Abstract
Objectives
Systematically review the available literature regarding the caries-preventive effect of probiotics.
Data, sources and study selection
An electronic search was conducted in three databases (PubMed MEDLINE, ISI Web of Science and Cochrane Library) to identify all suitable studies. The outcomes had to be presented as the effect of probiotics on the incidence of caries or on the levels of mutans streptococci and/or Lactobacillus species. Human studies, written in English, with at least 15 participants, comparing a probiotic product with a placebo/no probiotic were included. Where possible, a meta-analysis was performed to obtain quantitative data.
Results
Since only two articles presented useful data on the caries incidence, we focused on the surrogate endpoints: mutans streptococci and/or Lactobacillus counts. The meta-analysis showed that when the probiotic and control group are compared after treatment, significantly more patients in the probiotic group had low mutans streptococci (<105 CFU/ml) counts and significantly less patients had high (>106 CFU/ml) counts. Regarding the Lactobacillus counts, comparing the probiotic and control group at the end of the probiotic use, no significant differences could be observed, neither in low (<104 CFU/ml) nor in high Lactobacillus (>106 CFU/ml) counts.
Conclusions
Within the limitations of the available data, it may be concluded that probiotics decrease the mutans streptococci counts. This suggests that probiotics could have a positive effect in the prevention of caries.
Clinical relevance
There is insufficient evidence that probiotics can prevent caries, but they can reduce the mutans streptococci counts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dental caries is one of the most common preventable diseases and affects people of all ages [1]. It results from a disturbance in the ecological balance at the tooth surface which ultimately leads to loss of tooth mineral [2]. Endogenous, acidogenic bacteria (largely Streptococcus mutans, Streptococcus sobrinus and Lactobacillus spp. [3–7]) are of importance since they produce organic acids which demineralize the hard tissues [1, 4, 8–10]. Besides cariogenic bacteria, a susceptible host and nutrients are considered as essential elements in the aetiology of dental caries [11]. Furthermore, the time factor is important for the production of acids and the subsequent demineralization of tooth structures [11].
Current preventive strategies for dental caries target the host factors, dietary factors and the removal of the plaque biofilm. They encompass mainly the use of topical fluorides, dietary monitoring, and mechanical and chemical plaque control [3]. Recently, the caries preventive effect of probiotics has been suggested [12–14]. Probiotics are defined by the WHO as living microorganisms that confer a health benefit for the host when administered in sufficient amounts (www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf) [17]. Although their mechanisms of action are still poorly understood, it is known that probiotics can produce substances such as bacteriocins against pathogenic bacteria [15, 16]. Furthermore, they can stimulate local immunity, modulate the inflammatory response, modify the environment and compete with pathogens for binding sites and nutrients [15, 16].
To date, the effect of probiotics on systemic health and medical disorders is elaborately described [17]. Positive effects have been shown not only in the field of gastrointestinal diseases, e.g. for diarrhoea, inflammatory bowel disease and irritable bowel syndrome, but also for atopic diseases and cancer [17]. Over the recent years, an increasing interest in probiotics from an oral health perspective has emerged. The effect of probiotics on halitosis [18–20], candidiasis [21, 22] and periodontitis [23–31] (for review, see Teughels et al., 2011 [15]) has been investigated. Additionally, several papers have examined the effect of probiotics on caries. Recently, a systematic review showed that probiotics have the capacity to reduce mutans streptococci counts in short-term. However, a meta-analysis evaluating this effect has not yet been carried out. Therefore, this study aimed at systematically evaluating the current literature by means of a meta-analysis. The primary outcome variable of interest was caries incidence and as secondary parameters the surrogate endpoints, mutans streptococci and lactobacilli counts, were analysed.
Materials and methods
This systematic review was conducted in accordance with the guidelines of the Transparent Reporting of Systematic Reviews and Meta-Analyses (PRISMA) [32].
Focused PICO question
What is, in healthy humans, the effect of probiotics compared to a placebo just after its usage on caries incidence and on the level of mutans streptococci and lactobacilli spp. in the oral cavity?
Search strategy
A computerized literature search of PubMed MEDLINE, ISI Web of KnowledgeSM and the Cochrane databases was performed in order to identify all studies concerning caries and probiotics regardless of their publication status. These searches were restricted till June 2013. Additional hand searches were performed and included the following: (1) bibliographies of previous reviews on the subject [12–14, 16, 33–35], (2) bibliographies of all publications cited in these articles and (3) cited reference searches of the publications considered using the ISI Web of KnowledgeSM.
Search terms
Although there are some differences, no differentiation was made between probiotics and replacement therapy (also known as bacteriotherapy or bacterial interference) given the confusion regarding the use of these terms [15]. The following search was used: “probiotic OR replacement therapy OR bacterial interference OR bacteriotherapy” AND “dental caries OR tooth decay OR cariogenic bacteria OR Streptococcus mutans” OR “lactobacilli and dental”.
