Abstract
The aim of the present work was to isolate probiotic bacteria from the intestinal tract of healthy freshwater prawn Macrobrachium rosenbergii and to examine the effect of the isolated probiotic Bacillus vireti 01 in controlling Pseudomonas aeruginosa infection. This is probably the first report on the isolation of probiotic B. vireti 01 from the intestine of M. rosenbergii. The compounds present in B. vireti 01 were identified using GC-MS analysis. The effect of B. vireti 01-incorporated diet on survival and antioxidant enzymes was studied in M. rosenbergii for 2 weeks. Decreased mortality was observed in M. rosenbergii which were administered with the probiotic diet compared to control diet. The antioxidant defence enzymes activities such as SOD, catalase and GSH were analysed in various organs of M. rosenbergii probiotic-treated and control groups. Antioxidant enzyme activities were considerably lowered (p < 0.01) in the muscles, hepatopancreas and gills of prawns infected by P. aeruginosa when compared to that of prawns fed with the probiotic-supplemented diet. The histopathological results suggest that the hepatopancreas, gills and muscles infected with P. aeruginosa were altered structurally. The result of the present work demonstrates that the probiotic B. vireti 01 could be used as a substitute to antibiotics for treating P. aeruginosa infection in prawns.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For the past few years, aquaculture has turned into the quickest developing food-producing sector and has a significant role in supplementing economic development and global food supply [1]. The culture of giant freshwater prawn Macrobrachium rosenbergii known as scampi is rapidly expanding in many countries. In India, M. rosenbergii is one of the most important commercial crustaceans as it has high demand in the domestic and export markets [2, 3]. One of the biggest problems in prawn hatcheries is the existence of infectious diseases of bacterial and viral origins which negatively influence prawn production. Pseudomonas aeruginosa is a Gram-negative rod-shaped bacterium which causes infections in both fish and crustacean species. Several species of the genus Pseudomonas have been reported to be opportunistic pathogens causing diseases in the host when subjected to stress [4]. P. aeruginosa was identified as harmful to M. rosenbergii [5]. Prakash and Karmagam [6] isolated the bacterial strain P. aeruginosa from M. rosenbergii farms of two different places namely Marakanam and Nelakarai (place 1) and Kanchipuram (place 2), and the percentages of infection were found to be 18.3 and 30%, respectively.
The use of antibiotics was the first line of treatment for bacterial diseases and also for maintenance of a healthy environment in hatcheries and prawn culture. An undesirable and unavoidable consequence of using antibiotics for treating bacterial infection may be the development of resistance to different antibiotics [7]. Frappaolo et al. [8] reported that the resistance developed was transferred to the pathogenic organism. This promoted a decrease in the effectiveness of antibiotic treatment. To improve and maintain a healthy environment for aquaculture to flourish, the use of probiotics has proved to be a great boon. Probiotics are microorganisms that can be used to increase the immune response, intestinal microbial balance and growth performance and also to prevent the entry of pathogens [9]. Prawn and shrimp possess non-specific immunological response. It is precisely because of this reason that the treatment of bacterial infection using probiotics might be a better alternative to give a broader spectrum and non-specific disease protection through immune modulation [10].
Probiotic bacteria have an effect on aquaculture production. The mechanism by which it works is not known. There are no reports on the exact mode of action of probiotic bacteria in vivo. Antagonistic compounds of chemical substances produced by microorganisms are toxic or inhibitory to microbes. The presence of bacteria-producing antibacterial compounds can inhibit the proliferation of pathogenic bacteria. A probable explanation for the curative effect of probiotic microorganism may be that the dietary supplementation of a probiotic with antioxidant and antagonistic properties might prevent the animal from oxidative stress caused by the pathogenic bacteria [11].
Every animal needs O2 for the generation of energy and also for the oxidation of food. Throughout this process, O2 undergoes tetravalent reduction to water. Partial reduction of O2 leads to the formation of reactive oxygen species (ROS), like superoxide anion (O2), hydrogen peroxides (H2O2) and hydroxyl radicals (OH•) which may cause cell damage. In normal cells, ROS production is detoxified by antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT) and reduced glutathione (GSH) [12]. Treating the aquatic animals with probiotic bacteria might help to reduce or even eliminate toxicity of ROS production by increasing or maintaining the antioxidants in the animals [13].
The aim of the current work was to isolate and identify probiotic bacteria from the intestine of M. rosenbergii and to study its effects on antioxidant defence system of M. rosenbergii when challenged with P. aeruginosa.
Materials and Methods
Collection of Experimental Animals
Healthy adult freshwater prawns M. rosenbergii with an average weight of 28 ± 3 g were procured from a commercial farm in Chengalpattu, India. The prawns which were collected were brought to the laboratory in live condition and were acclimatized under aerated conditions for 15 days at 27–28 °C. The collected animals were fed twice a day with commercial pellet feed (Taiyo Feed Mill Pvt. Ltd).
Isolation of Intestinal Bacteria
The intestinal microbes were isolated from the intestines of three healthy M. rosenbergii following the procedure of Jafari et al. [14]. They were first euthanized by immersion in tricainemethanesulfonate (MS-222). The intestines of the collected prawns were dissected under aseptic condition and washed thrice with normal saline (NaCl 0.85% w/v). They were then cut into small pieces and homogenized in a surface-sterilized mortar and pestle using 5 ml of 0.85% sterile saline solution [15]. The homogenized samples were then serially diluted (tenfold) with sterile saline solution. A diluted sample of 0.1 ml was spread aseptically on sterilized Tryptic soya agar (Himedia) followed by incubation at 37 °C for 48 h. The bacterial colonies observed over Tryptic soya agar plates were maintained under refrigeration at a temperature of 4 °C for further studies.
