Abstract
This study investigated the compound probiotics and heat-killed compound probiotics on antioxidants, plasma biochemical parameters, intestinal histopathology, and microbiome of koi fish, Cyprinus carpio haematopterus. Koi fish (15.9 ± 0.63 g) were fed with five different diets: C (control, basal diet without probiotics), D3 (3% compound probiotics), D6 (6% compound probiotics), lnaD3 (3% heat-killed compound probiotics), and lnaD6 (6% heat-killed compound probiotics). After 28 days of the feeding trial, results showed that the activities of acid phosphatase (ACP), alkaline phosphatase (AKP), catalase (CAT), and total superoxide dismutase (T-SOD) were significantly improved in koi fish fed with heat-killed compound probiotics compared to the control group (P < 0.05). Interestingly, total activities of glutamyl oxaloacetic transaminase (GOT), total protein (TP), albumin (TA), globulin (GLB), and triglyceride (TG) were significantly improved in koi fish fed with compound probiotics compared to the control group (P < 0.05). The intestinal muscular and intestinal villi height of lnaD3 was significantly higher than other groups (P < 0.05). In addition, compound probiotics and heat-killed compound probiotics increased the diversity of intestinal microbes. Therefore, dietary supplementation with heat-killed compound probiotics can be an effective measure for improving nonspecific immune response, intestinal morphology, and the flora of koi fish.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Koi fish, Cyprinus carpio haematopterus, are popular worldwide due to their diverse colors and long-life span (Wang et al. 2021a, b, c). Currently, koi fish are essential for the import and export of ornamental fish and have been widely cultured in China (Putri & Dewi 2019). However, with the intensification of the aquaculture environment, the resistance of their immune system to various diseases has been disrupted. Moreover, these diseases affect aquaculture development and cause significant economic losses (Amenyogbe et al. 2020). Antibiotics are often used to prevent and treat aquatic diseases in traditional aquaculture (Yeganeh et al. 2021). However, the misuse of antibiotics can lead to the development of resistance in pathogens, thus making treatment progressively less effective (Čížek et al. 2010; Santos & Ramos 2016). In addition, the misuse of antibiotics disrupts the intestinal flora and alters the microbiological system, which in turn affects the growth, digestion, and immunity of koi fish (Rekecki et al. 2009; Maynard et al. 2012). For these reasons, alternative methods are required to reduce the use of antibiotics in aquaculture.
According to previous studies, probiotics can enhance immune response (El-Saadony et al. 2021; Islam et al. 2021; Jahan et al. 2021; Haque et al. 2021; Khalafalla et al. 2020) and improve digestion through enzyme activities to improve growth performance of aquatic animals (Giri et al. 2020; Van al. 2019a; Elsabagh et al. 2018; Dowidar et al. 2018; Saputra et al. 2016; Saravanan et al. 2021). Meanwhile, studies have demonstrated that probiotics could decrease mortality and increase immunity in shrimp, Litopenaeus vannamei, and sea cucumber, Apostichopus japonicus (Chiu et al. 2021; Feng et al. 2020). Therefore, probiotics are recommended to be added to the diet as a functional additive to replace antibiotics to reduce resistance (Yeganeh et al. 2021). However, the application of live bacteria may adversely affect the environment due to the interaction of probiotics with the ecosystem (Dawood et al. 2019). In addition, live probiotics may lose their activity during transportation or in animal intestines. Therefore, replacing live probiotics with dead bacteria is significant because it can benefit the host (Wang et al. 2018).
Probiotics are usually treated by heat (Van et al. 2019b; Giri et al. 2020), so inactivated probiotics can withstand high temperatures while preparing fish feed without affecting their function (Díaz-Rosales et al. 2006; Salinas et al. 2008). It has been demonstrated that dead probiotic cells also have components and metabolites that can exert the same effects as live probiotics (Giri et al. 2020). Although the elevated temperature alters the composition of probiotics, the inactivated probiotics can stimulate immune responses in the host (Yang et al. 2019; Pan et al. 2008). In addition, heat-killed probiotics can affect intestinal bacteria to improve host growth. The heat-killed Lactobacillus plantarum is a candidate as one of the functional additives for fish. Studies have shown that heat-killed Lactobacillus plantarum is effective in the host in improving growth performance, survival rate, cytokine gene expression, and bacterial resistance (Biswas et al. 2013; Dawood et al. 2015; Van et al. 2019b; Duc et al. 2020). Notably, in another study, it has been reported that heat-killed Pseudomonas aeruginosa more effectively than other forms of Pseudomonas aeruginosa (Giri et al. 2011). Recently, Wu et al. (2020) reported that after supplementing the diet with two kinds of heat-inactivated probiotics, the compound heat-killed probiotics could increase the 45 expressions of genes related to growth, inflammation, and non-specific immunity. Furthermore, Frouël et al. (2008) found that the mixture of probiotics can improve the endocytosis vesicles of intestinal epithelial cells. Thus, it is important to study the specific effects of heat-killed compound probiotics, especially on the antioxidative status and intestinal health of koi fish.
This study evaluated the effects of compound probiotics and heat-killed compound probiotics on the antioxidative capacity, plasma biochemical parameters, intestinal morphology, and microbiota of koi fish. The result of this study might provide necessary information for applying heat-killed compound probiotics in the sustainable development of koi fish culture.
Materials and methods
Experimental diets and probiotic supplements
Fish meal, soybean meal, and wheat gluten were the primary protein sources to control koi fish diets. Fish oil and soybean oil were the primary lipid sources for the control diets. The main components of probiotics are Lactobacillus plantarum, Lactobacillus acidophilus, Pseudomonas aeruginosa, etc., and the effective viable count was 4.0 × 108 CFU/g. The live compound probiotics were inactivated using an automatic temperature control system ventilated oven (DK-s26; Shanghai Samsung Laboratory Instrument Co. Ltd.) at temperatures from 121 to 126 °C for 20 min (Segawa et al. 2008).
In this study, besides the control group C, four experimental diets were prepared with different concentrations of probiotics, two of which contained two levels of compound probiotics (D3 (3% compound probiotics) and D6 (6% compound probiotics)), and the other two contained two levels of heat-killed compound probiotics (lnaD3 (3% heat-killed compound probiotics) and lnaD6 (6% heat-killed compound probiotics)). These components were mixed with water and extruded into 1.5 mm pellets (Pinzheng Equipment Co., Ltd., Changzhou, China). Natural drying to ensure probiotic activity then was put in the − 20 °C immediately. The ingredients and proximate composition of the diets are presented in Table 1.