Eligibility criteria
Following criteria were used for inclusion: studies in the English language conducted in humans. The intervention must comprise the use of a probiotic versus a placebo or no probiotic. For our primary parameter of interest, the results had to be presented as the effect of probiotics on the incidence of caries. For the indirect effect, the outcome measures had to be presented as the levels of mutans streptococci and/or Lactobacillus species, which are surrogate endpoints in the development of caries. The evaluation of all parameters had to take place before and just after using the probiotic. Only controlled clinical trials with at least 15 participants for each group were included.
Exclusion criteria
Studies that explicitly mentioned that the patients were wearing fixed orthodontic appliances were excluded, because this may facilitate the establishment and growth of cariogenic streptococci strains [36]. Studies with only a positive control group were excluded.
Risk of bias assessment
A quality assessment was conducted to evaluate the methodological quality of the selected studies. This was based on the randomized controlled trial (RCT) checklist of the Cochrane Center, the CONSORT guidelines [37], the Delphi list [38] and the checklist as proposed by Van der Weijden et al. [39]. Seven criteria from these lists were selected to assess risk of bias, namely random allocation, blinding of the participants and personnel, blinding of outcome assessment, defined inclusion and exclusion criteria, identical treatment between groups except for the intervention, incomplete outcome data and selective reporting.
When all these criteria were assessed as low risk of bias, the article was classified as having a low risk of bias. When one or two of these criteria were assessed as high risk of bias or unclear, the study was regarded to have a moderate potential risk of bias. The risk of potential bias was high, when three or more criteria had a high or unclear risk of bias. Two reviewers assessed the risk of bias independently (VD, IL).
Data extraction
Two reviewers (VD, IL) independently screened the titles and subsequent the abstracts of all articles found. When there was disagreement or when an abstract contained insufficient information, the full text of the paper was reviewed. The final inclusion of studies was then made by discussion. Thereafter, both reviewers extracted the data separately from the selected papers. This information was transferred to a data extraction sheet. The following characteristics were abstracted from each study: first author, year of publication, age of subjects, study design, length of treatment, number of subjects in each treatment group, vehicle, type and amount of probiotic used, publication bias and original author’s conclusion.
Data analysis
Concerning the levels of mutans streptococci and lactobacilli species, the intergroup comparisons after treatment and intragroup comparisons, as described by the authors, were placed in a table. All available microbiological data regarding the mutans streptococci and Lactobacillus counts were arranged in groups in analogy with the interpretation charts of these chairside tests. The microbiological results from the studies using specific agars were placed in a table with the baseline and post-treatment counts (expressed as mean and standard deviation) (this tables are available online).
Where possible, a meta-analysis for binary outcomes was performed regarding the number of patients in the probiotic versus the control group in the clusters with the highest and lowest bacterial counts both before and after treatment. Fixed effects were applied. Relative risks were calculated and they were, together with their corresponding confidence intervals, displayed in forest plots. Comparisons were made between placebo control (C) and probiotic groups (P) before and after treatment. In addition, for the control and probiotics group, data collected before treatment were also compared with those from after the treatment. For all studies, the microbiological levels at baseline and at the end of the probiotic usage (post-intervention) were used for this review. When data were missing, incomplete or ambiguous, the authors were contacted.
Results
Search and selection
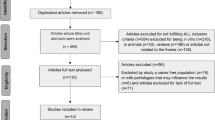
The electronic searches through the MEDLINE, Cochrane and ISI Web of KnowledgeSM retrieved 725 unique articles as summarized in Fig. 1. Of these, 690 were removed after a first selection and 35 articles were read full text for eligibility. Three studies were excluded because they appeared to be in vitro studies [20, 40, 41] Three studies did not have a control group [30, 42, 43]. One study had only a positive control as control group [44]. One study combined the use of a probiotic with the use of fluor [45]. And two studies combined the use of a probiotic with the use of xylitol [46, 47]. One study was conducted in patients wearing fixed orthodontic appliances [48]. And finally, five studies included less than 15 patients in each group [49–53]. This resulted in the retrieval of 19 publications. For two of these studies, we took only the data into consideration from the probiotic and/or test groups that met the inclusion criteria [54, 55].
Outcome results
Table 1 summarizes the study characteristics and their outcomes. The selected papers were substantially heterogeneous in their set-up, duration, used probiotics, mode of application and the assessment criteria. The number and the age of the participants varied among the studies.
Characteristics of the study design
Since only two articles that met our inclusion criteria were found that used caries incidence as outcome measure, it was decided to mainly focus on mutans streptococci and Lactobacillus counts. All included studies had as outcome measures mutans streptococci and/or Lactobacillus counts or the prevalence of patients having low, medium or high counts of either mutans streptococci or lactobacilli. All studies had an evaluation moment immediately post-intervention. Five studies had an extra evaluation moment some weeks later [21, 56–59].
