Abstract
The present study investigated the effect of enriched Artemia with Bacillus subtilis on growth performance, reproductive factors, proximate composition, intestinal microflora, and resistance to Aeromonas hydrophila of ornamental fish, Poecilia latipinna. Using a completely randomized design, the experiment included three groups. The first group was fed with commercial food without any probiotic. The second group was fed with unenriched Artemia, and the last group consumed long-time enriched Artemia with Bacillus subtilis. The bacteria B. subtilis with a density of 1 × 105 CFU mL−1 was added daily to Artemia culture medium. The total microflora and Bacillus subtilis counts were significantly increased in enriched Artemia compared to the unenriched group (P < 0.05). In fish fed groups, growth factors did not show any significant difference (P > 0.05). The maximum relative fecundity (28.65 ± 2.52 egg number g−1), fry production (62.93 ± 4.6 individual per female), and fry survival (70.97 ± 1.56%) obtained in the third group were found to be significantly more than those in the first and the second groups. Moreover, intestinal bacterial count for Bacillus revealed that the higher concentration of bacteria was significantly related to the third group (6.24 ± 0.11 log CFU g−1) (P < 0.05). Maximum protein and fat contents were observed in fish fed with Bacillus-enriched Artemia; however, no significant difference was found between control and unenriched Artemia groups (P > 0.05). The highest amount of ash was observed in fish fed with commercial food without any probiotic (P < 0.05). At the end of the feeding period, each of the three groups along with positive group (oxytetracycline 100 mg kg−1 of commercial food) was exposed to A. hydrophila (BCCM5/LMG3770) bacteria intraperitoneally. Based on the results, the lowest cumulative mortality was significantly found in group three (68.75 ± 3.6%) and positive group (62.5 ± 7.0%) compared to control and unenriched Artemia groups (P < 0.05). Hence, B. subtilis with a concentration of 1 × 105 CFU mL−1 during the period of Artemia culturing can improve the reproductive parameters, intestinal microflora, and resistance to pathogenic bacteria of Poecilia latipinna.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ornamental fish is important for the development of aquaculture in developing countries. Reproduction and cultivation of the ornamental fish species have increased dramatically and have received attention in a number of studies. Considering that the economic value of the ornamental fish is high, to support their sustainability, it is important to study various aspects of ex situ cultivation of broodstock in order to avoid relying on animals in the wild. With over 2500 species, freshwater aquarium fish trade is the biggest section of fish industry. New methods for the cultivation and reproduction of ornamental fish have been investigated worldwide in the recent decades [1].
Health and nutrition of the ornamental fish are of paramount importance in ornamental fish trade. Probiotics are microorganisms enhancing fish health via microbial balance of the host gut [2]. Probiotics have no negative effects [3], using several mechanisms such as production of essential digestive enzymes and vitamins and increase access to minerals, and trace elements [4] play a key role in preventing and controlling pathogens, thereby promoting growth performance, increasing larval survival, enhancing immunity, and improving tolerance as confirmed by findings of many studies [3, 5,6,7,8,9,10]. The Bacillus group is one of the most common probiotics used in aquaculture, which have beneficial impacts on fish [11,12,13] and shrimp [14,15,16]. The role of probiotics in increasing growth in ornamental fish, including Poecilia reticulata, Xiphophorus helleri [11, 17], and Cyprinus carpio koi [18, 19], was investigated by adding B. subtilis to their diets. The use of appropriate live food in the culture of ornamental fish is nutritionally significant. In this regard, Artemia is important due to its size, high nutritional value, non-selective feeding, and its role as carriers in the transfer of various nutrients and bacteria to fish [20]. A number of studies [5, 21] reported that Artemia had the ability to bio-encapsulate (short-time enrichment) bacteria, but this kind of enrichment causes disturbance [22] in the overall nutrition or energy balance in live food. There is also a force-feeding in this type of enrichment in the form of “gorging” in aquaculture. Nonetheless, a long-term enrichment during Artemia culture can be more effective. The selection of probiotic bacteria in the long-term enrichment is important because if the probiotic bacteria show antagonistic relationship with algae, it will not deem to be suitable for nutrition. In order to confirm the hypothesis that the use of the long-term enrichment in growing Artemia can have a positive effect on target animals, the present study examined the influence of long-time enriched Artemia with Bacillus subtilis on the growth performance, reproduction, proximate composition, gastrointestinal microflora, and resistance to Aeromonas hydrophila of ornamental fish Poecilia latipinna.
Materials and Methods
Preparation of Probiotic and Algae
Bacillus subtilis (strain number: IBRC-M 10742) was obtained from the National Center for Genetic and Biological Diseases of Iran and was grown in tryptic soy broth (TSB) medium (Merck, Germany) at 37 °C and aerobic condition in a shaking incubator (160 rpm) [23]. Bacteria number in each bacterial suspension was determined based on colony unit (CFU) per milliliter [24]. To feed Artemia franciscana, the cyst of this Artemia was obtained from Iran Artemia Co., Kerman, Iran, and algae, Dunaliella tertiolecta, was obtained from Artemia and Aquaculture Institute, Urmia, Iran. According to the method proposed by Sorgeloos [25] Valene, culture medium was used to culture algae. The number of algae was counted using a homocytometric lam, and its number per milliliter was determined.
