
No adverse reaction of topical treatment was observed during 4-week treatment.
Alternation of S. salivarius Relative Abundance after Treatment
Finally, there were 6 pairs of valid data of each group as illustrated in Fig. 5. The relative abundance of S. salivarius increased significantly after treatment in both groups. The increase was more significant in group B.
Discussion
OLP is considered a T cell-mediated inflammatory disease. Both intrinsic and extrinsic antigens induce the infiltration of immunocyte, mainly T cells [3]. Currently, there are many treatments for OLP focusing on relieving pain and preventing mucosa erosion. Topical application of corticosteroids is still the preferred clinical treatment for OLP because of its powerful anti-inflammatory effects. However, it has risks of secondary candida infections in long-term application, and some patients show no response to corticosteroids [23]. Immunosuppressive agents, such as pimecrolimus and tacrolimus, are widely used in the treatment of OLP as an alternative therapy. But sometimes they may cause adverse effects including burning sensation, pruritus, pain, or erythema [24]. Other treatment options include laser therapy, cryotherapy, biologics that regulate cytokines, etc., with few related clinical researches [4].
Our previous study has shown that ratio of genus Streptococcus decreased significantly in OLP patients [8]. In the present study, our group further utilized RT-PCR to detect the relative abundance of Streptococcus at species level. HSC-3 cells, with property of unlimited proliferation, were isolated from a poorly differentiated oral squamous cell carcinoma of tongue in a 64-year-old man and can be used in study of inflammatory pathways [25,26,27]. There is no specific OLP cell model or animal model; hence, HSC-3 cells were used as cell model to study the role of S. salivarius in the inactivation of NF-κB pathway. We found that S. salivarius was significantly decreased in OLP lesions, and supernatant of S. salivarius could inhibit NF-κB pathway activation. The results are consistent with other researchers [17, 28]. Cosseau et al. and Wescombe et al. have demonstrated that S. salivarius K12 can downregulate inflammatory responses by inhibiting the NF-kB pathway and suppressing IL-8 secretion, which contributes to immunomodulatory and host defense process [14, 17]. Couvigny et al. have found that S. salivarius inhibits NF-κB pathway and PPARγ transcriptional activity in human intestinal epithelial cells. It can affect both inflammatory response and metabolic regulation of its host, suggesting that S. salivarius plays an important role in maintaining host metabolism and immune balance, as one of the commensal bacteria of oral mucosal epithelium [29].
Modern microecological studies have found that changes in the composition of symbiotic flora, that is, imbalance between beneficial bacteria and pathogenic bacteria, are closely related to occurrence and development of various local and systemic diseases [30]. Restoring local microecological balance by regulating beneficial bacteria has become a new idea or new target for treatment of many diseases [31]. Several researchers conducted research on prevention of dental caries using probiotics and found that probiotics could reduce Streptococcus mutans, one of the cariogenic bacteria [32]. Seminario-Amez et al. indicated effects of probiotics in improving bleeding on probing, probing depth and gingival index [33].
S. salivarius K12, as a widely used probiotic, has been proven to inhibit activity of many microorganisms, reduce colonization of pathogenic bacteria in oral cavity and respiratory tract, and promote balance of host microbiota. Thus, it has the effect of prolonged prevention and treatment of chronic infectious diseases [14, 15]. Although another S. salivarius strain was used in the in vitro experiments, we chose S. salivarius K12 for clinical trials because of its extensive clinical applications and safety. Cosseau et al. [17] found that S. salivarius K12 downregulated inflammatory responses by inhibiting the NF-κB pathway, resulting in the attenuation of some innate immunity pathways involved in the pro-inflammatory responses of epithelial cells. Kaci et al. [18] indicated that supernatant of S. salivarius K12 downregulated NF-κB activation and the secretion of the pro-inflammatory chemokine IL-8. Burton et al. proved its safety for human by detecting drug resistance, metabolic profile, and toxic genes of S. salivarius K12, and through further evaluation including animal and human clinical trials [34]. Clinical trials have also confirmed that there is almost no exact adverse reaction of S. salivarius K12 lozenge [15]. Di Pierro et al. found that S. salivarius K12 might reduce incidence of pharyngitis and acute otitis media in pediatric subjects with non-recurrent streptococcal infection [35]. S. salivarius K12 proved to be effective against Streptococcus pyogenes and bacterial species involved in oral malodour in in vitro studies [36, 37]. S. salivarius can protect human body against cariogenic microorganisms by producing various bacteriocins [38].