Characteristics of the study population
Three studies did not mention if there were untreated caries lesions present in their study population [21, 56, 58]. This was an exclusion criterion for 13 studies [44, 54, 57, 59–68]. In three studies, caries was present in the study population [55, 69, 70]. Often, specific patient groups were used in the studies. Certain studies focused on specific age groups such as children [56, 58, 64–66, 69, 71] or elderly people [55]. Cildir and coworkers explicitly focused on operated cleft lip/palate children [64]. Other studies targeted patient groups with moderate to high (>104 CFU) [56, 58, 68] or high (≥105 CFU/ml saliva) [56, 57, 59] salivary mutans streptococci counts. One study selected only female subjects who were studying to become a dental hygienist [60]. Another study recruited their subjects from the University of Helsinki, Helsinki area polytechnic schools and Valio Ltd. personnel in Helsinki [21].
Type of probiotic and way of administration
Nine studies did not report on the time between brushing and the use of the probiotic product [44, 55, 59, 60, 65, 66, 68, 69, 71], but the other authors suggest to wait 1 h after administration of the probiotic. One study [58] suggested using the probiotic lozenges after brushing.
Microbiological changes
Mutans streptococci
Tables 3 and 4 of the online appendix show the raw microbiological data. Table 5 in the online appendix shows the post-intervention microbiological results presented as intergroup comparison after treatment and intragroup comparison.
Twelve studies reported a significant reduction in mutans streptococci when a probiotic was used [54, 56, 57, 60–63, 65–67, 70, 71]. A decrease of the mutans streptococci counts could also be observed in one control group [57]. However, in this study, a pre-treatment with a chlorhexidine mouthwash was performed. In contrast, four studies reported no significant differences in mutans streptococci counts [55, 59, 64, 68], albeit one of them [55] described a tendency to reduced counts. No study reported an increase in mutans streptococci numbers when probiotics were used.
In contrast to the intragroup comparisons, the intergroup comparisons were made in only a few studies. Four authors investigated the intergroup comparison only at the beginning of the study [54, 62, 64, 70], to examine whether they start with similar groups concerning the microbiological counts. Three studies found a significant difference at the end of the study between the mutans streptococci counts in the probiotic versus control group [63, 65, 71], this difference was not noticed at baseline. In contrast, six studies could not detect a statistically significant difference, neither at baseline nor at the end of the probiotic usage [21, 56, 58, 59, 67, 68].
Lactobacillus species
With regard to the Lactobacillus counts, the results are even more divergent. One study described decreased lactobacilli counts in one of their two probiotic groups [63]. In contrast, two studies observed a significant increase in lactobacilli counts [57, 68]. Although the majority of studies did not find significant differences in lactobacilli counts between the probiotic group and the control group [54, 55, 61, 62, 64, 66, 67, 70].
Concerning the intergroup comparison, four authors investigated this only at the beginning of the study [54, 62, 66, 70], to examine whether they start with similar groups concerning the microbiological counts. One study found a significant reduction of the Lactobacillus counts in the probiotic group compared with the control group [63]. Four studies could not detect a statistically significant difference, neither at baseline nor at the end of the probiotic usage [21, 58, 67, 68].
Meta-analysis
Of these, twelve articles could be used for a meta-analysis since data could be unambiguously extracted regarding the number of patients having low, medium or high counts of either mutans streptococci or lactobacilli.
The variety with which the available data were presented made it impossible to include all the studies in a meta-analysis. Most studies used a chairside test for evaluating the microbial counts, dividing the patients into groups with low, moderate or high microbial counts. However, the study of Petersson et al. (2011) could not be used, because in this paper, there is only mentioned in how much patients’ mutans streptococci or lactobacilli are detected [55]. Additionally, two studies which used cultivation methods presented their data accordingly to the data obtained with chairside tests and could be included in the meta-analysis [65, 67]. Of the remaining studies that made use of cultivation methods, only from two studies it was possible to unambiguously extract all necessary data as median and standard deviation, which is too little to perform a meaningful meta-analysis.
The results of this meta-analysis are displayed in Table 6 and 7 of the online appendix, respectively, as the intergroup and the intragroup analysis.
This meta-analysis showed that when the number of patients with the highest mutans streptococci counts for the probiotic group was compared before and after treatment, a significant decrease could be observed (RR = 0.37; 95 % CI 0.25 to 0.53). This could not be observed in the control group (RR = 0.86; 95 % CI 0.63 to 1.17). When comparing the number of patients with low mutans streptococci counts before and after intervention in the probiotic group, a significant increase was noted (RR = 1.33; 95 % CI 1.22 to 1.44). In the control group, a similar but more modest effect could be seen (RR = 1.12; 95 % CI 1.02 to 1.22).
Intergroup comparisons showed that when the probiotic and control groups were compared after treatment, significantly more patients in the probiotic groups had low mutans streptococci (<105 CFU/ml) counts (RR = 1.14; 95 % CI 1.06 to 1.23) (Fig. 2) and significantly less patients in the probiotic group had high (>106 CFU/ml) counts (RR = 0.55; 95 % CI 0.37 to 0.82) (Fig. 3). This pronounced significant difference was not present at baseline (respectively, RR = 0.95; 95 % CI 0.87 to 1.05 and RR = 1.35; 95 % CI 1.02 to 1.78).