Artemia franciscana Supplementation with Probiotic
Artemia franciscana cysts were hatched through the method suggested by Sorgeloos [25]. Filtered and autoclaved sea water (FASW) with a salinity of 30 ppt, temperature of 28 °C, pH of 8–8.5, light intensity of 2000 lx, and DO of 6 mg L−1 was selected as standard conditions for Artemia culture. According to Coutteau et al. [26] technique, Artemia was fed with 30% algae and 70% wheat bran in growing period (15 days). Based on Niu et al. [27] method, the minimum concentration of B. subtilis having a positive effect on disease response was 1 × 105 CFU mL−1, so during the growth period, B. subtilis with a density of 1 × 105 CFU mL−1 was daily added to Artemia culture tank. After 15 days of feeding Artemia with the mixture of algae at a density of 3 × 106 cell mL−1 and wheat bran, the grown Artemia were harvested for fish feeding and were stored at − 20 °C.
Experimental Design
Using a completely randomized design, three groups were included in this study. The first group was fed with commercial food without any probiotic (T1). The second group was fed with unenriched Artemia (T2), and the last group consumed enriched Artemia with Bacillus subtilis (T3). A total of 400 molly fish, Poecilia latipinna, with an average weight of 1.2 ± 0.2 g were obtained from Urmia Ornamental Fish Reproduction Center. The fish were fed at 4% body weight with the commercial food (Bio-Mar, France, with 46% crude protein and 6% crude lipid) or frozen Artemia (wet weight) for 60 days twice daily. On the 30th and 60th day, nine fish were randomly selected from each treatment and anesthetized with a solution of cloves powder (200 ppm) [28], and their total length and weight were measured by a ruler with a precision of 1 mm and by a digital scale with a precision of 0.001 g, respectively.
Bacterial Counts of Artemia and Fish Intestine
In order to evaluate colonization of bacteria in enriched Artemia and the intestinal tract of fish, the samples were harvested from each treatment. To eliminate the surface bacterial populations, first, the samples were placed in 0.1% benzalkonium chloride solution for 60 s. After homogenization of the Artemia and fish intestine, serial dilutions were prepared at a range of 10−1 to 10−3 with sterile saline solution (NaCl, 0.87 w/v, 1 mol). Under sterile conditions, 0.1 mL from each dilution was transferred to a plate containing tryptic soy agar (TSA) medium [29, 30]. They were incubated at 37 °C for 5 days, and finally, the number of bacteria in each sample was counted according to the log CFU g−1 of intestine or Artemia [31]. After counting colonies, Bacillus subtilis was identified with gram-positive staining and different biochemical tests (e.g., positive catalase, positive citrate, negative indole, positive movement, positive nitrite reduction, positive spores, and variable oxidase) by Holt method [32].
Reproductive Performance
Breeders were identified based on specifications such as abdominal bulge, anal appearance, black and white spots on the abdomen, and specific behavior of the fish. They were transmitted to the maternity ward that had previously been filled with fresh water. During spawning period, born larvae pass from the maternity ward and go down to avoid being eaten by the breeders. After larvae being born, the mother was removed, and the length and weight of the fish and born larvae were recorded.
Several parameters including relative fecundity (total number of born larvae in the experimental period/weight of brood stocks), reproductive efficiency (after 3 days, the number of healthy larvae/all born larvae ×100), larval survival (after a 3-day period, total live larvae/total number of born larvae), and the number of healthy, dead, and deformed larvae were used to determine reproductive performance [33].
Aeromonas hydrophila Challenging Test
After 60 days of feeding with enriched Artemia, experimental infection with strain of A. hydrophila (BCCM5/LMG3770) was induced [28]. Bacteria A. hydrophila were obtained from Belgian Coordinated Collections of Microorganism, Belgium, and cultured for 24 h at 27 °C in BHI medium (Merck, Darmstadt, Germany). A total of 16 fish were selected from each group, and then, 0.1 mL of A. hydrophila with 1.1 × 107 CFU mL−1 (10 times of LD50 concentration) [34] was injected intraperitoneally to the samples in all three groups and the positive group (oxytetracycline 100 mg kg−1 of commercial food). After injection, fish were examined twice daily in aquariums of 30 L for 8 days in terms of mortality and symptoms. The typical signs of infection including irregular hemorrhages, ulcers, and sero-hemorrhagic fluids in abdominal were observed. Water quality parameters were similar to those recorded during the nutritional trial. Daily death was recorded, and bacteriological examination was carried out to confirm infection in dead fish.
Proximate Composition Analysis
According to AOAC [35], body composition of fish fed with different treatments was investigated. The moisture and dry matter content of carcasses were determined using weight difference. By the Kjeldahl method and nitrogen coefficient of 6.25, crude protein was calculated, and the fat content was determined by dissolving fat in ether. The amount of ash by burning fish samples in 600 °C for 2 h was determined.