In this research, we observed that S. salivarius K12 was effective in the treatment of OLP. After 4-week treatment, sign scores and VAS scores of two groups both significantly decreased, which indicated that probiotics therapy was able to relieve OLP patients’ pain and improve symptoms. Intergroup comparison showed that triamcinolone acetonide dental paste seemed to work better in pain relief than the probiotics and also attained lower sign scores, although there was no statistical difference. The relative abundance of S. salivarius increased significantly after treatment, which indicated that enhanced levels of S. salivarius may be correlated with clinical improvement. Our research provided evidence of positive effects of S. salivarius K12 in OLP patients. As OLP is a chronic disease and long-term use of S. salivarius K12 lozenges showed no adverse effect, it is suggested that probiotics, especially long-term used, may contribute to the prolonged prevention and remission of OLP as a complementary treatment.
There were some limitations of this study. First, genus Streptococcus contains a variety of species, in which we only focused on S. Salivarius. Alteration and effect of other species remains to be determined. Besides, notable weaknesses included the small sample size, the lack of adequate blinding, and the short observation period. More detailed trials with long follow-up period and standardized usage/dosage are expected.
Conclusion
In conclusion, to the authors’ knowledge, this is the first study on the efficacy of S. salivarius K12 lozenge in treatment of OLP. We showed that relative abundance of S. salivarius in OLP patients was significantly decreased. The supernatants of S. salivarius could inhibit NF-κB pathway activation. Topical application of Streptococcus salivarius K12 seemed to be effective in treatment of OLP, especially with promising potential in long-term use. More detailed clinical studies with long follow-up period and standardized usage/dosage are expected to acquire definite conclusions.
References
Gupta S, Jawanda MK (2015) Oral lichen planus: an update on etiology, pathogenesis, clinical presentation, diagnosis and management. Indian J Dermatol 60(3):222–229. https://doi.org/10.4103/0019-5154.156315
Aghbari SMH, Abushouk AI, Attia A, Elmaraezy A, Menshawy A, Ahmed MS, Elsaadany BA, Ahmed EM (2017) Malignant transformation of oral lichen planus and oral lichenoid lesions: a meta-analysis of 20095 patient data. Oral Oncol 68:92–102. https://doi.org/10.1016/j.oraloncology.2017.03.012
Alrashdan MS, Cirillo N, McCullough M (2016) Oral lichen planus: a literature review and update. Arch Dermatol Res 308(8):539–551. https://doi.org/10.1007/s00403-016-1667-2
Gupta S, Ghosh S, Gupta S (2017) Interventions for the management of oral lichen planus: a review of the conventional and novel therapies. Oral Dis 23(8):1029–1042. https://doi.org/10.1111/odi.12634
Kundu P, Blacher E, Elinav E, Pettersson S (2017) Our gut microbiome: the evolving inner self. Cell 171(7):1481–1493. https://doi.org/10.1016/j.cell.2017.11.024
Sears CL, Pardoll DM (2011) Perspective: alpha-bugs, their microbial partners, and the link to colon cancer. J Infect Dis 203(3):306–311. https://doi.org/10.1093/jinfdis/jiq061
Honda K, Littman DR (2016) The microbiota in adaptive immune homeostasis and disease. Nature 535(7610):75–84. https://doi.org/10.1038/nature18848
He Y, Gong D, Shi C, Shao F, Shi J, Fei J (2017) Dysbiosis of oral buccal mucosa microbiota in patients with oral lichen planus. Oral Dis 23(5):674–682. https://doi.org/10.1111/odi.12657
Wang K, Miao T, Lu W, He J, Cui B, Li J, Li Y, Xiao L (2015) Analysis of oral microbial community and Th17-associated cytokines in saliva of patients with oral lichen planus. Microbiol Immunol 59(3):105–113. https://doi.org/10.1111/1348-0421.12232
Wang K, Lu W, Tu Q, Ge Y, He J, Zhou Y, Gou Y, Van Nostrand JD, Qin Y, Li J, Zhou J, Li Y, Xiao L, Zhou X (2016) Preliminary analysis of salivary microbiome and their potential roles in oral lichen planus. Sci Rep 6:22943. https://doi.org/10.1038/srep22943