When the number of patients with high Lactobacillus counts was compared before and after treatment, no significant difference could be noticed (control: RR = 0.71; 95 % CI 0.43 to 1.17 and probiotic: RR = 0.88; 95 % CI 0.59 to 1.31). Also, for the group with the lowest lactobacilli counts, no significant differences could be noted when comparing the number of patients in this group both before and after treatment (control: RR = 0.98; 95 % CI 0.83 to 1.16 and probiotic: RR = 1.13; 95 % CI 0.93 to 1.38).
Differences in low counts (<104 CFU/ml) of lactobacilli are noted when comparing the probiotic with the control group at baseline (RR = 0.80; 95 % CI 0.67 to 0.97); this could not be detected at the end of the treatment (RR = 0.93; 95 % CI 0.78 to 1.10) (Fig. 4). When we compare the probiotic and the control group for the patients with high Lactobacillus (>106 CFU/ml) counts, no statistically significant difference could be observed, neither at baseline (RR = 1.24; 95 % CI 0.82 to 1.87) nor after treatment (RR = 1.70; 95 % CI 1.05 to 2.75) (Fig. 5).
Risk of bias assessment
An evaluation of the risk criteria showed that two studies had a low potential risk of bias [66, 67]. The estimated risk of bias was moderate for ten papers [54–56, 58, 61, 62, 64, 68–70] and high for seven papers [21, 57, 59, 60, 63, 65, 71]. See Table 2.
Discussion
The aim of this review was to evaluate the effect of probiotics in the prevention of caries. Seeing the multitude of factors that play a role in the aetiology of caries, long-term studies with the incidence of caries as primary outcome measure are needed. However, only two articles that met the inclusion criteria reported on the effect of probiotics just after its usage, reporting the incidence of caries as an outcome measure [55, 69]. On the other hand, it became clear from the initial searches that a wide variety of articles reporting on the effects of probiotics assessed the level of caries-associated bacteria, namely mutans streptococci and lactobacilli. This is probably because caries is a relative slow process and probiotics are often used for a relatively short period of time. Lack of funding for long-term clinical trials involving probiotics seems a reasonable explanation. Although caries incidence should be the preferred hard endpoint of such studies, the lack of studies using this endpoint forced us to rephrase our anticipated focused question “What is the impact of probiotics in healthy humans on the incidence of caries in the oral cavity when compared to a placebo” to “What is the impact of probiotics in healthy humans on the level of the surrogate outcome parameters mutans streptococci and lactobacilli counts in the oral cavity when compared to a placebo” in order to obtain a meaningful result. It should be noted that there exists controversy regarding the value of surrogate endpoints, such as mutans streptococci levels, as a predictor for caries. Some studies report a poor correlation between mutans streptococci levels and risk for caries development [72] while others find correlations [73–76].
Nineteen articles were included in the final, descriptive analysis. These studies often utilized small sample sizes, no follow-up and frequently did not describe how randomization and blinding were performed. Additionally, there was a considerable variation in the study parameters, such as used probiotic strain, mode of application, length of the studies and outcome measures. This caused serious restrictions on reviewing the literature in a quantitative way. Twelve articles could be included into the meta-analysis. Because of the way the available data were presented, it was only possible to perform a meta-analysis concerning the groups with the lowest and highest mutans streptococci and lactobacilli counts. For this, we used the results from research conducted with chairside tests and with conventional cultivation methods on selective agar plates. A significant correlation concerning the mutans streptococci and the lactobacilli counts has already been shown for these two methods [77–79]. However, to date, there are more sensitive and specific techniques available, such as qPCR.
Taking the above-mentioned limitations into account, this meta-analysis showed that when comparing the probiotic and control group, significantly more patients in the probiotic group had low mutans streptococci (<105 CFU/ml) counts and significantly less patients had high (>106 CFU/ml) counts. Regarding the Lactobacillus counts, comparing the probiotic and control group at the end of the probiotic use, no significant differences could be observed, neither in low counts (<104 CFU/ml) nor in high Lactobacillus (>106 CFU/ml) counts. The heterogeneity of the used probiotics did not allow a subanalysis concerning the used probiotic strains.