Statistical Analysis
First, data normalization and homogeneity of variances were investigated using Shapiro-Wilk and Levene’s tests. Then, the data were analyzed through one-way ANOVA, and the difference between means was assessed by Duncan’s test. The minimum significance level for all tests was set to be P < 0.05. SPSS software (version 21) was employed for statistical analysis. Charts were also plotted in the Excel software (version 2013).
Results
Artemia Bacterial Count
The bacterial counts of enriched Artemia and unenriched Artemia are shown in Table 1. The Bacillus subtilis count in enriched Artemia was significantly higher than that of unenriched Artemia (P < 0.05). In addition, the enriched Artemia had significantly higher total bacterial count than unenriched Artemia (P < 0.05).
Growth Factors
The results of survival and length and weight indices of male and female fish are presented in Table 2. Based on statistical analysis, no significant differences were found between groups (P > 0.05). In both males and females, the survival rate in the control group was less than that of the other two groups, and the length gain of male samples was more than that of female ones.
Reproductive Factors
The results of reproductive factor in fish fed with different treatments are illustrated in Table 3. Maximal fry production (62.93 ± 4.6 individual per female), relative fecundity (28.65 ± 2.52 number per weight of female), and fry survival (70.97 ± 1.56%) were found to be significantly higher in fish fed with enriched Artemia with B. subtilis than other groups. About larval indices, no abnormal larvae were observed in group 3, and the dead larvae in this treatment were less than other treatments. However, there was no significant difference among the treatments (P > 0.05).
Intestinal Bacterial Count
The bacterial count of fish intestinal is shown in Table 4. In group 3, the Bacillus count (6.24 ± 0.11 log CFU g−1) significantly was higher than that of others (P < 0.05). Additionally, the highest total bacterial count (7.00 ± 0.02 log CFU g−1) was significantly observed in group 3 (P < 0.05).
Proximate Compositions
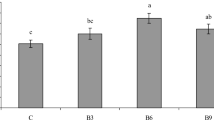
The results of body composition of the fish fed different treatments are presented in Fig. 1. The maximum protein and fat contents were observed in group 3, but no significant difference was found between other treatments (P > 0.05). The highest amount of ash (14%) was significantly observed in the control group (P < 0.05). Fish fed with enriched Artemia with B. subtilis significantly showed the lowest moisture content compared to other groups (P < 0.05).
Fish Mortality in Challenge of A. hydrophila
After injection of A. hydrophila, the symptoms of disease and mortality were recorded for 8 days (Fig. 2). The lowest cumulative mortality (68.75 ± 3.6%) was recorded in group 3 compared to the control group with 81.25 ± 3.6% mortality (P < 0.05). No significant difference was observed in cumulative mortality between group 3 and the positive group (tetracycline) (P > 0.05).
Discussion
Special care must be taken to ensure health and nutrition of fishes including ornamental fish, as this type of fish, in suboptimal conditions or during intensification of aquaculture, tends to overcome opportunistic bacteria. Probiotics are bio-compounds optimizing the colonization of intestine micro-biota in fish and enhancing immunity, survival, and growth performance [3, 5,6,7,8,9,10]. The positive effects of probiotics on growth performance, reproductive factors, and immune response of fish and shellfish species are reported [2, 8, 36,37,38,39,40,41,42,43]. Probiotic bacteria produce components such as fatty acids, vitamins, and enzymes (protease, lipase, and amylase) through stimulating appetite and enhancing microbial metabolism, thereby improving fish feeding [8]. By increasing digestibility, enhancing absorption of ingested food, and having antibacterial properties, probiotics increase feeding efficiency in fish [17].
In this study, 60 days after feeding, no significant differences were observed in fish growth indices among groups. In line with our results, Bacillus pumilus and Bacillus clausii with 108 CFU g−1 of diet had not significant effect on growth indices of Epinephelus coioides after 60 days [44]. Contrariwise, the use of Bacillus subtilis efficiently increased the growth performance of live-bearing ornamental fishes [17]. Furthermore, employing enriched rotifer or Artemia with a combination of Bacillus pumilus, B. subtilis, and B. licheniformis efficiently improved body weight and growth rate in sea bass Sparus auruta [41].
The insignificant effect of probiotics on length and weight of the fish examined in this study can be attributed to the use of fish with a high weight. Nonetheless, employing B. subtilis had no negative influence on the fish growth indices. The impact of B. subtilis probiotic on guppies (Poecilia reticulata and P. sphenops) and swordtail species (Xiphophorus helleri and X. maculatus) had been investigated [17]. An increase in length and weight in treated fish was ascribed to an increased activity of amylase and protease enzymes. In clownfish, Amphiprion ocellaris, larval development was doubled by adding probiotic bacteria to water and live feed compared to control treatment [45].