Pathogens Used To Study In Vitro Activity
Four bacterial pathogens, Vibrio parahaemolyticus (MTCC451), Aeromonas salmonicida (NCTC10408), Pseudomonas aeruginosa (MTCC1688) and Citrobacter freundii (MTCC 6736) were used to study the inhibitory activity of the isolated intestinal bacteria.
In Vitro Activity by Well Diffusion Method
Inhibition of the pathogenic bacterium by the four isolated intestinal bacteria was tested by well diffusion assay [16]. Briefly, 100 μl of different pathogenic strains V. parahaemolyticus, A. salmonicida, P. aeruginosa and C. freundii (108 CFU/ml) were swabbed on Muller-Hinton agar (Himedia) plate. A well of 7 mm diameter was made over the agar surface, and it was filled with 50 μl of a cell-free supernatant of the four isolated intestinal bacterial strains. Cell-free supernatants of the isolated intestinal bacterial strains were prepared by centrifugation at 8000 rpm for 10 min. After addition, the plates were incubated at 37 °C for 48 h and the diameter of inhibition zones formed around the well was measured [17].
Identification of Isolated Intestinal Bacteria
The morphological and biochemical characteristics of the intestinal bacteria which showed zone of inhibition were studied using Bergey’s Manual of Systematic Bacteriology, Eighth Edition [18]. Preliminary identification of the bacteria isolated was based on this. The isolated bacterium was further confirmed by 16S ribosomal RNA (rRNA) sequence analysis. The gene encoding the 16S rRNA sequence was amplified using gene-specific forward and reverse primers 5′ TACGCATTTCACCGCTAC 3′ and 5′GGCTTCGGCTATCACTTAC 3′, respectively. The primer was designed using Primer Premier Software, and the expected size of amplicon was 497 bp.
Identification of Compounds by Gas Chromatograpy-Mass Spectrometry Analysis
Twenty-five millilitres of the isolate which showed zone of inhibition against the pathogen was cultured at conc. 108 CFU/ml and enabled to grow in nutrient broth till its exponential stage. The bacterial culture was then spun at 8000 rpm for 10 min in a cooling centrifuge. The supernatant was collected and lyophilised to remove the moisture content to obtain a dried pellet. It was then taken for GC-MS analysis. The Clarus 680 GC which was used in the analysis was used as a fused silica column, packed with Elite-5MS (5% biphenyl/95% dimethylpolysiloxane, 30 m × 0.25 mm ID × 250 μm df). The components were separated using helium as a carrier gas at a constant flow of 1 ml/min. The injector temperature was set at 260 °C during the chromatographic run. One microlitre of the sample was injected to the column. The temperature of the oven was set at 60 °C for 2 min. It was then increased to 300 °C at the rate of 10 °C/min and 300 °C, where it was held for 6 min. The mass detector conditions were as follows: transfer line temperature 240 °C; ion source temperature 240 °C; and ionization mode electron impact at 70 eV, scan time 0.2 s and scan interval 0.1 s. The final results were compared with the database of spectrum of known components stored in the GC-MS NIST (2008) library [19].
Virulence Factor
The phenotypic test was performed to study the safety characteristics of isolated intestinal bacteria. An intestinal bacterium was used to evaluate the production of virulence factors like protease production and lipase production following the method of Barbosa et al. [20]. Results were interpreted by observing the clear zone around the colonies.
Biotoxicity Test of Intestinal Bacteria
Twenty microlitres (108 CFU/ml) of isolated intestinal strain was intraperitoneally injected into five prawns with an average weight of 25 ± 2 g to evaluate the toxic effect of isolated probiotic B. vireti 01. The same volume of sterile saline (0.85% NaCl) was injected into a control group. The experiments were conducted in triplicates. Prawns were maintained at a temperature of 23–27 °C with continuous aeration. They were monitored daily for 10 days after intraperitoneal injection to observe mortality rate and also to detect clinical signs [17].
Bacterial Pathogenicity
Pathogenicity was tested according to the modified method of Thanigaivel et al. [21] and Thomas et al. [22]. Two groups of animals were taken to study the pathogenicity of the bacterial strain P. aeruginosa in healthy freshwater prawns. One group of animals (nine prawns) was injected with 30 μl of bacterial suspension of P. aeruginosa (108 cells/ml) into the abdominal segment of the prawns. The bacterial suspension was prepared by centrifugation at 7000 rpm for 10 min and washed twice with sterile PBS. Another group of animals (nine prawns) was used as control which was injected with sterile PBS buffer. The mortality rate and symptoms were recorded daily.
Diet Preparation
The method of Rengpipat et al. [10] was followed. The intestinal bacterial strain was harvested by spinning at 6000 rpm for 10 min at 4 °C in a cooling centrifuge. It was then washed with sterile saline 0.9% (w/v). The suspension was then resuspended in sterile saline to obtain a final concentration of 108 cells/ml. B. vireti 01 probiotic suspension (108 cells/ml) was then mixed thoroughly with the normal diet. After mixing with the normal diet, it was made into a pellet, which was further used for the experiments.