These data suggests that probiotics could have a positive effect in the prevention of caries. These results are in agreement with the three available articles that describe, just after the usage of a probiotic, caries incidence as primary outcome measure. Nase and coworkers (2001) evaluated the children’s oral health according to the WHO criteria [69]. They combined these clinical results with the microbiological findings to develop a caries risk index. They claimed that milk containing Lactobacillus rhamnosus GG reduced the risk of caries significantly. This effect was particularly clear in the group with the 3–4-year-olds. Stecksen-Blicks et al. (2009) evaluated the effect of milk supplemented with L. rhamnosus and fluoride on enamel and dentine caries at the level of the canines and molars [45]. After 21 months, there was a statistically significant difference in caries activity between the two groups, with a preventive fraction of 75 %. Unfortunately, with this study design, one cannot determine if the positive effect is attributable to fluor, the probiotic or the combination of both. Petersson et al. (2011) investigated caries, in particular, root surface caries in older patients [55]. This paper showed that daily milk supplemented with fluoride and/or probiotic bacteria may reverse primary root caries lesions in older adults. The combination of a fluor and a probiotic showed better results than when only one of those two products was administered. However, more long-term studies with caries activity as primary outcome are needed. Besides, it is useful to investigate whether the effect of the probiotic continues after treatment, because it is believed that the effect of the probiotic will disappear when the patient discontinues its use and that the probiotic treatment does not induce a definitive shift towards a less pathogenic microbiota [15]. The currently available literature about the short-term follow-up after probiotic usage is contradicting, in regard to both the mutans streptococci and the Lactobacillus counts several weeks after probiotic therapy. However, it is remarkable that Aminabadi and coworkers (2011), when comparing the group that only received a probiotic with the group that received a probiotic and was pre-treated with a chlorhexidine mouthwash, showed in the latter group significantly lower mutans streptococci counts and increased Lactobacillus counts post-follow-up. This was not the case in the group that solely had the probiotic yoghurt. These results were not confirmed in the study by Keller and coworkers (2012) nor by Burton and coworkers (2013) [58, 59], yet it can be considered useful to remove the established biofilm before using the probiotic since probiotics have difficulties exerting their beneficial effects on an already matured biofilm [80]. On the other hand, two recent long-term studies demonstrated that the use of a probiotic in infancy compared to a placebo or the use of xylitol/sorbitol showed no difference in the occurrence of dental caries few years after the cessation of their usage [47, 81]. Furthermore, their microbiological data support the view that probiotic bacteria are only temporary colonizers, even in young children.
Finally, future studies need to focus on the best way of administration, the used bacteria and the optimal concentration.
Conclusion
Within the limitations of the available data, it may be concluded that probiotics can have a positive effect on reducing the mutans streptococci counts as long as they are being used. This may indicate a possible positive effect of probiotics on the development of caries. There is a need for examining the positive effect of these products with caries development as primary outcome and for determining the most appropriate species, treatment time, the ideal concentration and vehicle.
References
Selwitz RH, Ismail AI, Pitts NB (2007) Dental caries. Lancet 369:51–59. doi:10.1016/S0140-6736(07)60031-2
Michalek SM, Katz J, Childers NK (2001) A vaccine against dental caries: an overview. BioDrugs Clin Immunother Biopharm Gene Ther 15:501–508
Fejerskov O (2004) Changing paradigms in concepts on dental caries: consequences for oral health care. Caries Res 38:182–191. doi:10.1159/000077753
Caufield PW, Li Y, Dasanayake A (2005) Dental caries: an infectious and transmissible disease. Compend Contin Educ Dent Jamesburg NJ 26:10–16
Loesche WJ (1986) Role of Streptococcus mutans in human dental decay. Microbiol Rev 50:353–380
Hajishengallis G, Michalek SM (1999) Current status of a mucosal vaccine against dental caries. Oral Microbiol Immunol 14:1–20
Tanzer JM, Livingston J, Thompson AM (2001) The microbiology of primary dental caries in humans. J Dent Educ 65:1028–1037
Featherstone JD (2000) The science and practice of caries prevention. J Am Dent Assoc 1939 131:887–899