Due to the non-selective nutritional behavior of Artemia, it has the capability of transmitting probiotics to fish [46,47,48]. In this study, the total microflora and B. subtilis counts in on-growing Artemia with probiotic were increased, indicating the ability to colonize probiotics by Artemia. Hence, by changing the microbial flora of the intestinal tract, probiotic-enriched Artemia increases the growth and survival of target organisms [49]. The intestinal microflora of fish fed with Bacillus-enriched Artemia indicated that Bacillus was effectively colonized in the gastrointestinal tract after 60 days. The number of colonies of Bacillus was highest in the third group. It seems that there was competition between useful bacteria (Bacillus) and harmful ones, and Bacillus succeeded in replacing itself with other microorganisms by killing harmful bacteria by producing bacteriocins components [50]. Additionally, it was reported that using Lactobacillus delbrueckii (probiotic bacteria) led to bacterial colonization in the intestine of sea bass, Dicentrarchus labrax, and T cell production and intestine immune system were improved [51].
The nauplius of Artemia urmiana was found to have a high potential for enrichment with B. subtilis [52]. It was reported that if microorganisms were used for a long time, they could be colonized in the digestive system. For example, by using Bacillus sp. during 20 days, the microflora of the intestine was increased 500 times than normal condition [53].
Based on Table 2, reproductive factors in terms of high number of fry per fish, high number of fry per gram of fish, high fry survival, reduction of abnormal fry, and dead fry in fish fed with Bacillus-enriched Artemia were higher than those in other groups. B vitamins promoting maturation of intestinal secretory cells and synthesizing essential components in the host gut, probiotic bacteria enhance the efficiency of protein and fat conversions in diet of broodstock [11]. Likewise, Ghosh et al. [11] reported that including B. subtilis in ornamental fishes’ diet led to the production of B vitamins, thereby increasing larval production, fecundity, and larval survival and declining the number of dead larvae and abnormal larvae. Furthermore, the gonadosomatic index, fingerling production, and relative fecundity were increased in the ornamental fish, X. helleri, fed with PrimaLac (commercial probiotic containing four species of lactic acid bacteria) [54]. Essential fatty acids, B vitamins, and proteins support oocyte development, high rate of vitellogenesis energy [55], and spawning activities [56] in fish fed with probiotic. Avella et al. [57] indicated that continuous administration of external probiotics affects host development; sexual maturity in Danio rerio was observed by 2 months feeding with Lactobacillus rhamnosus. Munro et al. [58] showed that bacterial flora had an essential role in larval survival, although there may be no relationship between the number of bacteria in the intestine and the survival rate of larvae. Gram-positive bacteria lead to an increased survival, uniformity of size, and high growth of larvae in fish [59]. The synthesis of vitamin B12 and B1 by B. subtilis could be a reason for the reduced abnormal and dead larvae number in the group fed with Bacillus-enriched Artemia. Previous studies had reported the positive effect of thiamine in dropping the number of abnormal fry in Poecilia sphenops, P. reticulata, Xiphophorus helleri, X. maculatus [11], Atlantic salmon [60], and the Pacific salmon [61].
The enhancement of the immune system’s efficiency by probiotics had been reported in various aquatic species. Probiotic bacteria with positive effect on immunological components such as white blood cells (monocytes, lymphocytes, neutrophils, and macrophages) increase immune response [7, 43]. In this study, Bacillus-enriched Artemia reduced about 13% of cumulative mortality after 8 days of exposure to A. hydrophila compared to the control group (Fig. 2). By adding B. subtilis to Artemia culture media, the density of Bacillus group bacteria was increased in fish intestine (Table 3), confirming the presence of this bacterium in the intestinal mucosa. Bacillus group bacteria prevented the establishment of A. hydrophila in the fish body and in turn, reduced mortality when exposed to pathogens. It was stated that successful probiotics were those that have the ability to colonize in the intestinal mucus, to prevent the placement of pathogens, and to clean infected digestive tract [62]. It is indicated that probiotic bacteria secrete compounds that have the potential to inhibit pathogenic bacteria [63]. Gerard et al. [64] recorded the production of a peptide antibiotic by Bacillus sp. and stated that this antibiotic prevents pathogens’ growth.
According to Fig. 1, the highest moisture content was related to the control treatment. In line with findings of this study, using Bacillus in Huso huso diet, Jafaryan et al. [65] showed a significant difference in moisture content among different groups. In contrast, using Saccharomyces cerevisiae in Nile Tilapia, Oreochromis niloticus, Allam [66] did not find a significant difference in moisture content of fish fed with yeast and control treatments. Based on Fig. 1, the percentage of crude protein in Bacillus-enriched Artemia group increased, but the increase was not significant. Tukmechi and Bandboni [9] showed that S. cerevisiae had an effect on increasing the protein content of rainbow trout larvae. Jafaryan et al. [65] found a significant difference in crude protein content of the larvae of fish fed with Bacillus sp. Similarly, Allam [66] reported a significant increase in the amount of crude protein in Nile Tilapia, Oreochromis niloticus, fed with 10% yeast compared to the control group. He attributed protein increase in these fish to a rise in the total serum protein. It also was found that crude protein significantly increased in common carp when fed on yeast [67]. In the present study, the percentage of fat in all groups did not show any significant difference. In line with the results of our study, using 10% and 20% yeast in the Nile Tilapia diet, Allam [66] showed that there was no significant difference in the crude fat content compared to that of the control group.