Experimental Design and Bacterial Challenge Study
Healthy adult prawns were acclimatized in fresh water for 1 week before the experiment by feeding with commercial feed. Prawns were distributed into nine tanks, each tank containing five prawns with 15,000 ml water. The prawns in three tanks were fed a commercial diet (group 1), prawns in another three tanks were fed with probiotic-supplemented diet (group 2) and prawns in the remaining three tanks were used as a control group and fed with commercial diet during the entire experimental period. Prawns were fed twice a day at the rate of 6% body weight per day for 2 weeks.
After 2 weeks of feeding experiment, prawns in group 1 and group 2 fed with commercial feed and probiotic-supplemented feed, respectively, were intraperitoneally challenged with 30 μl of bacterial suspension of P. aeruginosa (108 CFU/ml), whereas the control group was not challenged with P. aeruginosa. Mortality rates were then recorded each day for 14 days following the injection. The guts of the prawns were collected to enumerate the total amount of P. aeruginosa and B. vireti 01 after the challenge with P. aeruginosa (group 1 and group 2) by growing on Muller-Hinton agar plates [23].
In Vivo Antioxidant Enzyme Activity
Hepatopancreas, gills and muscles were excised from the two groups of animals (one fed with normal diet and the other fed with probiotic-supplemented diet) challenged with P. aeruginosa. Prawns not challenged with P. aeruginosa were used as a control group. The group of animals supplemented with normal feed and then challenged with P. aeruginosa and the group supplemented with probiotic feed and then challenged with P. aeruginosa are hereafter mentioned as the infected and treated groups, respectively. Hepatopancreas, gills and muscles were excised from survived and dead animals of control, infected and treated groups and homogenized in phosphate buffer using pestle and mortar. The homogenates of different organs were then centrifuged at 6000 rpm for 10 min at 4 °C and the supernatants obtained were stored at − 80 °C for further analysis. The levels of the different antioxidant enzymes such as protein, superoxide dismutase (SOD), catalase (CAT) and reduced glutathione (GSH) were measured.
Protein Estimation
The Bradford method [24] was used to determine the protein concentration in different groups. Bovine serum albumin was used as a standard.
SOD Assay
SOD was calculated according to the method of Kono [25]. The reaction mixture containing 1.3 ml of 50 mM sodium carbonate buffer (pH 10.0), 500 μl of 96 μM nitro blue tetrazolium (NBT) and 100 μl of Triton X-100 (0.6%) was taken. Then, 100 μl of 20 mM hydroxylamine hydrochloride (pH 6.0) was added to initiate the reaction. The enzyme extract of 70 μl was added after 2 min. The percentage of inhibition was recorded as an increase in absorbance at 540 nm. Formation of the purple colour was used as an indicator of NBT reduction by superoxide radicals.
Catalase Assay
Catalase activity was determined by using a UV spectrophotometer according to the method of Xu et al. [26]. Ten microlitres of the sample was added to 3.0 ml of H2O2 prepared in phosphate buffer, pH 7.0 at 25 °C. The change in H2O2 absorbance in 60 s was measured with a UV-220 spectrophotometer at 250 nm and expressed in units of enzyme activities per gramme of tissue wet weight.
Reduced Glutathione Assay
The procedure of Ellman [27] was followed to evaluate the reduced GSH level. The homogenates were precipitated by the addition of an equal volume of 5% trichloroacetic acid (TCA). The mixture was then allowed to stand for 5 min and thereafter centrifuged for 10 min at 6000 rpm. Two hundred microlitres of the supernatant was collected and transferred to fresh tubes. Then, 1.8 ml of the Ellman’s reagent (5, 5′-dithio bis2-nitrobenzoic acid (0.1 mM) prepared in 0.3 M phosphate buffer with 1% of sodium citrate solution) was added. All the test tubes were then made up to 2 ml. After the completion of the total reaction, the absorbance of the solution was measured at 412 nm against that of the blank. The sample absorbance was compared with a standard curve generated from known GSH.
Histopathology Analysis
Histopathological analysis of the hepatopancreas, gills and muscles of M. rosenbergii was carried out for both treated and infected groups. Both groups were compared with the control group that was not challenged with P. aeruginosa. A small portion of different organs was taken and fixed in 10% formalin solution for histopathology studies. Samples were then stained with haematoxylin and eosin (H and E).
Statistical Analysis
The results were analysed and data are expressed as the mean ± standard error (S.E). Statistical analyses were done on all the data using two-way ANOVA. A value of p < 0.01 was considered to be statistically significant. Percentage survival of prawns challenged with the bacterial pathogen was analysed using Kaplan-Meier survival analysis [28].
Results
In Vitro Activity of Isolated Intestinal Bacteria
A total of four different bacterial isolates was obtained from the intestines of the prawns. Among the four isolates, one bacterial isolate showed a zone of inhibition against P. aeruginosa. This was chosen for further investigation due to its antibacterial activity. The supernatant of the isolated strain showed an inhibition zone of 4 mm diameter against P. aeruginosa. No zone of inhibition was observed for the isolated strain against other pathogens, namely, V. parahaemolyticus, A. salmonicida and C. freundii.
Identification of Intestinal Bacteria
Based on the morphological and biochemical characteristics, the isolated bacteria were identified as Bacillus sp. Morphological characteristics revealed that the isolated bacterium was Gram positive and motile and had short rods. The isolated intestinal bacteria were further confirmed by 16S rRNA gene sequencing as B. vireti 01-like organisms with 99% identity. The GenBank accession number of the identified strain is KU601617. The PCR result revealed an amplification of 497 bp (data not shown).