Featherstone JDB (2004) The continuum of dental caries—evidence for a dynamic disease process. J Dent Res 83:C39–C42, Spec No C
Marsh P, Martin MV, Lewis MAO (2009) Oral microbiology. Churchill-Livingston
Anderson MH, Shi W (2006) A probiotic approach to caries management. Pediatr Dent 28:151–153, discussion 192–198
Teughels W, Van Essche M, Sliepen I, Quirynen M (2008) Probiotics and oral healthcare. Periodontol 2000 48:111–147. doi:10.1111/j.1600-0757.2008.00254.x
Meurman JH (2005) Probiotics: do they have a role in oral medicine and dentistry? Eur J Oral Sci 113:188–196. doi:10.1111/j.1600-0722.2005.00191.x
Meurman JH, Stamatova I (2007) Probiotics: contributions to oral health. Oral Dis 13:443–451. doi:10.1111/j.1601-0825.2007.01386.x
Teughels W, Loozen G, Quirynen M (2011) Do probiotics offer opportunities to manipulate the periodontal oral microbiota? J Clin Periodontol 38(Suppl 11):159–177. doi:10.1111/j.1600-051X.2010.01665.x
Choudhari S, Mopagar V (2011) Probiotic way of dental caries prevention. Int. J. Contemp. Dent. 2:
Broekaert IJ, Walker WA (2006) Probiotics and chronic disease. J Clin Gastroenterol 40:270–274
Henker J, Schuster F, Nissler K (2001) Successful treatment of gut-caused halitosis with a suspension of living non-pathogenic Escherichia coli bacteria—a case report. Eur J Pediatr 160:592–594
Burton JP, Wescombe PA, Cadieux PA, Tagg JR (2011) Beneficial microbes for the oral cavity: Time to harness the oral streptococci? Benefic Microbes 2:93–101
Kang M-S, Kim B-G, Chung J et al (2006) Inhibitory effect of Weissella cibariaisolates on the production of volatile sulphur compounds. J Clin Periodontol 33:226–232
Ahola AJ, Yli-Knuuttila H, Suomalainen T et al (2002) Short-term consumption of probiotic-containing cheese and its effect on dental caries risk factors. Arch Oral Biol 47:799–804
Hatakka K, Ahola AJ, Yli-Knuuttila H et al (2007) Probiotics reduce the prevalence of oral candida in the elderly—a randomized controlled trial. J Dent Res 86:125–130
Teughels W, Newman MG, Coucke W et al (2007) Guiding periodontal pocket recolonization: a proof of concept. J Dent Res 86:1078–1082
Nackaerts O, Jacobs R, Quirynen M et al (2008) Replacement therapy for periodontitis: pilot radiographic evaluation in a dog model. J Clin Periodontol 35:1048–1052. doi:10.1111/j.1600-051X.2008.01333.x
Krasse P, Carlsson B, Dahl C et al (2006) Decreased gum bleeding and reduced gingivitis by the probiotic Lactobacillus reuteri. Swed Dent J 30:55–60
Twetman L, Larsen U, Fiehn N-E et al (2009) Coaggregation between probiotic bacteria and caries-associated strains: an in vitro study. Acta Odontol Scand 67:284–288
Shimauchi H, Mayanagi G, Nakaya S et al (2008) Improvement of periodontal condition by probiotics with Lactobacillus salivarius WB21: a randomized, double-blind, placebo-controlled study. J Clin Periodontol 35:897–905. doi:10.1111/j.1600-051X.2008.01306.x
Mayanagi G, Kimura M, Nakaya S et al (2009) Probiotic effects of orally administered Lactobacillus salivarius WB21-containing tablets on periodontopathic bacteria: a double-blinded, placebo-controlled, randomized clinical trial. J Clin Periodontol 36:506–513. doi:10.1111/j.1600-051X.2009.01392.x
Staab B, Eick S, Knöfler G, Jentsch H (2009) The influence of a probiotic milk drink on the development of gingivitis: a pilot study. J Clin Periodontol 36:850–856. doi:10.1111/j.1600-051X.2009.01459.x
Zahradnik RT, Magnusson I, Walker C et al (2009) Preliminary assessment of safety and effectiveness in humans of ProBiora3(trademark) a probiotic mouthwash. J Appl Microbiol 107:682–690
Riccia DND, Bizzini F, Perilli MG et al (2007) Anti-inflammatory effects of Lactobacillus brevis (CD2) on periodontal disease. Oral Dis 13:376–385. doi:10.1111/j.1601-0825.2006.01291.x
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097. doi:10.1371/journal.pmed.1000097
Stamatova I, Meurman JH (2009) Probiotics and periodontal disease. Periodontology 2000 51:141–151. doi:10.1111/j.1600-0757.2009.00305.x
Bonifait L, Chandad F, Grenier D (2009) Probiotics for oral health: myth or reality? J Can Dent Assoc 75:585–590
Twetman S (2012) Are we ready for caries prevention through bacteriotherapy? Braz Oral Res 26(Suppl 1):64–70
Ahn S-J, Lim B-S, Lee S-J (2007) Prevalence of cariogenic streptococci on incisor brackets detected by polymerase chain reaction. Am J Orthod Dentofac Orthop Off Publ Am Assoc Orthod Const Soc Am Board Orthod 131:736–741. doi:10.1016/j.ajodo.2005.06.036
Schulz KF, Altman DG, Moher D (2010) CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol 63:834–840. doi:10.1016/j.jclinepi.2010.02.005
Verhagen AP, de Vet HC, de Bie RA et al (1998) The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol 51:1235–1241
Van der Weijden F, Dell’Acqua F, Slot DE (2009) Alveolar bone dimensional changes of post-extraction sockets in humans: a systematic review. J Clin Periodontol 36:1048–1058. doi:10.1111/j.1600-051X.2009.01482.x