Experimental daily management and sample collection
All animal experiments complied with the Guidelines of the Care and Use of Laboratory Animals in China, and the study was approved by the ethics committee of Dalian Ocean University. Koi fish were purchased in an ornamental fish commercial hatchery (Fushan Koi Farm, Liaoning, China) and acclimated for 15 days in the tanks. After acclimatization, 300 uniform individuals of Koi fish with similar weights (15.9 ± 0.63 g) were randomly distributed into 15 tanks (capacity: 40 L).
During the experiment, the koi fish were kept in freshwater tanks at 23 °C with 12 h dark and 12 h light, and the pH was 7.8–8.0. Air pumps were used to maintain an adequate oxygen content in the water. The fish were fed at 3% of their body weight at 8:00 and 18:00. The residue and feces were cleaned every day at 17:00 and replaced 1/3 of the water. The feeding trial continued for 28 days.
At the end of the trial, the koi fish were starved for 24 h and weighed body. All koi fish were anesthetized with eugenol after being put on the ice plate. The blood was collected through the caudal vein, then put the blood sample in the tube and liquid nitrogen. In addition, the koi fish was opened with scissors and disinfection with 75% alcohol. Collected gut tracts and liver were quickly frozen in liquid nitrogen. All samples were kept at − 80 °C until further analysis.
Non-specific immune parameters analysis
The levels of total acid phosphatase (ACP and spectrophotometric method), alkaline phosphatase (AKP and visible light colorimetry), catalase (CAT and visible light colorimetry), and superoxide dismutase (T-SOD and hydroxylamine method) in the liver of the koi fish were measured using kits purchased by Nanjing Jiancheng Institute of Biological Engineering (Nanjing Jiancheng Bioengineering Institute). All the parameters were performed using visible spectrophotometry (Shanghai Tianmei Scientific instrument Co., Ltd., VIS7200A). ACP and AKP colorimetric measurement wavelengths are 520 nm; CAT colorimetric measurement wavelengths are 405 nm; and T-SOD colorimetric measurement wavelengths are 550 nm. The sample pretreatment, reagent preparation, and determination steps were carried out strictly with the operating instructions.
Blood assays
After the fed trial, the koi fish from each treatment were anesthetized with eugenol (5 mg/L). Then, the blood was collected from the caudal vein using a plastic syringe (syringes were pre-washed with sodium heparin solution (1 g/L) to determine the hemoglobin level). The levels of total activities of glutamyl oxaloacetic transaminase (GOT), glutamic-pyruvic acid transaminase (GPT), total protein (TP), albumin (TA), globulin (GLB), and triglyceride (TG) in the blood of the koi fish were measured by 7600–110 automatic biochemical analyzer (Hitachi, Japan).
Intestinal morphology
The intestines were fixed in Bouin’s fixative solution for 24 h and were dehydrated using a series of graded ethanol concentrations in the DIAPATH dehydrator (Donatello). Following that, samples were embedded into melted paraffin wax using an embedding (JB-P5, Wuhan Junjie Electronics Co., Ltd) and sectioned into a 4.0-µm thick slice. Finally, the sections were stained with hematoxylin and eosin. The stained tissue sections were observed using an Olympus CX23 microscope (Olympus Industry Co., Ltd., Guangzhou, China).
Microbial study
Gut samples were taken from the refrigerator at − 80 °C and were subjected to intestinal flora determination. According to the manufacturer’s instructions, the total genomic DNA was extracted from intestinal samples using the bacterial DNA kit (E.Z.N.A bacterial Mag-Bind SoilDNA Kit OMEGA). Next, Qubit 3.0 DNA detection kit was used to accurately quantify genomic DNA to determine the amount of DNA used in the PCR reaction. Universal primers 805R (5t-GACTACHVGGGTATCTAA GATCC-3AC and 341F (5A-CCTACGGGNGGCWGCAG-3CTACGGGNGGCWGCAGA GATCC 3.0 V3-V4 hypervariable region of the 16S rRNA gene, respectively. The following PCR reaction conditions were used: 94 °C heat preservation for 3 min, followed by 94 °C heat preservation for 30 s, 45 °C for 20 s, and 65 °C for 30 s for five cycles; 94 °C for 20 s, 55 °C for 20 s, and 72 °C for 30 s for 20 cycles; finally, an extension for 5 min at 72 °C was performed. Purified PCR products were analyzed by 2% gel electrophoresis to check the library size, and the Qubit3.0 fluorescence quantitative instrument was used to determine the concentration of the library. High-throughput sequencing was performed using the Illumina MiSeq platform. Finally, chimeric sequence detection and OTU selection at 97% sequence similarity were conducted using USEARCH v6.1 64.
Statistical analysis
The statistical analysis was performed by the SPSS20.0 (SPSS, Chicago, IL, USA) software. All data are expressed as mean ± standard error. Two-way ANOVA was employed to test the effects of different probiotics and concentrations and their interactions, excluding the control diet. Data from the test group were compared using Duncan’s multiple comparisons (one-way ANOVA). P < 0.05 was considered statistically significant.
Results
There was no mortality or abnormally behaved fish in each dietary group during a feeding trial. Furthermore, all dietary groups showed similar specific growth rates (2.18–2.66) and feed intake. Only the relative growth rate of the lnaD6 groups was significantly higher than the other groups (P < 0.05).
Hepatopancreatic immune enzyme activity assays
The immune enzymes of the koi fish fed with different diets for 28 days are given in Table 2. In two-way ANOVA, an interactive effect was found in test groups on the ACP and AKP. The ACP activity of the D3 and lnaD3 groups was significantly higher than that of the D6 group (P < 0.05). The ACP activity of the lnaD6 group was significantly higher than that of the D3, D6, and lnaD3 groups (P < 0.05). The AKP activity of the lnaD6 group was significantly lower than that of the D3, D6, and lnaD3 groups (P < 0.05). In the two independent samples’ test, the ACP activity of the D3, lnaD3, and lnaD6 groups was significantly higher than the control group (P < 0.05). The AKP activity of the D3, D6, lnaD3, and lnaD6 groups was significantly higher than the control group (P < 0.05).
In two-way ANOVA, interactive effects were found in test groups on the CAT and T-SOD. The CAT activity of the lnaD6 group was significantly higher than that of the D3, D6, and lnaD3 groups (P < 0.05). Similar trends were found in T-SOD. T-SOD was higher in the lnaD6 group than in the D3, D6, and lnaD3 groups (P < 0.05). In the two independent samples’ test, the CAT activity of the D3, lnaD3, and lnaD6 groups was significantly higher than the control group (P < 0.05). The T-SOD activity of D3, D6, lnaD3, and lnaD6 was significantly higher than the control group (P < 0.05) (Table 2).