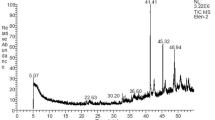
Identification of Compounds by GC-MS Analysis
The culture supernatant of B. vireti 01 grown in nutrient broth was subjected to GC-MS analysis to identify the compounds present in the strain. From the result of GC-MS analysis, major peaks were detected and the compounds present in the peak were identified using a Wiley library. The activities of the compounds, molecular formula and molecular weight are listed in Table 1. Gas chromatography-mass spectrum analysis of the B. vireti 01 probiotic showed major peaks for pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-, which is an efficient antimicrobial agent, and 9-octadecenamide, (Z)-, which is an antioxidant and antimicrobial agent.
Virulence Factor
B. vireti 01 was used to evaluate the production of protease and lipase activity. A clear zone was seen around the colonies (data not shown). This indicates that the isolated intestinal bacterium has the ability to produce digestive enzymes such as protease and lipase.
Biotoxicity Test of Intestinal Bacteria
Clinical symptoms or mortalities were not observed following injection of B. vireti 01 isolated from the intestine. This confirms the non-pathogenicity of the isolated probiotic strain and its safety when injected intraperitoneally into tested freshwater prawns.
Bacterial Pathogenicity
In the present study, M. rosenbergii, which were intraperitoneally injected with P. aeroginosa, exhibited clinical signs such as reddish lesion at the uropod, darkening of the dorsal portion and black spots over the head region. The internal organs such as the gills, hepatopancreas and muscles were damaged. The rate of mortality was also very high in Pseudomonas-infected prawns (90%).
Bacterial Challenge Study
After 2 weeks of the feeding experiment, the percentage of survival was observed in both groups of prawns challenged with P. aeruginosa for 14 days (Fig. 1). Mortality was observed in prawns which were not treated with the isolated probiotic (group 1), whereas in unchallenged control groups, there was mortality in the case of one animal only. The mortality rate was found to be reduced in prawns treated with the probiotic B. vireti 01 (group 2). The probiotic strain was found to be effective in preventing the development of P. aeruginosa infection in M. rosenbergii. The survival percentage was found to be 70% in groups fed with the probiotic-supplemented diet (group 2), whereas survival percentage was found to be 10% in groups fed the commercial diet (group 1). In the P. aeruginosa-challenged group of animals (group 1) fed with commercial diet, 8.3 × 105 CFU/ml of P. aeruginosa was found in the dead animals, whereas in the probiotic-treated group (group 2), only 0.8 × 105 CFU/ml of P. aeruginosa was observed. In the probiotic-treated group, 16.3 × 105 CFU/ml of B. vireti 01 was found in the gut.
Survival of prawns following administration of P. aeruginosa. Control group: prawns fed with commercial diet and unchallenged with P. aeruginosa. Group 1: prawns fed with commercial diet and challenged with P. aeruginosa. Group 2: prawns fed with probiotic-supplemented diet and challenged with P. aeruginosa
In Vivo Antioxidant Activity
The antioxidant enzyme activity of the muscles, hepatopancreas and gills of the control, pathogen-infected and probiotic-treated prawns is illustrated in Fig. 2.
(a) Protein concentration, super oxide dismutase (SOD) activity, catalase (CAT) activity and reduced glutathione (GSH) activity in different organs of M. rosenbergii control, infected and treated groups. In the figures (a, b, c and d) α - indicates significant difference between the control and treated groups of prawns. β- Indicates significant difference between the control and infected groups of prawns. ns- Indicates no significant difference. In the figure, Control represents prawns with commercial diet which was not challenged with Infected represents prawns (group 1) fed with commercial diet and challenged with P. aeruginosa. Treated represents prawns (group 2) fed with probiotic-supplemented diet and challenged with P. aeruginosa
Protein Estimation
Protein concentration was found to be significantly very low in infected groups when compared to that in probiotic-treated and control groups. The concentration of protein present in control, infected and treated groups is shown in Fig. 2a.
SOD Assay
In the present study, the activity of SOD in response to probiotic treatment was analysed in the hepatopancreas, gills and muscles of the prawns based on the percentage of inhibition. In the hepatopancreas and muscles, the activity of SOD was found to be significantly decreased (p < 0.01) with respect to that of the control. Similarly, SOD activity was decreased in the gills of the infected prawns, whereas, it showed statistically no significant changes (p > 0.01) in the gills of the treated prawns. When SOD activity was compared in the infected and the treated prawn organs, it showed an increase in the prawns fed with probiotic-supplemented diet. The SOD activity in different organs of control, infected and treated groups is shown in Fig. 2b.
Catalase Assay
The activity of catalase enzyme in the muscles, hepatopancreas and gills of the control, infected and treated group is shown in Fig. 2c. The catalase activity was significantly very high (p < 0.01) in the muscles, hepatopancreas and gills of the prawns which were fed with the probiotic diet (24.77, 35.09 and 34.40 units/min/g, respectively). However, in prawns fed with a normal diet, the catalase activity was found to be 11.35 ± 0.34, 16.51 and 4.12 units/mins/g, respectively, in the hepatopancreas, gills and muscles. Catalase activity was found to be very low in the hepatopancreas, gills and muscles of the prawns infected by P. aeruginosa. The administration of probiotic-supplemented diet increased the catalase enzyme activity in M. rosenbergii more than the normal diet.