In conclusion, the use of Bacillus-enriched Artemia in feeding Poecilia latipinna had a positive impact on gastrointestinal tract microflora, reproductive factors, and resistance to A. hydrophila. According to the results of this study, the concentration of 1 × 105 CFU mL−1 of B. subtilis can be used indirectly through Artemia culture to increase productivity and resistance to pathogens in fish.
References
Langroudi HE, Mousavi SH, Falahatkar B, Moradkhani Z (2009) Effect of diets containing artemia enriched with unsaturated fatty acids and vitamin C on angel fish Pterophyllum scalare propagation. Int Aquat Res 1:67–72
Wang Y-B, Li J-R, Lin J (2008) Probiotics in aquaculture: challenges and outlook. Aquaculture 281(1–4):1–4. https://doi.org/10.1016/j.aquaculture.2008.06.002
Merrifield DL, Dimitroglou A, Foey A, Davies SJ, Baker RT, Bøgwald J, Castex M, Ringø E (2010) The current status and future focus of probiotic and prebiotic applications for salmonids. Aquaculture 302(1):1–18. https://doi.org/10.1016/j.aquaculture.2010.02.007
Holzapfel WH, Haberer P, Snel J, Schillinger U (1998) Overview of gut flora and probiotics. Int J Food Microbiol 41(2):85–101. https://doi.org/10.1016/S0168-1605(98)00044-0
Gatesoupe F-J (1994) Lactic acid bacteria increase the resistance of turbot larvae, Scophthalmus maximus, against pathogenic Vibrio. Aquat Living Resour 7(4):277–282. https://doi.org/10.1051/alr:1994030
Balcázar JL, De Blas I, Ruiz-Zarzuela I, Cunningham D, Vendrell D, Muzquiz JL (2006) The role of probiotics in aquaculture. Vet Microbiol 114(3):173–186. https://doi.org/10.1016/j.vetmic.2006.01.009
Nayak S (2010) Probiotics and immunity: a fish perspective. Fish Shellfish Immun 29(1):2–14. https://doi.org/10.1016/j.fsi.2010.02.017
Dimitroglou A, Merrifield DL, Carnevali O, Picchietti S, Avella M, Daniels C, Güroy D, Davies SJ (2011) Microbial manipulations to improve fish health and production–a Mediterranean perspective. Fish Shellfish Immun 30(1):1–16. https://doi.org/10.1016/j.fsi.2010.08.009
Tukmechi A, Bandboni M (2014) Effects of Saccharomyces cerevisiae supplementation on immune response, hematological parameters, body composition and disease resistance in rainbow trout, Oncorhynchus mykiss (Walbaum, 1792). J Appl Ichthyol 30(1):55–61. https://doi.org/10.1111/jai.12314
Azimirad M, Meshkini S, Ahmadifard N, Hoseinifar SH (2016) The effects of feeding with synbiotic (Pediococcus acidilactici and fructooligosaccharide) enriched adult Artemia on skin mucus immune responses, stress resistance, intestinal microbiota and performance of angelfish (Pterophyllum scalare). Fish Shellfish Immun 54:516–522. https://doi.org/10.1016/j.fsi.2016.05.001
Ghosh S, Sinha A, Sahu C (2007) Effect of probiotic on reproductive performance in female livebearing ornamental fish. Aquac Res 38(5):518–526. https://doi.org/10.1111/j.1365-2109.2007.01696.x
González-Félix ML, Gatlin DM III, Urquidez-Bejarano P, de la Reé-Rodríguez C, Duarte-Rodríguez L, Sánchez F, Casas-Reyes A, Yamamoto FY, Ochoa-Leyva A, Perez-Velazquez M (2018) Effects of commercial dietary prebiotic and probiotic supplements on growth, innate immune responses, and intestinal microbiota and histology of Totoaba macdonaldi. Aquaculture 491:239–251. https://doi.org/10.1016/j.aquaculture.2018.03.031
Yi Y, Zhang Z, Zhao F, Liu H, Yu L, Zha J, Wang G (2018) Probiotic potential of Bacillus velezensis JW: antimicrobial activity against fish pathogenic bacteria and immune enhancement effects on Carassius auratus. Fish Shellfish Immun 78:322–330. https://doi.org/10.1016/j.fsi.2018.04.055
Tseng D-Y, Ho P-L, Huang S-Y, Cheng S-C, Shiu Y-L, Chiu C-S, Liu C-H (2009) Enhancement of immunity and disease resistance in the white shrimp, Litopenaeus vannamei, by the probiotic, Bacillus subtilis E20. Fish Shellfish Immun 26(2):339–344. https://doi.org/10.1016/j.fsi.2008.12.003
Liu K-F, Chiu C-H, Shiu Y-L, Cheng W, Liu C-H (2010) Effects of the probiotic, Bacillus subtilis E20, on the survival, development, stress tolerance, and immune status of white shrimp, Litopenaeus vannamei larvae. Fish Shellfish Immun 28(5):837–844. https://doi.org/10.1016/j.fsi.2010.01.012