Blood hematology and biochemistry
The blood index of the koi fish fed with different diets for 28 days is given in Table 3. In addition to GLB, interactive effects were found in two-way ANOVA of other indicators. GOT, GPT, TP, and TA were significantly lower in the lnaD6 group than in D3, D6, and lnaD3 groups (P < 0.05). TG in D6 was significantly lower than in D3, lnaD3, and lnaD6 groups (P < 0.05). In the two independent samples’ test, the GOT, TP, and GLB of the D3 and D6 groups were significantly higher than the control group (P < 0.05), on the contrary, the GOT, TP, and GLB of lnaD3 and lnaD6 groups were significantly lower than the control group (P < 0.05). The GPT of the D3, D6, and lnaD3 groups was significantly higher than the control group (P < 0.05), and the GPT of the lnaD6 group was significantly lower than the control group (P < 0.05). The TA of the D3 and D6 groups was significantly higher than the control group (P < 0.05), and the TA of the lnaD6 group was significantly lower than the control group (P < 0.05). All test groups in TG were significantly lower than the control group (P < 0.05).
Intestinal morphology
In two-way ANOVA, interactive effects were found on the intestinal muscular in test groups. The intestinal muscular thickness of the lnaD3 group was significantly higher than that of the D3, D6, and lnaD6 groups (P < 0.05). The intestinal muscular thickness was significantly lower in the lnaD6 group than in D3, D6, and lnaD3 groups (P < 0.05).
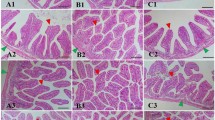
In the two independent samples’ test, the intestinal villi height of the lnaD3 group was significantly higher than the control group (P < 0.05). The intestinal villus width of the lnaD6 group was significantly higher than in the control group (P < 0.05). All the test groups were significantly higher than the control group (P < 0.05) (Fig. 1) (Table 4).
Gut microflora
Richness and diversity
Figure 2 indicates that the sample dilution curve tended to be flat. The coverage of all the sequenced samples ranged up to 0.999 in all the treatments, demonstrating that the sequencing result had a sufficient amount of sequencing data (Table 5). The Shannon index, Chao1, Ace estimators, and Simpson index represented the richness and alpha diversity of the intestinal microbiota of the koi fish gut microbial community. The order of Shannon, Chao1, and Ace estimators of all treatment groups was higher than that of the control group. The Simpson order index of all treatment groups was lower than that of the control group.
Composition of intestinal microbiota
The relative abundance of the intestinal flora of each group at the phylum level is shown in Fig. 3. Results showed that the most dominant bacteria were Fusobacteria in the intestine of fish at the phylum level. The relative abundance of Bacteroidetes in the D3, lnaD3, and lnaD6 groups was higher than that in the control group. Notably, the relative abundance of Proteobacteria increased in the lnaD6 group.
At the genus level in Fig. 4, the Cetobacterium was dominant in the intestines of fish. The relative abundance of Bacteroides increased in the D3, lnaD3, and lnaD6 groups. Moreover, the relative abundance of Aeromonas was increased in lanD6.
Discussion
This study explores the interactive effects of activity and concentration of probiotics in aquaculture. Probiotics can increase teleost fish immunity in vivo (Aly et al. 2008; Balcázar et al. 2007; Daz-Rosales et al. 2009).
ACP and AKP are probiotic indicators that have an impact on aquatic animal health (Yi et al. 2018). ACP is an enzyme found in lysosomes. It is a macrophage activation marker that can eliminate exogenous microorganisms (Li et al. 2019). In this experiment, koi fish fed on diets containing heat-killed compound probiotics had higher ACP activity than the other groups. This was consistent with the results for carp Labeo rohita (Giri et al. 2013) and hybrid grouper Epinephelus lanceolatus anceolafuscoguttatus us(Li et al. 2019). However, koi fish fed on diets containing 6% compound probiotics had lower ACP activity than the control group. Thus, an overdose of probiotics is likely to harm the immune system of koi fish.
AKP is an extracellular enzyme that is activated by macrophages (Kuebutornye et al. 2020). AKP activity in koi fish fed compound probiotics in the D6 group was significantly higher than AKP activity in koi fish fed the control diet in this experiment. According to Wang et al. (2021a), live Lactococcus lactis Z-2 dramatically boosted the AKP activity in carp Cyprinus carpio. Similar findings were made by Yi et al. (2018) who discovered that feeding fish velezensis JW can enhance AKP activity. Additionally, like live probiotics, heat-killed probiotics can enhance the stimulation of immunological responses (Pan et al. 2008). The AKP activity of the lnaD3 group fed with heat-killed compound probiotics was significantly higher than the control group. However, the AKP activity of the lnaD6 group fed with heat-killed compound probiotics was significantly lower than other test groups. Even though live and heat-killed probiotics elicit the same immune response, the underlying mechanisms of action may differ. (Taverniti & Guglielmetti 2011).
CAT is an antioxidant enzyme found in the fish’s first line of oxidant defense (Kuebutornye et al. 2020). In the meantime, CAT can mitigate the damage by decomposing excess hydrogen peroxide (Wang et al. 2021b). Yang et al. (2020) discovered that feeding living and heat-killed B. cereus can improve CAT activity, with live probiotics outperforming heat-killed probiotics. On the contrary, our study found that heat-killed compound probiotics-supplemented groups had significantly higher CAT activity than other groups. This inconsistency could be attributed to different methods of killing probiotics and treatment duration (Munoz-Atienza et al. 2015). SOD is a type of living organism with enzymatic defenses (Wang et al. 2017). According to Gayed et al. (2021), feeding probiotics can significantly increase SOD activity. Similarly, our findings indicated that feeding probiotics or heat-killed probiotics could improve SOD activity significantly more than the control group. It is worth noting that the lnaD6 group fed with heat-killed probiotics was significantly higher than in other groups. This finding demonstrated that heat-killed compound probiotics can improve SOD activity more than live probiotics. The evidence presented above suggests that a diet supplemented with heat-killed compound probiotics can improve the health of koi fish. Overall, feeding 3% heat-killed probiotics can improve the immunity of koi fish.
Hematologic parameters can reflect fish liver health (Lemaire et al. 1991; Megarani et al. 2020). Meanwhile, hematologic parameters are an important guide for determining fish health (Kesbiç et al. 2020). Dawood et al. (2015, 2019) reported that fish fed with heat-killed probiotics can decrease the GOT and GPT levels. This is consistent with the results of the present study. Serum GOT and GPT of the koi fish fed with 6% heat-killed probiotics were lower than other groups. The GOT and GPT will be higher when the liver is destroyed (Lemaire et al. 1991). As a result, the fish-fed heat-killed compound probiotics appeared to be in good health. Blood protein profiles (TP, ALB, and GLO) are produced in the liver and are considered markers of fish humoral immunity (Patriche et al. 2009). Tan et al. (2017) found that blood protein profiles can reflect the body’s protein metabolism and the liver’s functional status and that elevated levels of TP and ALB in the liver are a sign that the liver’s capacity for protein synthesis has risen. Dawood et al. (2019) discovered that TP levels were significantly higher in fish-fed heat-killed probiotics. Conversely, some reports claimed that probiotics had little effect on TP (Shelby et al. 2006; Abd El-Rhman et al. 2009). In contrast to previous studies, we discovered that TP was significantly higher in koi fish-fed probiotics and reduced in koi fish-fed heat-killed compound probiotics. It is thought to be because different probiotics have different durations of action and effects. Dawood et al. (2015) discovered that heat-killed probiotics can reduce TG levels. We came to the same conclusion in our report. Probiotics can significantly reduce TG levels. In fish, TG could be involved in the regulation of blood lipid derivatives (Falcinelli et al. 2015). This result indicated that the fish had low plasma triglyceride levels, and compound probiotics in the feed could improve koi fish fat metabolism (Dawood et al. 2015).