Reduced Glutathione Assay
The GSH level in the control, infected and treated groups of different organs is shown in Fig. 2d. Prawns fed with the B. vireti 01-supplemented diet and normal diet showed enhanced GSH level than infected prawns. GSH activity was found to be 0.0187 ± 0.0002, 0.0213 ± 0.0002 and 0.0213 ± 0.0006 units/min/g in the muscles, hepatopancreas and gills of the infected groups, respectively. GSH activity was found to be increased in the hepatopancreas, gills and muscles of the treated group (0.0234 ± 0.0006, 0.0250 ± 0.0004 and 0.0219 ± 0.0008 units/min/g, respectively).
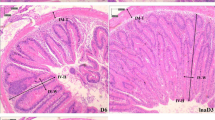
Histopathological Analysis
Histopathological changes in the hepatopancreas, gills and muscles of the treated, infected and control groups are shown in Fig. 3. In the gills, a uniform arrangement of lamellae with interlamellar spaces was observed in control prawns. In the infected gills, damaged and fused lamellae were observed (Fig. 3a–c). The hepatopancreas of the infected prawns exhibited a disrupted epithelial lining, appearance of a large number of vacuoles in the tubular epithelial cells and abnormal lumen. Control showed the normal architecture of tubule and lumen. In the prawns treated with probiotic, normal architecture of hepatopancreas tubule and lumen was seen (Fig. 3d, e, f). The muscles of control prawns and treated prawns showed a normal skeletal muscle structure (muscle fibre) whereas infected muscles showed muscle necrosis and haemocytic infiltration (Fig. 3g, h, i).
Discussion
In the present study, we have isolated B. vireti 01 from the intestine of the freshwater prawn M. rosenbergii which showed antibacterial activity against P. aeruginosa. The isolated strain was confirmed as B. vireti 01 by 16S rRNA gene sequencing with 99% identity. There are only a few reports on the use of B. vireti 01. Heyrman et al. [29] isolated the strain B. vireti from the soil of hay fields, in an agricultural research area (The Netherlands). Mohandass et al. [30] also isolated B. cereus and B. vireti strains from the soil around a petrochemical industry which were reported to biodegrade benzo[a]pyrene. Based on our literature survey, we find that there are no previous reports on the isolation of B. vireti 01 from the intestine of freshwater M. rosenbergii. Hence, this could probably be the first report on the isolation of B. vireti 01 from the intestine of M. rosenbergii.
The GC-MS analysis showed that pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-, 9-octadecenamide, (Z)-, cis-vaccenic acid, D-leucine and 2,5-piperazinedione, 3,6-bis(2-methylpropyl) were present in the strain of B. vireti 01. Among these, the major compounds present in B. vireti 01 were pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro- and 9-octadecenamide, (Z). Both the compounds were found to exhibit antimicrobial activity. The other two compounds, 2, 5-piperazinedione and 3, 6-bis (2-methylpropyl), exhibited antibacterial activity. The work of Gopi et al. [31] suggests that the compound isolated from Bacillus species pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro- could be effectively used as a potential antioxidant to reduce the oxidative damages induced by oxygen radicals.
B. vireti 01 did not cause any significant mortality when injected intraperitoneally into healthy prawns. The results of antimicrobial tests confirm that the isolated probiotic strain is effective (4 mm) in inhibiting the pathogen P. aeruginosa in in vitro condition. Phenotypic and pathogenicity studies proved that isolated B. vireti 01 are safe and harmless when added to prawn feed. This suggests that this probiotic can be used for the treatment of Pseudomonas infection in M. rosenbergii.
In the present study, M. rosenbergii injected with P. aeruginosa exhibited clinical signs such as reddish lesion at the uropod and darkening of the dorsal portion. The internal organs such as the gills, hepatopancreas and muscles were also damaged. Ramalingam and Ramarani [32] studied the pathogenicity of P. aeruginosa in M. rosenbergii and observed clinical symptoms such as the appearance of translucent abdominal musculature, slight darkening of the dorsal portion and presence of black spots on the uropod. These results agree with our observations. Thomas et al. [33] stated that P. aeruginosa causes infection in Oreochromis mossambicus and observed symptoms of scale loss, fin erosion and signs of septicaemia.
The mortality of the freshwater prawns challenged with P. aeruginosa was significantly reduced (p < 0.0001) when supplemented with the probiotic-supplemented diet. Balcázar et al. [34] found that an adult shrimp Litopenaeus vannamei when fed with a diet supplemented with probiotic strains isolated from the intestinal tract of adult shrimp indicated effectiveness in reducing disease caused by Vibrio parahaemolyticus. Chai et al. [35] observed significantly higher survival in the shrimp L. vannamei fed with probiotic Bacillus PC465 diet when compared with those fed on control diet. Chiu et al. [36] also observed a similar survival improvement when the probiotic Lactobacillus plantarum was administered to L. vannamei challenged with Vibrio alginolyticus. Our results which confirmed that B. vireti 01 increased the rate of survival and resistance of M. rosenbergii when infected with P. aeruginosa agree with the reports of the workers mentioned above who have recorded similar findings with other probiotic bacteria.
Under normal physiological status, the antioxidant defence systems together with SOD, catalase and GSH can be affected by way of a slight oxidative stress as a compensatory response. Thus, the ROS should be considered to be responsible for protecting the organisms from oxidative damage [37].