Simark-Mattsson C, Emilson C-G, Håkansson EG et al (2007) Lactobacillus-mediated interference of mutans streptococci in caries-free vs. caries-active subjects. Eur J Oral Sci 115:308–314. doi:10.1111/j.1600-0722.2007.00458.x
Khanafari A, Porgham SH, Ebrahimi MT (2012) Investigation of probiotic chocolate effect on Streptococcus mutans growth inhibition. Jundishapur J Microbiol 5:590–597
Hillman JD, Dzuback AL, Andrews SW (1987) Colonization of the human oral cavity by a Streptococcus mutans mutant producing increased bacteriocin. J Dent Res 66:1092–1094
Glavina D, Gorseta K, Skrinjarić I et al (2012) Effect of LGG yoghurt on Streptococcus mutans and Lactobacillus spp. salivary counts in children. Coll Antropol 36:129–132
Chuang L-C, Huang C-S, Ou-Yang L-W, Lin S-Y (2011) Probiotic Lactobacillus paracasei effect on cariogenic bacterial flora. Clin Oral Investig 15:471–476. doi:10.1007/s00784-010-0423-9
Stecksen-Blicks C, Sjostrom I, Twetman S (2009) Effect of long-term consumption of milk supplemented with probiotic lactobacilli and fluoride on dental caries and general health in preschool children: a cluster-randomized study. Caries Res 43:374–381
Taipale T, Pienihäkkinen K, Salminen S et al (2012) Bifidobacterium animalis subsp. lactis BB-12 administration in early childhood: a randomized clinical trial of effects on oral colonization by mutans streptococci and the probiotic. Caries Res 46:69–77. doi:10.1159/000335567
Taipale T, Pienihäkkinen K, Alanen P et al (2013) Administration of Bifidobacterium animalis subsp. lactis BB-12 in early childhood: a post-trial effect on caries occurrence at four years of age. Caries Res 47:364–372. doi:10.1159/000348424
Cildir SK, Germec D, Sandalli N et al (2009) Reduction of salivary mutans streptococci in orthodontic patients during daily consumption of yoghurt containing probiotic bacteria. Eur J Orthod 31:407–411
Marttinen A, Haukioja A, Karjalainen S et al (2011) Short-term consumption of probiotic lactobacilli has no effect on acid production of supragingival plaque. Clin Oral Investig. doi:10.1007/s00784-011-0584-1
Montalto M, Vastola M, Marigo L et al (2004) Probiotic treatment increases salivary counts of lactobacilli: a double-blind, randomized, controlled study. Digestion 69:53–56. doi:10.1159/000076559
Caglar E, Kuscu OO, Cildir SK et al (2008) A probiotic lozenge administered medical device and its effect on salivary mutans streptococci and lactobacilli. Int J Paediatr Dent Br Paedodontic Soc Int Assoc Dent Child 18:35–39. doi:10.1111/j.1365-263X.2007.00866.x
Lexner MO, Blomqvist S, Dahlén G, Twetman S (2010) Microbiological profiles in saliva and supragingival plaque from caries-active adolescents before and after a short-term daily intake of milk supplemented with probiotic bacteria—a pilot study. Oral Health Prev Dent 8:383–388
Sinkiewicz G, Cronholm S, Ljunggren L et al (2010) Influence of dietary supplementation with Lactobacillus reuteri on the oral flora of healthy subjects. Swed Dent J 34:197–206
Caglar E, Kavaloglu SC, Kuscu OO et al (2007) Effect of chewing gums containing xylitol or probiotic bacteria on salivary mutans streptococci and lactobacilli. Clin Oral Investig 11:425–429. doi:10.1007/s00784-007-0129-9
Petersson LG, Magnusson K, Hakestam U et al (2011) Reversal of primary root caries lesions after daily intake of milk supplemented with fluoride and probiotic lactobacilli in older adults. Acta Odontol Scand 69:321–327. doi:10.3109/00016357.2011.568962
Juneja A, Kakade A (2012) Evaluating the effect of probiotic containing milk on salivary mutans streptococci levels. J Clin Pediatr Dent 37:9–14
Aminabadi NA, Erfanparast L, Ebrahimi A, Oskouei SG (2011) Effect of chlorhexidine pretreatment on the stability of salivary lactobacilli probiotic in six- to twelve-year-old children: a randomized controlled trial. Caries Res 45:148–154. doi:10.1159/000325741
Burton JP, Drummond BK, Chilcott CN et al (2013) The influence of the probiotic Streptococcus salivarius M18 on indices of dental health in children: a randomised double-blind placebo-controlled trial. J Med Microbiol. doi:10.1099/jmm.0.056663-0
Keller MK, Hasslöf P, Dahlén G et al (2012) Probiotic supplements (Lactobacillus reuteri DSM 17938 and ATCC PTA 5289) do not affect regrowth of mutans streptococci after full-mouth disinfection with chlorhexidine: a randomized controlled multicenter trial. Caries Res 46:140–146. doi:10.1159/000337098
Nikawa H, Makihira S, Fukushima H et al (2004) Lactobacillus reuteri in bovine milk fermented decreases the oral carriage of mutans streptococci. Int J Food Microbiol 95:219–223
Caglar E, Sandalli N, Twetman S et al (2005) Effect of yogurt with Bifidobacterium DN-173 010 on salivary mutans streptococci and lactobacilli in young adults. Acta Odontol Scand 63:317–320