The effects of different probiotics on intestinal morphology have been investigated in many fish species (Lee et al. 2017; Ramos et al. 2015, 2017; Zhu et al. 2021). The intestine is a vital digestive and defense organ in fish (Mohammadian et al. 2022). Studies have revealed that B. cereus and B. subtilis can protect the intestinal barrier (Xue et al. 2020). When Nile tilapia Oreochromis niloticus were fed cerevisiae, the fish’s intestinal mucosal fold, lamina propria, and enterocyte width all increased (Islam et al. 2021). However, Ramos et al. (2017) observed no significant effect on the mid-intestine villi length using probiotic formula. Unlike other results, our findings showed that intestinal villi height and intestinal villi width were lower in D3- and D6-fed probiotics. Differences in results could be attributed to differences in digestive system location and probiotic species measured (Demirci et al. 2021). In our study, the intestinal villi height and intestinal muscular thickness were higher in the lnaD3 group fed with heat-killed compound probiotics. Dawood et al. (2019) fed the tilapia Oreochromis niloticus with the heat-killed Lactobacillus plantarum and found villus length significantly increased. Hien et al. observed that in snakehead Channa striata fed with heat-killed Lactobacillus plantarum, the height and length of the intestinal villi were significantly higher than those fed the control diet (Hien et al. 2021). Nofouzi et al. found that the intestinal villus length of the rainbow trout Oncorhynchus mykiss was mainly enhanced in the heat-killed Tsukamurella inchonensis group (Nofouzi et al. 2019). Increasing the length of the intestinal villi may increase the absorption surface area, resulting in improved nutrient utilization and growth performance (Khojasteh, 2012). The increase in muscle thickness can enhance the digestion and absorption capacity of the intestinal tract (Peng et al. 2022). The findings showed that heat-killed probiotics can improve digestion by increasing the height of the fish intestinal villi and the thickness of the intestinal muscular layer.
Probiotics can influence changes in the intestinal microbial community (Nayak 2010). Moreover, dominant intestinal bacteria are required to maintain intestinal homeostasis (Guangxin et al. 2022; Zhu et al. 2021). In the current study, probiotic supplements or heat-killed probiotics increased the richness of gut microbiota in fish. Carnevali et al. (2017) investigated that the higher richness indicated implies that probiotics could improve organisms’ ability to adapt to bacterial diversity. Proteobacteria, Firmicutes, and Actinobacteria are the primary bacteria found in the fish gut (Wu et al. 2012). Fusobacteria and Bacteroidetes were the dominant phyla in all groups in this study. At the phylum level, the addition of heat-killed compound probiotics increased the relative abundance of Bacteroidetes and Proteobacteria. Bacteroidetes have digestive enzymes, according to previous research (Karlsson et al. 2011). Furthermore, Proteobacteria can promote nutrient cycling and are linked to the immune system (Gomez et al. 2013; Cardona et al. 2016). This demonstrates that heat-killed compound probiotics benefit the digestive system and immunity.
Interestingly, Aeromonas was discovered in the intestines of the lnaD6 group at the genus level. Many extracellular proteins can be produced by Aeromonas, including amylase, chitinase, elastase, aerolysin, nuclease, gelatinase, lecithinase, lipase, and protease (Aberoum et al. 2010). As a result, heat-killed compound probiotics may affect the digestive ability of koi fish. In addition, the Shewanella was found in D3, D6, and lnaD6. Several studies have explored the antimicrobial activity of Shewanella species against various fish pathogens (Zadeh et al. 2010; Díaz-Rosales et al. 2009; Makridis et al. 2008). From this point of view, the application of compound probiotics and heat-killed compound probiotics can improve koi fish resistance to pathogenic bacteria.
In conclusion, the effects of compound probiotics and heat-killed probiotics on the antioxidative capacity, plasma biochemical parameters, intestinal morphology, and microbiota of koi fish were demonstrated in this study. The appropriate amount of heat-killed compound probiotics are better than compound probiotics based on the species, feed composition, type of probiotics, and experimental environment of koi fish. However, the number of probiotics used should be noted. As a result, heat-killed compound probiotics could be used in the healthy management of koi fish aquaculture. However, more research is needed to determine the mechanism of action of heat-killed compound probiotics.
Data availability
All data, models, and code generated or used during the study appear in the submitted article.