Balcázar JL, Rojas-Luna T (2007) Inhibitory activity of probiotic Bacillus subtilis UTM 126 against Vibrio species confers protection against vibriosis in juvenile shrimp (Litopenaeus vannamei). Curr Microbiol 55(5):409–412. https://doi.org/10.1007/s00284-007-9000-0
Ghosh S, Sinha A, Sahu C (2008) Dietary probiotic supplementation in growth and health of live-bearing ornamental fishes. Aquac Nutr 14(4):289–299. https://doi.org/10.1111/j.1365-2095.2007.00529.x
Lin S, Mao S, Guan Y, Luo L, Luo L, Pan Y (2012) Effects of dietary chitosan oligosaccharides and Bacillus coagulans on the growth, innate immunity and resistance of koi (Cyprinus carpio koi). Aquaculture 342:36–41. https://doi.org/10.1016/j.aquaculture.2012.02.009
He S, Liu W, Zhou Z, Mao W, Ren P, Marubashi T, Ringø E (2011) Evaluation of probiotic strain Bacillus subtilis C-3102 as a feed supplement for koi carp (Cyprinus carpio). J Aquac Res Dev S1:005:1–7. https://doi.org/10.4172/2155-9546.S4171-4005
Makridis P, Bergh Ø, Skjermo J, Vadstein O (2001) Addition of bacteria bioencapsulated in Artemia metanauplii to a rearing system for halibut larvae. Aquac Int 9(3):225–235. https://doi.org/10.1023/A:1016815929846
Gatesoupe F-J (1991) Managing the dietary value of Artemia for larval turbot, Scophthalmus maximus; the effect of enrichment and distribution techniques. Aquac Eng 10(2):111–119. https://doi.org/10.1016/0144-8609(91)90004-4
Negm RK, Cobcroft JM, Brown MR, Nowak BF, Battaglene SC (2014) Performance and skeletal abnormality of striped trumpeter Latris lineata larvae and post larvae fed vitamin A enriched Artemia. Aquaculture 422:115–123. https://doi.org/10.1016/j.aquaculture.2013.11.008
Soltanian S, Dhont J, Sorgeloos P, Bossier P (2007) Influence of different yeast cell-wall mutants on performance and protection against pathogenic bacteria (Vibrio campbellii) in gnotobiotically-grown Artemia. Fish Shellfish Immun 23(1):141–153. https://doi.org/10.1016/j.fsi.2006.09.013
Gomez-Gil B, Herrera-Vega MA, Abreu-Grobois FA, Roque A (1998) Bioencapsulation of two different Vibrio species in nauplii of the brine shrimp (Artemia franciscana). Appl Environ Microbiol 64(6):2318–2322
Sorgeloos P (1986) Manual for the culture and use of brine shrimp Artemia in aquaculture. State University of Ghent, Faculty of Agriculture, Ghent 319 p
Coutteau P, Lavens P, Sorgeloos P (1990) Baker’s yeast as a potential substitute for live algae in aquaculture diets: Artemia as a case study. J World Aquacult Soc 21(1):1–9. https://doi.org/10.1111/j.1749-7345.1990.tb00947.x
Niu Y, Defoirdt T, Baruah K, Van de Wiele T, Dong S, Bossier P (2014) Bacillus sp. LT3 improves the survival of gnotobiotic brine shrimp (Artemia franciscana) larvae challenged with Vibrio campbellii by enhancing the innate immune response and by decreasing the activity of shrimp-associated vibrios. Vet Microbiol 173(3):279–288. https://doi.org/10.1016/j.vetmic.2014.08.007
Tukmechi A, HRR A, Manaffar R, Sheikhzadeh N (2011) Dietary administration of beta-mercapto-ethanol treated Saccharomyces cerevisiae enhanced the growth, innate immune response and disease resistance of the rainbow trout, Oncorhynchus mykiss. Fish Shellfish Immun 30(3):923–928. https://doi.org/10.1016/j.fsi.2011.01.016
Utiswannakul P, Sangchai S, Rengpipat S (2011) Enhanced growth of black tiger shrimp Penaeus monodon by dietary supplementation with Bacillus (BP11) as a probiotic. J Aquac Res Dev. https://doi.org/10.4172/2155-9546.S4171-4006
Mahious A, Gatesoupe F, Hervi M, Metailler R, Ollevier F (2006) Effect of dietary inulin and oligosaccharides as prebiotics for weaning turbot, Psetta maxima (Linnaeus, C. 1758). Aquac Int 14(3):219. https://doi.org/10.1007/s10499-005-9003-4
Sneath PH (1986) Endospore-forming Gram-positive rods and cocci. In: Bergey’s manual of systemic bacteriology, vol 2. Williams & Wilkins, pp 1104–1207
Cappuccino N, Mackay R, Eisner C (2002) Spread of the invasive alien vine Vincetoxicum rossicum: tradeoffs between seed dispersability and seed quality. Am Midl Nat 148(2):263–270. https://doi.org/10.1674/0003-0031(2002)148[0263:SOTIAV]2.0.CO;2
Chitra G, Krishnaveni N (2013) Effect of probiotics on reproductive performance in female livebearing ornamental fish Poecilia sphenops. Int J Pure Appl Zool 1(3):235–245
Misra CK, Das BK, Mukherjee SC, Pattnaik P (2006) Effect of long term administration of dietary β-glucan on immunity, growth and survival of Labeo rohita fingerlings. Aquaculture 255:82–94. https://doi.org/10.1016/j.aquaculture.2005.12.009