The hepatopancreas, gills and muscles of M. rosenbergii infected with P. aeruginosa showed a decrease in the antioxidant enzymes such as SOD, catalase and GSH activity indicating that the bacterial infection induces the mobilization of the antioxidant defence system and oxidative stress development leading to oxidative damage to tissues [38]. Mathew et al. [39] found a decrease in the antioxidant enzyme activities of SOD, catalase, glutathione peroxidise (Gpx) and glutathione S-transferase (GST) in the digestive gland, muscles and haemolymph of Penaeus monodon following white spot syndrome virus (WSSV) infection.
The major antioxidant defence enzyme superoxide dismutase generated detoxifies toxic superoxide anion radicals. In this study, the SOD activity was considerably lowered in the muscles, hepatopancreas and gills of Pseudomonas-infected prawns when compared to control and treated prawns. Sarathi et al. [40] and Mohankumar and Ramasamy [41] observed that the activity of SOD was considerably lowered in WSSV-infected Fenneropenaeus indicus when compared with that in control animals. Raghu et al. [42] observed enhanced level of SOD and catalase activity in different organs of P. monodon supplemented with two probiotic bacteria Bacillus coagulans and B. firmus.
Catalase is the major primary antioxidant defence component which is present in the cells. It is responsible for the catalytic decomposition of H2O2, which is toxic to the cells. The H2O2 in turn converts to oxygen and water molecules. In this work, catalase activity in prawns fed with probiotic-supplemented diet was enhanced when compared to that in the infected and control groups. Catalase activity was higher in the hepatopancreas of a probiotic-treated group than that in the gills and muscles. Borković et al. [43] also reported that CAT activities were greater in the hepatopancreas compared to those in the gills and muscles. Kumar et al. [44] reported that shrimps fed with a probiotic-incorporated diet such as Bacillus subtilis and Lactobacillus rhamnosus showed maximum catalase activity than those fed with the control diet. Zhang et al. [45] observed that supplementation of probiotic Bacillus licheniformis significantly increased the antioxidant activities such as SOD and CAT in both liver and plasma of triangular bream.
Reduced GSH is the important antioxidant enzyme associated with its metabolism. It provides a significant defence against ROS-induced cellular damage [46]. GSH interacts with ROS and acts as a chain-breaking antioxidant [47]. Our results showed that GSH level was decreased in the hepatopancreas, gills and muscles of prawns infected with P. aeruginosa as compared to that of control and probiotic-supplemented prawns. Mohankumar and Ramasamy [41] found a significant reduction in the activities of reduced glutathione in the haemolymph, hepatopancreas, gills and muscles of WSSV-infected F. indicus.
From the histopathological results, we concluded that, in the infected groups, the muscles, hepatopancreas and gills underwent structural changes and damages. In probiotic-treated groups, in the muscles, hepatopancreas and gills, structural changes were restored to near normal. This preliminary result indicates that isolated probiotic B. vireti 01 has antibacterial activity and that it can be effectively used to treat infection in prawns. Thomas et al. [22] observed the histological changes in the gill, liver and skin of O. mossambicus treated with lime oil nanoemulsion against the P. aeruginosa infection.
The probable mechanism of action of the isolated probiotic strain when administered orally with the diet might be that they might attach themselves to the surface of the gastrointestinal tract and colonize. This prevents the colonization and proliferation of P. aeruginosa in the gastrointestinal tract. In general, the process by which probiotic bacteria inhibit the colonization of pathogenic bacteria in the gastrointestinal tract is called colonization resistance [48]. The probiotic bacteria produce antioxidant, antibacterial and antimicrobial compounds such as pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-, 9-octadecenamide, (Z)- and 2,5-piperazinedione, 3,6-bis(2-methylpropyl)- that may inhibit the growth of pathogenic bacteria and reduce the oxidative stress caused by the pathogen. These compounds not only reduce the amount of viable cells but may also affect the bacterial mechanism or toxin production. This might be the probable mechanism by which the probiotic bacteria act against the pathogen. This corroborates with the report of Nwanna [48] who reported that the probiotic bacteria produce a number of substances that inhibit the growth of pathogenic bacteria. Our results suggest that B. vireti 01 can be used as a promising probiotic for inhibiting the pathogenic bacteria P. aeruginosa and also for reducing the oxidative damage or stress generated by bacterial infection.
Conclusion
The results of this study indicated that infection caused by Pseudomonas produced significant alterations in the antioxidant defence system resulting in decreased antioxidant enzyme activity in freshwater prawn M. rosenbergii. Our results showed that probiotic B. vireti 01 isolated from the intestine of M. rosenbergii mixed with the basal diet and administered to M. rosenbergii challenged with P. aeruginosa infection maintained its antioxidant enzyme activity and decreased the mortality rate. Our report may probably be the first report on the isolation of probiotic bacteria B. vireti 01 from the intestine of M. rosenbergii. In the prawn industry, administration of a probiotic-enriched diet with antimicrobial properties may be a promising disease prevention novel strategy to increase the resistance to freshwater prawn pathogens. GC-MS analysis was performed to understand the compounds present in the probiotic strain. The present studies clearly demonstrate the protective effect of a probiotic-supplemented diet against P. aeruginosa infection in M. rosenbergii. Further analysis or other analyses have to be performed to improve the detection threshold and to enlarge the detection to molecules other than the volatile ones.