References
Abd El-Rhman AM, Khattab YA, Shalaby AM (2009) Micrococcus luteus and Pseudomonas species as probiotics for promoting the growth performance and health of Nile tilapia. Oreochromis Niloticus Shellfish Immunol 27(2):175–180. https://doi.org/10.1016/j.fsi.2009.03.020
Aberoum A, Jooyandeh H (2010) A review on occurrence and characterization of the Aeromonas species from marine fishes. World J Fish Mar Sci 2(6):519–523. https://doi.org/10.1016/s0025-326x(02)00143-1
Aly SM, Ahmed YA-G, Ghareeb AA-A, Mohamed MF (2008) Studies on Bacillus subtilis and Lactobacillus acidophilus, as potential probiotics, on the immune response and resistance of Tilapia nilotica (Oreochromis niloticus) to challenge infections. Fish Shellfish Immunol 25(1–2):128–136. https://doi.org/10.1016/j.fsi.2008.03.013
Amenyogbe E, Chen G, Wang Z, Huang J, Huang B, Li H (2020) The exploitation of probiotics, prebiotics and synbiotics in aquaculture: present study, limitations and future directions: a review. Aquac Int 28(3):1017–1041. https://doi.org/10.1007/s10499-020-00509-0
Balcázar JL, De Blas I, Ruiz-Zarzuela I, Vendrell D, Gironés O, Muzquiz JL (2007) Enhancement of the immune response and protection induced by probiotic lactic acid bacteria against furunculosis in rainbow trout (Oncorhynchus mykiss). FEMS Immunol Med Microbiol 51(1):185–193. https://doi.org/10.1111/j.1574-695X.2007.00294.x
Biswas G, Korenaga H, Nagamine R, Takayama H, Kawahara S, Takeda S, Kikuchi Y, Dashnyam B, Kono T, Sakai M (2013) Cytokine responses in the Japanese pufferfish (Takifugu rubripes) head kidney cells induced with heat-killed probiotics isolated from the Mongolian dairy products. Fish Shellfish Immunol 34(5):1170–1177. https://doi.org/10.1016/j.fsi.2013.01.024
Cardona E, Gueguen Y, Magré K, Lorgeoux B, Piquemal D, Pierrat F, Noguier F, Saulnier D (2016) Bacterial community characterization of water and intestine of the shrimp Litopenaeus stylirostris in a biofloc system. BMC Microbiol 16(1):1–9. https://doi.org/10.1186/s12866-016-0770-z
Carnevali O, Maradonna F, Gioacchini G (2017) Integrated control of fish metabolism, wellbeing and reproduction: the role of probiotic. Aquaculture 472:144–155. https://doi.org/10.1016/j.aquaculture.2016.03.037
Chiu S-T, Chu T-W, Simangunsong T, Ballantyne R, Chiu C-S, Liu C-H (2021) Probiotic, Lactobacillus pentosus BD6 boost the growth and health status of white shrimp, Litopenaeus vannamei via oral administration. Fish Shellfish Immunol 117:124–135. https://doi.org/10.1016/j.fsi.2021.07.024
Čížek A, Dolejská M, Sochorová R, Strachotová K, Piačková V, Veselý T (2010) Antimicrobial resistance and its genetic determinants in aeromonads isolated in ornamental (koi) carp (Cyprinus carpio koi) and common carp (Cyprinus carpio). Vet Microbiol 142(3–4):435–439. https://doi.org/10.1016/j.vetmic.2009.10.001
Dawood MA, Koshio S, Ishikawa M, Yokoyama S (2015) Effects of heat killed Lactobacillus plantarum (LP20) supplemental diets on growth performance, stress resistance and immune response of red sea bream, Pagrus major. Aquaculture 442:29–36. https://doi.org/10.1016/j.aquaculture.2015.02.005
Dawood MA, Magouz FI, Salem MF, Abdel-Daim HA (2019) Modulation of digestive enzyme activity, blood health, oxidative responses and growth-related gene expression in GIFT by heat-killed Lactobacillus plantarum (L-137). Aquaculture 505:127–136. https://doi.org/10.1016/j.aquaculture.2019.02.053
Demirci B, Terzi F, Kesbic OS, Acar U, Yilmaz S, Kesbic FI (2021) Does dietary incorporation level of pea protein isolate influence the digestive system morphology in rainbow trout ( Oncorhynchus mykiss )? Anat Histol Embryol 50(6):956–964. https://doi.org/10.1111/ahe.12740
Díaz-Rosales P, Salinas I, Rodríguez A, Cuesta A, Chabrillón M, Balebona MC, Moriñigo MÁ, Esteban MÁ, Meseguer J (2006) Gilthead seabream (Sparus aurata L.) innate immune response after dietary administration of heat-inactivated potential probiotics. Fish Shellfish Immunol 20(4):482–492. https://doi.org/10.1016/j.fsi.2005.06.007
Díaz-Rosales P, Arijo S, Chabrillón M, Alarcón F, Tapia-Paniagua S, Martínez-Manzanares E, Balebona M, Moriñigo M (2009) Effects of two closely related probiotics on respiratory burst activity of Senegalese sole (Solea senegalensis, Kaup) phagocytes, and protection against Photobacterium damselae subsp. piscicida. Aquaculture 293(1–2):16–21. https://doi.org/10.1016/j.aquaculture.2009.03.050
Dowidar M, Abd ElAzeem S, Khater A, Awad Somayah M, Metwally S (2018) Improvement of growth performance, immunity and disease resistance in Nile tilapia, Oreochromis niloticus, by using dietary probiotics supplementation. J AnimSci Vet Med 3(2):35–46. https://doi.org/10.31248/JASVM2018.076
Duc PM, Myo HN, Hoa TTT, Liem PT, Onoda S, Hien TTT (2020) Effects of heat killed Lactobacillus plantarum (HK L-137) supplemental diets on growth, survival and health of juvenile striped catfish, Pangasianodon hypophthalmus. Int J Sci Res Publ 10(3):761–767. https://doi.org/10.29322/ijsrp.10.03.2020.p9993
El-Saadony MT, Alagawany M, Patra AK, Kar I, Tiwari R, Dawood MA, Dhama K, Abdel-Latif HM (2021) The functionality of probiotics in aquaculture: an overview. Fish Shellfish Immunol 117:36–52. https://doi.org/10.1016/j.fsi.2021.07.007
Elsabagh M, Mohamed R, Moustafa EM, Hamza A, Farrag F, Decamp O, Dawood MA, Eltholth M (2018) Assessing the impact of Bacillus strains mixture probiotic on water quality, growth performance, blood profile and intestinal morphology of Nile tilapia. Oreochromis Niloticus Aquac Nutr 24(6):1613–1622. https://doi.org/10.1111/anu.12797
Falcinelli S, Picchietti S, Rodiles A, Cossignani L, Merrifield DL, Taddei AR, Maradonna F, Olivotto I, Gioacchini G, Carnevali O (2015) Lactobacillus rhamnosus lowers zebrafish lipid content by changing gut microbiota and host transcription of genes involved in lipid metabolism. Sci Rep 5(1):1–11. https://doi.org/10.1038/srep09336
Feng Z, Song X, Zhao L, Zhu W (2020) Isolation of probiotics and their effects on growth, antioxidant and non-specific immunity of sea cucumber Apostichopus japonicus. Fish Shellfish Immunol 106:1087–1094. https://doi.org/10.1016/j.fsi.2020.08.049