AOAC (1990) In: Horwitz W (ed) Official methods of analyses, 15th edn. Association of Official Analytical Chemists Inc., Arlington 445p
Douillet PA, Langdon CJ (1994) Use of a probiotic for the culture of larvae of the Pacific oyster (Crassostrea gigas Thunberg). Aquaculture 119(1):25–40. https://doi.org/10.1016/0044-8486(94)90441-3
Ghosh K, Sen SK, Ray AK (2003) Supplementation of an isolated fish gut bacterium, Bacillus circulans, in formulated diets for rohu, Labeo rohita, fingerlings. Isr J Aquac 55(1):13–21. http://hdl.handle.net/10524/19065
Carnevali O, Zamponi MC, Sulpizio R, Rollo A, Nardi M, Orpianesi C, Silvi S, Caggiano M, Polzonetti AM, Cresci A (2004) Administration of probiotic strain to improve sea bream wellness during development. Aquac Int 12(4–5):377–386. https://doi.org/10.1023/B:AQUI.0000042141.85977.bb
Abdel-Tawwab M, Abdel-Rahman AM, Ismael NE (2008) Evaluation of commercial live bakers’ yeast, Saccharomyces cerevisiae as a growth and immunity promoter for Fry Nile tilapia, Oreochromis niloticus (L.) challenged in situ with Aeromonas hydrophila. Aquaculture 280(1–4):185–189. https://doi.org/10.1016/j.aquaculture.2008.03.055
El-Rhman AMA, Khattab YA, Shalaby AM (2009) Micrococcus luteus and Pseudomonas species as probiotics for promoting the growth performance and health of Nile tilapia, Oreochromis niloticus. Fish Shellfish Immun 27(2):175–180. https://doi.org/10.1016/j.fsi.2009.03.020
Avella MA, Gioacchini G, Decamp O, Makridis P, Bracciatelli C, Carnevali O (2010a) Application of multi-species of Bacillus in sea bream larviculture. Aquaculture 305(1–4):12–19. https://doi.org/10.1016/j.aquaculture.2010.03.029
Nandi A, Banerjee G, Dan SK, Ghosh K, Ray AK (2018) Evaluation of in vivo probiotic efficiency of Bacillus amyloliquefaciens in Labeo rohita challenged by pathogenic strain of Aeromonas hydrophila MTCC 1739. Probiotics Antimicrob Proteins 10(2):391–398. https://doi.org/10.1007/s12602-017-9310-x
Adorian TJ, Jamali H, Farsani HG, Darvishi P, Hasanpour S, Bagheri T, Roozbehfar R (2018) Effects of probiotic bacteria Bacillus on growth performance, digestive enzyme activity, and hematological parameters of Asian sea bass, Lates calcarifer (Bloch). Probiotics Antimicrob Proteins. https://doi.org/10.1007/s12602-018-9393-z
Sun Y-Z, Yang H-L, Ma R-L, Lin W-Y (2010) Probiotic applications of two dominant gut Bacillus strains with antagonistic activity improved the growth performance and immune responses of grouper Epinephelus coioides. Fish Shellfish Immun 29(5):803–809. https://doi.org/10.1016/j.fsi.2010.07.018
Avella MA, Olivotto I, Silvi S, Place AR, Carnevali O (2010b) Effect of dietary probiotics on clownfish: a molecular approach to define how lactic acid bacteria modulate development in a marine fish. Am J Physiol-Reg 298(2):359–371. https://doi.org/10.1152/ajpregu.00300.2009
Patra S, Mohamed K (2003) Enrichment of Artemia nauplii with the probiotic yeast Saccharomyces boulardii and its resistance against a pathogenic Vibrio. Aquac Int 11(5):505–514. https://doi.org/10.1023/B:AQUI.0000004193.40039.54
Verschuere L, Rombaut G, Huys G, Dhont J, Sorgeloos P, Verstraete W (1999) Microbial control of the culture of Artemia juveniles through preemptive colonization by selected bacterial strains. Appl Environ Microbiol 65(6):2527–2533
Jamali H, Imani A, Abdollahi D, Roozbehfar R, Isari A (2015) Use of probiotic Bacillus spp. in rotifer (Brachionus plicatilis) and Artemia (Artemia urmiana) enrichment: effects on growth and survival of Pacific white shrimp, Litopenaeus vannamei, larvae. Probiotics Antimicrob Prot 7(2):118–125. https://doi.org/10.1007/s12602-015-9189-3
Ringø E, Birkbeck T (1999) Intestinal microflora of fish larvae and fry. Aquac Res 30(2):73–93. https://doi.org/10.1046/j.1365-2109.1999.00302.x
Kesarcodi-Watson A, Kaspar H, Lategan MJ, Gibson L (2008) Probiotics in aquaculture: the need, principles and mechanisms of action and screening processes. Aquaculture 274(1):1–14. https://doi.org/10.1016/j.aquaculture.2007.11.019