Ertugrul AS, Arslan U, Dursun R, Hakki SS (2013) Periodontopathogen profile of healthy and oral lichen planus patients with gingivitis or periodontitis. Int J Oral Sci 5(2):92–97. https://doi.org/10.1038/ijos.2013.30
Ma F, Zhou C, Wang J, Liu T, Liu J (2015) Probiotics in the treatment of peptic ulcer infected by Helicobacter pylory and its safety. Pak J Pharm Sci 28(3 Suppl):1087–1090
Fuchs-Tarlovsky V, Marquez-Barba MF, Sriram K (2016) Probiotics in dermatologic practice. Nutrition 32(3):289–295. https://doi.org/10.1016/j.nut.2015.09.001
Wescombe PA, Hale JD, Heng NC, Tagg JR (2012) Developing oral probiotics from Streptococcus salivarius. Future Microbiol 7(12):1355–1371. https://doi.org/10.2217/fmb.12.113
Zupancic K, Kriksic V, Kovacevic I, Kovacevic D (2017) Influence of oral probiotic Streptococcus salivarius K12 on ear and oral cavity health in humans: systematic review. Probiotics Antimicrob Proteins 9(2):102–110. https://doi.org/10.1007/s12602-017-9261-2
Ishijima SA, Hayama K, Burton JP, Reid G, Okada M, Matsushita Y, Abe S (2012) Effect of Streptococcus salivarius K12 on the in vitro growth of Candida albicans and its protective effect in an oral candidiasis model. Appl Environ Microbiol 78(7):2190–2199. https://doi.org/10.1128/aem.07055-11
Cosseau C, Devine DA, Dullaghan E, Gardy JL, Chikatamarla A, Gellatly S, Yu LL, Pistolic J, Falsafi R, Tagg J, Hancock RE (2008) The commensal Streptococcus salivarius K12 downregulates the innate immune responses of human epithelial cells and promotes host-microbe homeostasis. Infect Immun 76(9):4163–4175. https://doi.org/10.1128/iai.00188-08
Kaci G, Lakhdari O, Dore J, Ehrlich SD, Renault P, Blottiere HM, Delorme C (2011) Inhibition of the NF-kappaB pathway in human intestinal epithelial cells by commensal Streptococcus salivarius. Appl Environ Microbiol 77(13):4681–4684. https://doi.org/10.1128/aem.03021-10
Kramer IR, Lucas RB, Pindborg JJ, Sobin LH (1978) Definition of leukoplakia and related lesions: an aid to studies on oral precancer. Oral Surg Oral Med Oral Pathol 46(4):518–539
Hoshino T, Kawaguchi M, Shimizu N, Hoshino N, Ooshima T, Fujiwara T (2004) PCR detection and identification of oral streptococci in saliva samples using gtf genes. Diagn Microbiol Infect Dis 48(3):195–199. https://doi.org/10.1016/j.diagmicrobio.2003.10.002
Kaliakatsou F, Hodgson TA, Lewsey JD, Hegarty AM, Murphy AG, Porter SR (2002) Management of recalcitrant ulcerative oral lichen planus with topical tacrolimus. J Am Acad Dermatol 46(1):35–41. https://doi.org/10.1067/mjd.2002.120535
Malik U, Gupta S, Malik SD, Vashishth S, Zaheeruddin RMS (2012) Treatment of symptomatic oral lichen planus (OLP) with 0.1% tacrolimus powder in Oraguard-B - a pilot prospective study. Saudi Dent J 24(3–4):143–148. https://doi.org/10.1016/j.sdentj.2012.05.002
Mostafa D, Moussa E, Alnouaem M (2017) Evaluation of photodynamic therapy in treatment of oral erosive lichen planus in comparison with topically applied corticosteroids. Photodiagn Photodyn Ther 19:56–66. https://doi.org/10.1016/j.pdpdt.2017.04.014
Chamani G, Rad M, Zarei MR, Lotfi S, Sadeghi M, Ahmadi Z (2015) Efficacy of tacrolimus and clobetasol in the treatment of oral lichen planus: a systematic review and meta-analysis. Int J Dermatol 54(9):996–1004. https://doi.org/10.1111/ijd.12925
Momose F, Araida T, Negishi A, Ichijo H, Shioda S, Sasaki S (1989) Variant sublines with different metastatic potentials selected in nude mice from human oral squamous cell carcinomas. J Oral Pathol Med 18(7):391–395
Miyazaki Y, Yoshida N, Nozaki T, Inoue H, Kikuchi K, Kusama K (2015) Telomerase activity in the occurrence and progression of oral squamous cell carcinoma. J Oral Sci 57(4):295–303. https://doi.org/10.2334/josnusd.57.295
Takata H, Tomizawa K, Matsushita M, Matsui H (2004) 2-methoxyestradiol enhances p53 protein transduction therapy-associated inhibition of the proliferation of oral cancer cells through the suppression of NFkappaB activity. Acta med Okayama 58(4):181–187. https://doi.org/10.18926/amo/32086