Frouël S, Le Bihan E, Serpentini A, Lebel J, Koueta N, Nicolas J (2008) Preliminary study of the effects of commercial lactobacilli preparations on digestive metabolism of juvenile sea bass (Dicentrarchus labrax). Microbial Physiol 14(1–3):100–106. https://doi.org/10.1159/000106088
Gayed MA, Elabd H, Tageldin M, Abbass A (2021) Probiotic Zado®(Ruminococcus Flavefaciens) boosts hematology, immune, serum proteins, and growth profiles in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol Rep 2:100021. https://doi.org/10.1016/j.fsirep.2021.100021
Giri SS, Sukumaran V, Sen SS, Vinumonia J, Banu BN, Jena PK (2011) Antagonistic activity of cellular components of potential probiotic bacteria, isolated from the gut of Labeo rohita, against Aeromonas hydrophila. Probiotics Antimicrob Proteins 3(3):214–222. https://doi.org/10.1007/s12602-011-9078-3
Giri SS, Sukumaran V, Oviya M (2013) Potential probiotic Lactobacillus plantarum VSG3 improves the growth, immunity, and disease resistance of tropical freshwater fish. Labeo Rohita Fish Shellfish Immunol 34(2):660–666. https://doi.org/10.1016/j.fsi.2012.12.008
Giri SS, Jun JW, Yun S, Kim HJ, Kim SG, Kim SW, Woo KJ, Han SJ, Oh WT, Kwon J, Sukumaran V, Park SC (2020) Effects of dietary heat-killed Pseudomonas aeruginosa strain VSG2 on immune functions, antioxidant efficacy, and disease resistance in Cyprinus carpio. Aquaculture 514:734489. https://doi.org/10.1016/j.aquaculture.2019.734489
Gomez D, Sunyer JO, Salinas I (2013) The mucosal immune system of fish: the evolution of tolerating commensals while fighting pathogens. Fish Shellfish Immunol 35(6):1729–1739. https://doi.org/10.1016/j.fsi.2013.09.032
Guangxin G, Li K, Zhu Q, Zhao C, Li C, He Z, Hu S, Ren Y (2022) Improvements of immune genes and intestinal microbiota composition of turbot (Scophthalmus maximus) with dietary oregano oil and probiotics. Aquaculture 547:737442. https://doi.org/10.1016/j.aquaculture.2021.737442
Haque MM, Hasan NA, Eltholth MM, Saha P, Mely SS, Rahman T, Murray FJ (2021) Assessing the impacts of in-feed probiotic on the growth performance and health condition of pangasius (Pangasianodon hypophthalmus) in a farm trial. Aquac Rep 20:100699. https://doi.org/10.1016/j.aqrep.2021.100699
Islam SM, Rohani MF, Shahjahan M (2021) Probiotic yeast enhances growth performance of Nile tilapia (Oreochromis niloticus) through morphological modifications of intestine. Aquac Rep 21:100800. https://doi.org/10.1016/j.aqrep.2021.100800
Jahan N, Islam SM, Rohani MF, Hossain MT, Shahjahan M (2021) Probiotic yeast enhances growth performance of rohu (Labeo rohita) through upgrading hematology, and intestinal microbiota and morphology. Aquaculture 545:737243. https://doi.org/10.1016/j.aquaculture.2021.737243
Jiang H-F, Liu X-L, Chang Y-Q, Liu M-T, Wang G-X (2013) Effects of dietary supplementation of probiotic Shewanella colwelliana WA64, Shewanella olleyana WA65 on the innate immunity and disease resistance of abalone, Haliotis discus hannai Ino. Fish Shellfish Immunol 35(1):86–91. https://doi.org/10.1016/j.fsi.2013.04.009
Karlsson FH, Ussery DW, Nielsen J, Nookaew I (2011) A closer look at bacteroides: phylogenetic relationship and genomic implications of a life in the human gut. Microb Ecol 61(3):473–485. https://doi.org/10.1007/s00248-010-9796-1
Kesbiç OS, Yiğit M, Acar Ü (2016) Effects of tank color on growth performance and nitrogen excretion of European seabass (Dicentrarchus labrax) juveniles. Proc National Acad Sci, India Section b: Biol Sci 86(1):205–210. https://doi.org/10.1007/s40011-014-0441-5
Kesbiç OS, Acar Ü, Yilmaz S, Aydin ÖD (2020) Effects of bergamot (Citrus bergamia) peel oil-supplemented diets on growth performance, haematology and serum biochemical parameters of Nile tilapia (Oreochromis niloticus). Fish Physiol Biochem 46(1):103–110. https://doi.org/10.1007/s10695-019-00700-y
Khalafalla MM, Ibrahim SA, Zayed MM, Awad MN, Mohamed RA (2020) Effect of a dietary mixture of beneficial bacteria on growth performance, health condition, chemical composition, and water quality of Nile Tilapia, Oreochromis niloticus fingerlings. J Aquat Food Prod Technol 29(8):823–835. https://doi.org/10.1080/10498850.2020.1764685
Khojasteh B (2012) The morphology of the post-gastric alimentary canal in teleost fishes: A brief review. Int J Aquat Sci 3:71–88
Kuebutornye FK, Tang J, Cai J, Yu H, Wang Z, Abarike ED, Lu Y, Li Y, Afriyie G (2020) In vivo assessment of the probiotic potentials of three host-associated Bacillus species on growth performance, health status and disease resistance of Oreochromis niloticus against Streptococcus agalactiae. Aquaculture 527:735440. https://doi.org/10.1016/j.aquaculture.2020.735440
Lee S, Katya K, Park Y, Won S, Seong M, Hamidoghli A, Bai SC (2017) Comparative evaluation of dietary probiotics Bacillus subtilis WB60 and Lactobacillus plantarum KCTC3928 on the growth performance, immunological parameters, gut morphology and disease resistance in Japanese eel, Anguilla japonica. Fish Shellfish Immunol 61:201–210. https://doi.org/10.1016/j.fsi.2016.12.035
Lemaire P, Drai P, Mathieu A, Lemaire S, Carriere S, Giudicelli J, Lafaurie M (1991) Changes with different diets in plasma enzymes (GOT, GPT, LDH, ALP) and plasma lipids (cholesterol, triglycerides) of sea-bass (Dicentrarchus labrax). Aquaculture 93(1):63–75. https://doi.org/10.1016/0044-8486(91)90205-L
Li J, Wu Z-B, Zhang Z, Zha J-W, Qu S-Y, Qi X-Z, Wang G-X, Ling F (2019) Effects of potential probiotic Bacillusvelezensis K2 on growth, immunity and resistance to Vibrio harveyi infection of hybrid grouper (Epinephelus lanceolatus♂×E. fuscoguttatus♀. us Fish Shellfish Immunol 93:1047–1055
Makridis P, Martins S, Reis J, Dinis MT (2008) Use of probiotic bacteria in the rearing of Senegalese sole (Solea senegalensis) larvae. Aquac Res 39(6):627–634. https://doi.org/10.1111/j.1365-2109.2008.01933.x
Maynard CL, Elson CO, Hatton RD, Weaver CT (2012) Reciprocal interactions of the intestinal microbiota and immune system. Nature 489(7415):231–241. https://doi.org/10.1038/nature11551