Caglar E, Cildir SK, Ergeneli S et al (2006) Salivary mutans streptococci and lactobacilli levels after ingestion of the probiotic bacterium Lactobacillus reuteri ATCC 55730 by straws or tablets. Acta Odontol Scand 64:314–318. doi:10.1080/00016350600801709
Cogulu D, Topaloglu-Ak A, Caglar E et al (2010) Potential effects of a multistrain probiotic-kefir on salivary Streptococcus mutans and Lactobacillus spp. J Dent Sci 5:144–149. doi:10.1016/S1991-7902(10)60021-9
Cildir S, Sandalli N, Alp F, Caglar E (2011) A novel delivery system of probiotic drop and its effect on dental caries risk factors in cleft lip/palate children. Cleft Palate Craniofac J Off Publ Am Cleft Palate Craniofac Assoc. doi:10.1597/10-035
Jindal G, Pandey RK, Agarwal J, Singh M (2011) A comparative evaluation of probiotics on salivary mutans streptococci counts in Indian children. Eur Arch Paediatr Dent Off J Eur Acad Paediatr Dent 12:211–215
Singh RP, Damle SG, Chawla A (2011) Salivary mutans streptococci and lactobacilli modulations in young children on consumption of probiotic ice-cream containing Bifidobacterium lactis Bb12 and Lactobacillus acidophilus La5. Acta Odontol Scand 69:389–394
Mortazavi S, Akhlaghi N (2012) Salivary Streptococcus mutans and Lactobacilli levels following probiotic cheese consumption in adults: a double blind randomized clinical trial. J Res Med Sci 17:57–66
Keller MK, Twetman S (2012) Acid production in dental plaque after exposure to probiotic bacteria. BMC Oral Health. doi:10.1186/1472-6831-12-44
Nase L, Hatakka K, Savilahti E et al (2001) Effect of long-term consumption of a probiotic bacterium, Lactobacillus rhamnosus GG, in milk on dental caries and caries risk in children. Caries Res 35:412–420
Caglar E, Kuscu OO, Selvi Kuvvetli S et al (2008) Short-term effect of ice-cream containing Bifidobacterium lactis Bb-12 on the number of salivary mutans streptococci and lactobacilli. Acta Odontol Scand 66:154–158. doi:10.1080/00016350802089467
Sudhir R, Praveen P, Anantharaj A, Venkataraghavan K (2012) Assessment of the effect of probiotic curd consumption on salivary pH and streptococcus mutans counts. Niger Med J J Niger Med Assoc 53:135–139. doi:10.4103/0300-1652.104382
Caufield PW, Dasanayake AP, Li Y (2001) The antimicrobial approach to caries management. J Dent Educ 65:1091–1095
Aguilera Galaviz LA, Premoli G, Gonzalez A, Rodriguez RA (2005) Caries risk in children: determined by levels of mutans streptococci and Lactobacillus. J Clin Pediatr Dent 29:329–333
Parisotto TM, Steiner-Oliveira C, Silva CM et al (2010) Early childhood caries and mutans streptococci: a systematic review. Oral Health Prev Dent 8:59–70
Thenisch NL, Bachmann LM, Imfeld T et al (2006) Are mutans streptococci detected in preschool children a reliable predictive factor for dental caries risk? A systematic review. Caries Res 40:366–374. doi:10.1159/000094280
Gross EL, Beall CJ, Kutsch SR et al (2012) Beyond Streptococcus mutans: dental caries onset linked to multiple species by 16S rRNA community analysis. PloS One 7:e47722. doi:10.1371/journal.pone.0047722
Tanabe Y, Park JH, Tinanoff N et al (2006) Comparison of chairside microbiological screening systems and conventional selective media in children with and without visible dental caries. Pediatr Dent 28:363–368
Davenport ES, Day S, Hardie JM, Smith JM (1992) A comparison between commercial kits and conventional methods for enumeration of salivary mutans streptococci and lactobacilli. Community Dent Health 9:261–271
Karjalainen S, Söderling E, Pienihäkkinen K (2004) Validation and inter-examiner agreement of mutans streptococci levels in plaque and saliva of 10-year-old children using simple chair-side tests. Acta Odontol Scand 62:153–157. doi:10.1080/00016350410001559
Pham LC, Hoogenkamp MA, Exterkate RAM et al (2011) Effects of Lactobacillus rhamnosus GG on saliva-derived microcosms. Arch Oral Biol 56:136–147
Hasslöf P, West CE, Karlsson Videhult F et al (2013) Early intervention with probiotic Lactobacillus paracasei F19 has no long-term effect on caries experience. Caries Res 47:559–565. doi:10.1159/000350524
Acknowledgments
Funding source
The study was funded by grants of the Katholieke Universiteit Leuven and the Fund for Scientific Research Flanders.
Conflict of interest
The authors declare that they have no conflict of interest. However, Wim Teughels has received grants from the Katholieke Universiteit Leuven, the Fund for Scientific Research Flanders, BioGaia and Pierre Fabre Médicament for studies in the field of oral probiotics and periodontitis.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 108 kb)
Rights and permissions
About this article
Cite this article
Laleman, I., Detailleur, V., Slot, D.E. et al. Probiotics reduce mutans streptococci counts in humans: a systematic review and meta-analysis. Clin Oral Invest 18, 1539–1552 (2014). https://doi.org/10.1007/s00784-014-1228-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-014-1228-z