References
MujeebRahiman KM, Jesmi Y, Thomas AP, Mohamed Hatha AA (2010) Probiotic effect of Bacillus NL110 and Vibrio NE17 on the survival, growth performance and immune response of Macrobrachium rosenbergii (de Man). Aquac Res 41:120–134. https://doi.org/10.1111/j.1365-2109.2009.02473.x
MPEDA, Statistics of marine products exports (2010) The Marine Products Exports Development Authority, Kochi 360
FAO (2013) National aquaculture sector overview, India. Food and Agriculture, Organization of the United Nations
Panda S, Bandyopadhyay PK, Chatterjee SN (2015) Characterization of Pseudomonas aeruginosa PB112 (JN996498) isolated from infected Labeobata (Hamilton) by 16S rRNA gene sequence analysis and fatty acid methyl ester (FAME) analysis. Afr J Biotechnol 12:400–405
Ramalingam K, Ramarani S (2007) Effect of Pseudomonas aeruginosa on the giant freshwater prawn, Macrobrachium rosenbergii histopathotogical and electron microscopic study. J EnvBiol 28
Prakash M, Karmagam N (2013) A study on bacterial flora associated with fresh water prawn, Macrobrachium rosenbergii. Int J Curr Res Aca Rev 1:1–16
Defoirdt T, Sorgeloos P, Bossier P (2011) Alternatives to antibiotics for the control of bacterial disease in aquaculture. Curr Opin Microbiol 14:251–258
Frappaolo PJ, Guest GB (1986) Regulatory status of tetracyclines, penicillin and other antimicrobial microbials drugs in animal feeds. J AnimSci62: 86–92. https://doi.org/10.2527/1986.62Supplement_386s
Hai NV (2015) The use of probiotics in aquaculture. J Appl Microbiol 119:917–935
Rengpipat S, Rukpratanporn S, Piyatiratitivorakul S, Menasaveta P (2000) Immunity enhancement in black tiger shrimp (Penaeus monodon) by a probiont bacterium (Bacillus S11). Aquacult 191:271–288
Castex M, Lemaire P, Wabete N, Chim L (2010) Effect of probiotic Pediococcus acidilactici on antioxidant defences and oxidative stress of Litopenaeus stylirostris under Vibrio nigripulchritudo challenge. Fish Shellfish Immunol 28:622–631
Livingstone DR (2003) Oxidative stress in aquatic organisms in relation to pollution and aquaculture. Rev Med Vet 154:427–430
Wang Y, Wu Y, Wang Y, Xu H, Mei X, Yu D, Wang Y, Li W (2017) Antioxidant properties of probiotic bacteria. Nutrients 9:521
Jafari B, Rezaie A, Alizadeh S (2011) Isolation and identification of potentially probiotic bacteria from traditional dairy products of Ardabil region in Iran. Ann Biol Res 2:311–317
Rengpipat S, Rueangruklikhit T, Piyatiratitivorakul S (2008) Evaluations of lactic acid bacteria as probiotics for juvenile seabass Lates calcarifer. Aquac Res 39:134–143. https://doi.org/10.1111/j.1365-2109.2007.01864.x
Tejero-Sariñena S, Barlow J, Costabile A, Gibson GR, Rowland I (2012) In vitro evaluation of the antimicrobial activity of a range of probiotics against pathogens: evidence for the effects of organic acids. Anaerobe 18:530–538
Ramesh D, Vinothkanna A, Rai AK, Vignesh VS (2015) Isolation of potential probiotic Bacillus spp. and assessment of their subcellular components to induce immune responses in Labeo rohita against Aeromonas hydrophila. Fish Shellfish Immunol 45:268–276
Bergey’s manual of systematic bacteriology (1974) 8th Edition
Niku-Paavola ML, Laitila A, Mattila-Sandholm T, Haikara A (1999) New types of antimicrobial compounds produced by Lactobacillus plantarum. J Appl Microbiol 86:29–35
Barbosa J, Gibbs PA, Teixeira P (2010) Virulence factors among enterococci isolated from traditional fermented meat products produced in the north of Portugal. Food Control 21:651–656
Thanigaivel S, Vijayakumar S, Mukherjee A, ChandrasekaranN,Thomas J (2014) Antioxidant and antibacterial activity of Chaetomorpha antennina against shrimp pathogen Vibrio parahaemolyticus. Aquacult 433: 467–475
Thomas J, Madan N, NambiKSN,Majeed SA, Basha AN, Hameed AS (2013) Studies on ulcerative disease caused by Aeromonas caviae-like bacterium in Indian catfish, Clarias batrachus (Linn). Aquacult 376: 146–150
Pirarat N, Kobayashi T, Katagiri T, Maita M, Endo M (2006) Protective effects and mechanisms of a probiotic bacterium Lactobacillus rhamnosus against experimental Edwardsiella tarda infection in tilapia (Oreochromis niloticus). Vet Immunol Immunopathol 113:339–347
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Kono Y (1978) Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys 186:189–195. https://doi.org/10.1016/0003–9861(78)90479-4
Xu JB, Yuan XF, Lang PZ (1997) Determination of catalase activity and catalase inhibition by ultraviolet spectrophotometry. Envir Chem 16:73–76
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77. https://doi.org/10.1016/0003-9861(59)90090-6