Megarani DV, Hardian AB, Arifianto D, Santosa CM, Salasia SI (2020) Comparative morphology and morphometry of blood cells in zebrafish (Danio rerio), common carp (Cyprinus carpio carpio), and tilapia (Oreochromis niloticus). J Am Assoc Lab Anim Sci 59(6):673–680. https://doi.org/10.30802/aalas-jaalas-20-000013
Mohammadian T, Monjezi N, Peyghan R, Mohammadian B (2022) Effects of dietary probiotic supplements on growth, digestive enzymes activity, intestinal histomorphology and innate immunity of common carp (Cyprinus carpio): a field study. Aquaculture 549:737787. https://doi.org/10.1016/j.aquaculture.2021.737787
Munoz-Atienza E, Araújo C, Lluch N, Hernández PE, Herranz C, Cintas LM, Magadán S (2015) Different impact of heat-inactivated and viable lactic acid bacteria of aquatic origin on turbot (Scophthalmus maximus L.) head-kidney leucocytes. Fish Shellfish Immunol 44(1):214–223. https://doi.org/10.1016/j.fsi.2015.02.021
Nayak SK (2010) Probiotics and immunity: a fish perspective. Fish Shellfish Immunol 29(1):2–14. https://doi.org/10.1016/j.fsi.2010.02.017
Nofouzi K, Sheikhzadeh N, Varshoie H, Sharabyani SK, Jafarnezhad M, Shabanzadeh S, Ahmadifar E, Stanford J, Shahbazfar AA (2019) Beneficial effects of killed Tsukamurella inchonensis on rainbow trout (Oncorhynchus mykiss) growth, intestinal histology, immunological, and biochemical parameters. Fish Physiol Biochem 45(1):209–217. https://doi.org/10.1007/s10695-018-0555-4
Pan X, Wu T, Song Z, Tang H, Zhao Z (2008) Immune responses and enhanced disease resistance in Chinese drum, Miichthys miiuy (Basilewsky), after oral administration of live or dead cells of Clostridium butyrium CB2. J Fish Dis 31(9):679–686. https://doi.org/10.1111/j.1365-2761.2008.00955.x
Patriche T, Patriche N, Tenciu M (2009) Cyprinids total blood proteins determination. Lucrări Ştiinţifice Zootehnie Şi Biotechnologii 42(2):95–101
Peng Z, Yan L, Wei L, Gao X, Shi L, Ren T, Han Y (2022) Effect of dietary chicken gut meal levels on growth performance, plasma biochemical parameters, digestive ability and fillet quality of Cyprinus carpio. Aquac Rep 24:101183. https://doi.org/10.1016/j.aqrep.2022.101183
Putri FP, Dewi NN (2019) Growth monitoring of koi fish (Cypri nus carpio) in natural hatchery techniques in Umbulan, Pasuruan, East Java. IOP Conf Series: Earth Environ Sci 236(1):012016–012018. https://doi.org/10.1088/1755-1315/236/1/012016
Ramos MA, Gonçalves JFM, Batista S, Costas B, Pires MA, Rema P, Ozório ROA (2015) Growth, immune responses and intestinal morphology of rainbow trout (Oncorhynchus mykiss) supplemented with commercial probiotics. Fish Shellfish Immunol 45(1):19–26. https://doi.org/10.1016/j.fsi.2015.04.001
Ramos MA, Batista S, Pires MA, Silva AP, Pereira LF, Saavedra MJ, Ozório ROA, Rema P (2017) Dietary probiotic supplementation improves growth and the intestinal morphology of Nile tilapia. Animal 11(8):1259–1269. https://doi.org/10.1017/S1751731116002792
Rekecki A, Dierckens K, Laureau S, Boon N, Bossier P, Van den Broeck W (2009) Effect of germ-free rearing environment on gut development of larval sea bass (Dicentrarchus labrax L.). Aquaculture 293(1):8–15. https://doi.org/10.1016/j.aquaculture.2009.04.001
Salinas I, Abelli L, Bertoni F, Picchietti S, Roque A, Furones D, Cuesta A, Meseguer J, Esteban MÁ (2008) Monospecies and multispecies probiotic formulations produce different systemic and local immunostimulatory effects in the gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol 25(1):114–123. https://doi.org/10.1016/j.fsi.2008.03.011
Santos L, Ramos F (2016) Analytical strategies for the detection and quantification of antibiotic residues in aquaculture fishes: a review. Trends Food Sci Technol 52:16–30. https://doi.org/10.1016/j.tifs.2016.03.015
Saputra F, Shiu Y-L, Chen Y-C, Puspitasari AW, Danata RH, Liu C-H, Hu S-Y (2016) Dietary supplementation with xylanase-expressing B. áamyloliquefaciens R8 improves growth performance and enhances immunity against Aeromonas hydrophila in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 58:397–405. https://doi.org/10.1016/j.fsi.2016.09.046
Saravanan K, Sivaramakrishnan T, Praveenraj J, Kiruba-Sankar R, Haridas H, Kumar S, Varghese B (2021) Effects of single and multi-strain probiotics on the growth, hemato-immunological, enzymatic activity, gut morphology and disease resistance in Rohu Labeo rohita. Aquaculture 540:736749. https://doi.org/10.1016/j.aquaculture.2021.736749
Segawa S, Nakakita Y, Takata Y, Wakita Y, Kaneko T, Kaneda H, Watari J, Yasui H (2008) Effect of oral administration of heat-killed Lactobacillus brevis SBC8803 on total and ovalbumin-specific immunoglobulin E production through the improvement of Th1/Th2 balance. Int J Food Microbiol 121(1):1–10. https://doi.org/10.1016/j.ijfoodmicro.2007.10.004
Shelby RA, Lim C, Yildirim-Aksoy M, Delaney MA (2006) Effects of probiotic diet supplements on disease resistance and immune response of young Nile tilapia, Oreochromis niloticus. J Appl Aquac 18(2):23–34. https://doi.org/10.1300/j028v18n02_02
Tan X, Sun Z, Chen S, Chen S, Huang Z, Zhou C, Zou C, Liu Q, Ye H, Lin H, Ye C, Wang A (2017) Effects of dietary dandelion extracts on growth performance, body composition, plasma biochemical parameters, immune responses and disease resistance of juvenile golden pompano Trachinotus ovatus. Fish Shellfish Immunol 66:198–206. https://doi.org/10.1016/j.fsi.2017.05.028
Taverniti V, Guglielmetti S (2011) The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: proposal of paraprobiotic concept). Genes Nutr 6(3):261–274. https://doi.org/10.1007/s12263-011-0218-x
Van Doan H, Hoseinifar SH, Naraballobh W, Jaturasitha S, Tongsiri S, Chitmanat C, Ringø E (2019) Dietary inclusion of orange peels derived pectin and Lactobacillus plantarum for Nile tilapia (Oreochromis niloticus) cultured under indoor biofloc systems. Aquaculture 508:98–105. https://doi.org/10.1016/j.aquaculture.2019.03.067