Kaci G, Goudercourt D, Dennin V, Pot B, Dore J, Ehrlich SD, Renault P, Blottiere HM, Daniel C, Delorme C (2014) Anti-inflammatory properties of Streptococcus salivarius, a commensal bacterium of the oral cavity and digestive tract. Appl Environ Microbiol 80(3):928–934. https://doi.org/10.1128/aem.03133-13
Couvigny B, de Wouters T, Kaci G, Jacouton E, Delorme C, Dore J, Renault P, Blottiere HM, Guedon E, Lapaque N (2015) Commensal Streptococcus salivarius modulates PPARgamma transcriptional activity in human intestinal epithelial cells. PLoS One 10(5):e0125371. https://doi.org/10.1371/journal.pone.0125371
Round JL, Mazmanian SK (2009) The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 9(5):313–323. https://doi.org/10.1038/nri2515
Coyte KZ, Schluter J, Foster KR (2015) The ecology of the microbiome: networks, competition, and stability. Science 350(6261):663–666. https://doi.org/10.1126/science.aad2602
Laleman I, Detailleur V, Slot DE, Slomka V, Quirynen M, Teughels W (2014) Probiotics reduce mutans streptococci counts in humans: a systematic review and meta-analysis. Clin Oral Investig 18(6):1539–1552. https://doi.org/10.1007/s00784-014-1228-z
Seminario-Amez M, Lopez-Lopez J, Estrugo-Devesa A, Ayuso-Montero R, Jane-Salas E (2017) Probiotics and oral health: a systematic review. Med Oral Patol Oral Cir Bucal 22(3):e282–e288
Burton JP, Cowley S, Simon RR, McKinney J, Wescombe PA, Tagg JR (2011) Evaluation of safety and human tolerance of the oral probiotic Streptococcus salivarius K12: a randomized, placebo-controlled, double-blind study. Food Chem Toxicol 49(9):2356–2364. https://doi.org/10.1016/j.fct.2011.06.038
Di Pierro F, Risso P, Poggi E, Timitilli A, Bolloli S, Bruno M, Caneva E, Campus R, Giannattasio A (2018) Use of Streptococcus salivarius K12 to reduce the incidence of pharyngo-tonsillitis and acute otitis media in children: a retrospective analysis in not-recurrent pediatric subjects. Minerva Pediat 70(3):240–245. https://doi.org/10.23736/s0026-4946.18.05182-4
Humphreys GJ, McBain AJ (2019) Antagonistic effects of Streptococcus and Lactobacillus probiotics in pharyngeal biofilms. Lett Appl Microbiol 68(4):303–312. https://doi.org/10.1111/lam.13133
Masdea L, Kulik EM, Hauser-Gerspach I, Ramseier AM, Filippi A, Waltimo T (2012) Antimicrobial activity of Streptococcus salivarius K12 on bacteria involved in oral malodour. Arch Oral Biol 57(8):1041–1047. https://doi.org/10.1016/j.archoralbio.2012.02.011
Walker GV, Heng NC, Carne A, Tagg JR, Wescombe PA (2016) Salivaricin E and abundant dextranase activity may contribute to the anti-cariogenic potential of the probiotic candidate Streptococcus salivarius JH. Microbiology 162(3):476–486. https://doi.org/10.1099/mic.0.000237
Funding
This work was financially supported by the Natural Science Foundation of China (81870764).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Yuting Li, Fangyang Shao, and Yuan He. The first draft of the manuscript was written by Yuting Li and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
The research protocols were approved by the Ethics Committee of Faculty of Medicine for Human Studies, School of Medicine, Tongji University (Ethical Reference Number: 2017–45; SL2019SR21). The procedures followed were in accordance with the Helsinki Declaration of 1975, as revised in 1983.
Statement of Informed Consent
A written consent was obtained from every patient before initiating researches.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 16.4 kb)
Rights and permissions
About this article
Cite this article
Li, Y., Shao, F., Zheng, S. et al. Alteration of Streptococcus salivarius in Buccal Mucosa of Oral Lichen Planus and Controlled Clinical Trial in OLP Treatment. Probiotics & Antimicro. Prot. 12, 1340–1348 (2020). https://doi.org/10.1007/s12602-020-09664-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-020-09664-5