Picchietti S, Fausto AM, Randelli E, Carnevali O, Taddei AR, Buonocore F, Scapigliati G, Abelli L (2009) Early treatment with Lactobacillus delbrueckii strain induces an increase in intestinal T-cells and granulocytes and modulates immune-related genes of larval Dicentrarchus labrax (L.). Fish Shellfish Immun 26(3):368–376. https://doi.org/10.1016/j.fsi.2008.10.008
Dehghan M, Jafariyan H, Rezai MH, Amoozagar MA, Sahandi J (2011) Potential of the brine shrimp (Artemia urniana) enrichment with two species of Bacillus and yeast (Saccharomyces cerevisiae). World J Fish Marine Sci 3(6):523–528
Gatesoupe F-J (2008) Updating the importance of lactic acid bacteria in fish farming: natural occurrence and probiotic treatments. J Mol Microbiol Biotechnol 14(1–3):107–114. https://doi.org/10.1159/000106089
Hajibeglou A, Sudagar M (2010) Effect of dietary supplementation with probiotic on reproductive performance of female livebearing ornamental fish. Res J Anim Sci 4(4):103–107
Dahlgren B (1980) The effects of three different dietary protein levels on the fecundity in the guppy, Poecilia reticulata (Peters). J Fish Biol 16(1):83–97. https://doi.org/10.1111/j.1095-8649.1980.tb03688.x
Goldin BR, Gorbach SL (1992) Probiotics for humans. In: Probiotics. Springer, pp 355–376
Avella MA, Place A, Du S-J, Williams E, Silvi S, Zohar Y, Carnevali O (2012) Lactobacillus rhamnosus accelerates zebrafish backbone calcification and gonadal differentiation through effects on the GnRH and IGF systems. PLoS One 7(9):e45572. https://doi.org/10.1371/journal.pone.0045572
Munro P, Barbour A, Blrkbeck T (1994) Comparison of the gut bacterial flora of start-feeding larval turbot reared under different conditions. J Appl Microbiol 77(5):560–566. https://doi.org/10.1111/j.1365-2672.1994.tb04402.x
Kennedy BS, Tucker JW Jr, Neidig CL, Vermeer GK, Cooper VR, Jarrell JL, Sennett DG (1998) Bacterial management strategies for stock enhancement of warmwater marine fish: a case study with common snook (Centropomus undecimalis). B Mar Sci 62(2):573–588
Wooster GA, Bowser PR, Brown SB, Fisher JP (2000) Remediation of Cayuga syndrome in landlocked Atlantic Salmon Salmo salar using egg and sac-fry bath treatments of thiamine-hydrochloride. J World Aquac Soc 31(2):149–157. https://doi.org/10.1111/j.1749-7345.2000.tb00348.x
Hornung M, Miller L, Peterson R, Marcquenski S, Brown S (1998) Efficacy of various treatments conducted on Lake Michigan salmonid embryos in reducing early mortality syndrome. Early life stage mortality syndrome in fishes of the Great Lakes and Baltic Sea. In: McDonald G, Fitzsimons JD, Honeyfield DC (eds) Am Fish Soc. Bethesda. pp 124–134
Vine NG, Leukes WD, Kaiser H (2006) Probiotics in marine larviculture. FEMS Microbiol Rev 30(3):404–427. https://doi.org/10.1111/j.1574-6976.2006.00017.x
Nadella RK, Prakash RR, Dash G, Ramanathan SK, Kuttanappilly LV, Mothadaka MP (2018) Histopathological changes in giant freshwater prawn Macrobrachium rosenbergii (de Man 1879) fed with probiotic Bacillus licheniformis upon challenge with Vibrio alginolyticus. Aquac Res 49(1):81–92. https://doi.org/10.1111/are.13436
Gerard J, Lloyd R, Barsby T, Haden P, Kelly MT, Andersen RJ (1997) Massetolides A– H, antimycobacterial cyclic depsipeptides produced by two pseudomonads isolated from marine habitats. J Nat Prod 60(3):223–229. https://doi.org/10.1021/np9606456
Jafaryan H, Mehdi TM, Mohammad MN (2010) The effects of probiotic bacillus for promotion of growth and feeding parameters in beluga (Huso huso) larvae via feeding by bioencapsulated Artemia. AACL Bioflux 3(4):273–280
Allam HYH (2007) Physiological effects of some additives on growth, blood constituents and immunity in Nile tilapia (Oreochromis niloticus). PhD thesis. Fac of Agric, Assiut Univ, Egypt
Carvalho A, Escaffre A-M, Teles AO, Bergot P (1997) First feeding of common carp larvae on diets with high levels of protein hydrolysates. Aquac Int 5(4):361–367. https://doi.org/10.1023/A:1018368208323
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahmadifard, N., Rezaei Aminlooi, V., Tukmechi, A. et al. Evaluation of the Impacts of Long-Term Enriched Artemia with Bacillus subtilis on Growth Performance, Reproduction, Intestinal Microflora, and Resistance to Aeromonas hydrophila of Ornamental Fish Poecilia latipinna. Probiotics & Antimicro. Prot. 11, 957–965 (2019). https://doi.org/10.1007/s12602-018-9453-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-018-9453-4