Van Nguyen N, Onoda S, Van Khanh T, Hai PD, Trung NT, Hoang L, Koshio S (2019) Evaluation of dietary heat-killed Lactobacillus plantarum strain L-137 supplementation on growth performance, immunity and stress resistance of Nile tilapia (Oreochromis niloticus). Aquaculture 498:371–379. https://doi.org/10.1016/j.aquaculture.2018.08.081
Wang Y, Wu Y, Wang Y, Xu H, Mei X, Yu D, Wang Y, Li W (2017) Antioxidant properties of probiotic bacteria. Nutrients 9(5):521. https://doi.org/10.3390/nu9050521
Wang J, Yang HL, Xia HQ, Ye JD, Lu KL, Hu X, Feng Y, Ruan L, Sun YZ (2018) Supplementation of heat-inactivated Bacillus clausii DE 5 in diets for grouper, Epinephelus coioides, improves feed utilization, intestinal and systemic immune responses and not growth performance. Aquac Nutr 24(2):821–831. https://doi.org/10.1111/anu.12611
Wang J, Feng J, Liu S, Cai Z, Song D, Yang L, Nie G (2021) The probiotic properties of different preparations using Lactococcus lactis Z-2 on intestinal tract, blood and hepatopancreas in Cyprinus carpio. Aquaculture 543:736911. https://doi.org/10.1016/j.aquaculture.2021.736911
Wang J, Zhang D, Wang Y, Liu Z, Liu L, Shi C (2021b) Probiotic effects of the Bacillus velezensis GY65 strain in the mandarin fish Siniperca Chuatsi. Aquac Rep 21:100902
Wang Z, Zheng N, Liang J, Wang Q, Zu X, Wang H, Yuan H, Zhang R, Guo S, Liu Y, Zhou J (2021) Emodin resists to Cyprinid herpesvirus 3 replication via the pathways of Nrf2/Keap1-ARE and NF-κB in the ornamental koi carp (Cyprinus carpio haematopterus). Comp Biochem Physiol Part C: Toxicol Pharmacol 246:109023. https://doi.org/10.1016/j.cbpc.2021.109023
Wu S, Wang G, Angert ER, Wang W, Li W, Zou H (2012) Composition, diversity, and origin of the bacterial community in grass carp intestine. Plos One 7(2):e30440. https://doi.org/10.1371/journal.pone.0030440
Wu X, Teame T, Hao Q, Ding Q, Liu H, Ran C, Yang Y, Zhang Y, Zhou Z, Duan M (2020) Use of a paraprobiotic and postbiotic feed supplement (HWF™) improves the growth performance, composition and function of gut microbiota in hybrid sturgeon (Acipenser baerii x Acipenser schrenckii). Fish Shellfish Immunol 104:36–45. https://doi.org/10.1016/j.fsi.2020.05.054
Xue J, Shen K, Hu Y, Hu Y, Kumar V, Yang G, Wen C (2020) Effects of dietary Bacillus cereus, B. subtilis, Paracoccus marcusii, and Lactobacillus plantarum supplementation on the growth, immune response, antioxidant capacity, and intestinal health of juvenile grass carp (Ctenopharyngodon idellus). Aquac Rep 17:100387. https://doi.org/10.1016/j.aqrep.2020.100387
Yang H-L, Sun Y-Z, Hu X, Ye J-D, Lu K-L, Hu L-H, Zhang J-J (2019) Bacillus pumilus SE5 originated PG and LTA tuned the intestinal TLRs/MyD88 signaling and microbiota in grouper (Epinephelus coioides). Fish Shellfish Immunol 88:266–271. https://doi.org/10.1016/j.fsi.2019.03.005
Yang G, Shen K, Yu R, Wu Q, Yan Q, Chen W, Ding L, Kumar V, Wen C, Peng M (2020) Probiotic (Bacillus cereus) enhanced growth of Pengze crucian carp concurrent with modulating the antioxidant defense response and exerting beneficial impacts on inflammatory response via Nrf2 activation. Aquaculture 529:735691. https://doi.org/10.1016/j.aquaculture.2020.735691
Yeganeh S, Adel M, Nosratimovafagh A, Dawood MA (2021) The effect of Lactococcus lactis subsp. Lactis PTCC 1403 on the growth performance, digestive enzymes activity, antioxidative status, immune response, and disease resistance of rainbow trout (Oncorhynchus mykiss). Probiotics Antimicrob Proteins 13(6):1723–1733. https://doi.org/10.1007/s12602-021-09787-3
Yi Y, Zhang Z, Zhao F, Liu H, Yu L, Zha J, Wang G (2018) Probiotic potential of Bacillus velezensis JW: antimicrobial activity against fish pathogenic bacteria and immune enhancement effects on Carassius auratus. Fish Shellfish Immunol 78:322–330. https://doi.org/10.1016/j.fsi.2018.04.055
Zadeh SS, Saad CR, Christianus A, Kamarudin MS, Sijam K, Shamsudin MN, Neela VK (2010) Assessment of growth condition for a candidate probiotic, Shewanella algae, isolated from digestive system of a healthy juvenile Penaeus monodon. Aquacult Int 18(6):1017–1026. https://doi.org/10.1007/s10499-010-9319-6
Zhu C-Z, Li D, Chen W-J, Ban S-N, Liu T, Wen H, Jiang M (2021) Effects of dietary host-associated Lactococcus lactis on growth performance, disease resistance, intestinal morphology and intestinal microbiota of mandarin fish (Siniperca chuatsi). Aquaculture 540:736702. https://doi.org/10.1016/j.aquaculture.2021.736702
Hien, T. T. T., Tu, T. L. C., Carris, S. H., Onoda, S., Tuan, T. N., & Duc, P. M. (2021). Dietary supplementation with heat-killed Lactobacillus plantarum L-137 improves growth, immune response, and disease resistance of snakehead (Channa striata). 14(4).
Acknowledgements
Thanks to Dalian Shengtai Biology Science and Technology Co., Ltd for providing the compound probiotics.
Funding
This work was supported by the Development and Demonstration of Efficient Green Production Mode of Important Seawater Fish in Liaoning (grant numbers 2020JH1/10200002) and the Project for Dalian Youth Star of Science and Technology (grant numbers 2019RQ137).
Author information
Authors and Affiliations
Contributions
Lin Wu wrote the manuscript and collected the data. Yuzhe Han revised the manuscript. Tongjun Ren provided funding acquisition and revised the manuscript. Others provided help for this experiment.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The experimental processes in this research confirmed to the Guidelines of the Care and Use of Laboratory Animals in China, and the study was approved by the ethics committee of Dalian Ocean University.
Conflict of interest
The authors declare no competing interests.
Additional information
Handling editor: Gavin Burnell
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, L., Wang, L., Cui, S. et al. Effects of dietary compound probiotics and heat-killed compound probiotics on antioxidative capacity, plasma biochemical parameters, intestinal morphology, and microbiota of Cyprinus carpio haematopterus. Aquacult Int 31, 2199–2219 (2023). https://doi.org/10.1007/s10499-023-01080-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01080-0