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Heyrman J, Vanparys B, Logan NA, Balcaen A, Rodríguez-Díaz M, Felske A, De Vos P (2004)Bacillus novalis sp. nov., Bacillus vireti sp. nov., Bacillus soli sp. nov., Bacillus bataviensis sp. nov. and Bacillus drentensis sp. nov., from the Drentse A grasslands. Int J Syst Evol Microbiol 54: 47–57.doi: https://doi.org/10.1099/ijs.0.02723-0
Mohandass R, Rout P, Jiwal S, Sasikala C (2012) Biodegradation of benzo [a] pyrene by the mixed culture of Bacillus cereus and Bacillus vireti isolated from the petrochemical industry. J Env Biol 33:985
Gopi M, Dhayanithi NB, Devi KN, Kumar TTA (2014) Marine natural product, pyrrolo [−a] pyrazine–dione, hexahydro- (C7H10N2O2) of antioxidant properties from Bacillus species at Lakshadweep archipelago. J Coast Life Med 2:632–637. https://doi.org/10.12980/JCLM.2.201414J40
Ramalingam K, Ramarani S (2006) Pathogenic changes due to inoculation of gram-negative bacteria Pseudomonas aeruginosa (MTCC 1688) on host tissue proteins and enzymes of the giant freshwater prawn, Macrobrachium rosenbergii (De Man). J Env Biol 27:199–205
Thomas J, Thanigaivel S, Vijayakumar S, Acharya K, Shinge D, Seelan TSJ, Mukherjee A, Chandrasekaran N (2014) Pathogenecity of Pseudomonas aeruginosa in Oreochromis mossambicus and treatment using lime oil nanoemulsion. Colloids Surf B Biointerfaces 116:372–377
Balcázar JL, Rojas-Luna T, Cunningham DP (2007) Effect of the addition of four potential probiotic strains on the survival of pacific white shrimp (Litopenaeus vannamei) following immersion challenge with Vibrio parahaemolyticus. J Invertebr Pathol 96:147–150
Chai PC, Song XL, Chen GF, Xu H, Huang J (2016) Dietary supplementation of probiotic Bacillus PC465 isolated from the gut of Fenneropenaeus chinensis improves the health status and resistance of Litopenaeus vannamei against white spot syndrome virus. Fish Shellfish Immunol 54:602–611
Chiu CH, Guu YK, Liu CH, Pan TM, Cheng W (2007) Immune responses and gene expression in white shrimp, Litopenaeus vannamei, induced by Lactobacillus plantarum. Fish Shellfish Immunol 23:364–377
Livingstone DR (2001) Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Marine Poll Bull 42:656–666
Castex M, Lemaire P, Wabete N, Chim L (2010) Effect of probiotic Pediococcus acidilactici on antioxidant defences and oxidative stress of Litopenaeus stylirostris under Vibrio nigripulchritudo challenge. Fish Shellfish Immunol 28:622–631
Mathew S, Kumar KA, Anandan R, Nair PV, Devadasan K (2007) Changes in tissue defence system in white spot syndrome virus (WSSV) infected Penaeus monodon. Comp Biochem Physiol C Toxicol Pharmacol 145:315–320
Sarathi M, Ahmed VI, Venkatesan C, Balasubramanian G, Prabavathy J, Hameed AS (2007) Comparative study on immune response of Fenneropenaeus indicus to Vibrio alginolyticus and white spot syndrome virus. Aquacult 271:8–20
Mohankumar K, Ramasamy P (2006) White spot syndrome virus infection decreases the activity of antioxidant enzymes in Fenneropenaeus indicus. Virus Res 115:69–75
Raghu P, Rajikkannu M, Baburajan R, Deva A, Nandakumar R, Masilamni V, Prabhakaran K (2016) Effect of Bacillus coagulans and B. firmus incorporated probiotic diet on superoxide dismutase activity and catalase activity in Penaeus monodon. World Sci News 44:224–235
Borković SS, Pavlović SZ, Kovačević TB, Štajn AŠ, Petrović VM, Saičić ZS (2008) Antioxidant defence enzyme activities in hepatopancreas, gills and muscles of spiny cheek crayfish (Orconectes limosus) from the river Danube. Comp Biochem Physiol C Toxicol Pharmacol 147:122–128
Kumar PNJ, Jyothsna SA, Reddy MH, Sreevani SR (2013) Effect of Bacillus subtilis and Lactobacillus rhamnosus incorporated probiotic diet on growth pattern and enzymes in Penaeus vannamei. Int j life sci pharma res 3:6–11
Zhang CN, Li XF, Xu WN, Jiang GZ, Lu KL, Wang LN, Liu WB (2013) Combined effects of dietary fructooligosaccharide and Bacillus licheniformis on innate immunity, antioxidant capability and disease resistance of triangular bream (Megalobrama terminalis). Fish Shellfish Immunol 35:1380–1386
Grisham MB, McCord JM (1986) Chemistry and cytotoxicity of reactive oxygen metabolites. Physiol oxygen radicals:1–18
Cadenas E (1989) Biochemistry of oxygen toxicity. Annu Rev Biochem 58: 79–110.doi: https://doi.org/10.1146/annurev.bi.58.070189.000455
Nwanna L (2015) Use of probiotics in aquaculture. Appl Trop Agr 15:76–83
Acknowledgements
The authors are thankful to VIT University, Vellore for providing the facility to carry out the research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Vidhya Hindu, S., Chandrasekaran, N., Mukherjee, A. et al. Effect of Dietary Supplementation of Novel Probiotic Bacteria Bacillus vireti 01 on Antioxidant Defence System of Freshwater Prawn Challenged with Pseudomonas aeruginosa . Probiotics & Antimicro. Prot. 10, 356–366 (2018). https://doi.org/10.1007/s12602-017-9317-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-017-9317-3