Abstract
The gastrointestinal tract of pigs is densely populated with microorganisms that closely interact with the host and with ingested feed. Gut microbiota benefits the host by providing nutrients from dietary substrates and modulating the development and function of the digestive and immune systems. An optimized gastrointestinal microbiome is crucial for pigs’ health, and establishment of the microbiome in piglets is especially important for growth and disease resistance. However, the microbiome in the gastrointestinal tract of piglets is immature and easily influenced by the environment. Supplementing the microbiome of piglets with probiotic bacteria such as Lactobacillus could help create an optimized microbiome by improving the abundance and number of lactobacilli and other indigenous probiotic bacteria. Dominant indigenous probiotic bacteria could improve piglets’ growth and immunity through certain cascade signal transduction pathways. The piglet body provides a permissive habitat and nutrients for bacterial colonization and growth. In return, probiotic bacteria produce prebiotics such as short-chain fatty acids and bacteriocins that benefit piglets by enhancing their growth and reducing their risk of enteric infection by pathogens. A comprehensive understanding of the interactions between piglets and members of their gut microbiota will help develop new dietary interventions that can enhance piglets’ growth, protect piglets from enteric diseases caused by pathogenic bacteria, and maximize host feed utilization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microbiota in the gastrointestinal (GI) tract is an important environmental factor for health [1], because it has evolutionarily conserved roles in the metabolism, immunity, development, and behavior of the host [2, 3]. There is huge diversity in the bacterial species that constitute the intestinal microbiota: approximately 160 species of bacteria are present within every individual [4]. Considerable efforts have focused on cataloging the human gut microbiome and its relationship to complex diseases [5–7]. Many of these studies have been conducted in model organisms. Societal pressure to reduce the number of non-human primates and dogs used in biomedical research has led to an increase in the use of pigs. Pigs are easy to keep and collecting samples from pigs is simple. In addition, the anatomy and physiology of pigs have notable similarities to humans. These factors have helped to establish pigs as a model organism in research fields [8]. However, studies on the gut microbiota of pigs, and especially piglets, have been limited. In addition, factors that affect establishment of intervention on the gut microbiota in piglets and its relationship to pathogen defense have not been satisfactorily reported. The objective of this review was to evaluate the effect and potential mechanism of probiotic Lactobacillus supplementation in piglets and to provide the basis for supplementation of probiotic bacteria in human infants.
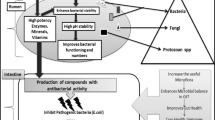
Probiotics contain live microorganisms and spores, and when administered in adequate amounts, confer health benefits to the host [9, 10]. The benefits of probiotic application in humans and animals include inhibition of pathogens [11, 12], improved digestive function [13], and modulation of immune responses [14, 15]. In animal agriculture, probiotics are thought to be an important potential alternative to the use of antibiotic growth promoters (AGP) [16, 17]. When used in piglets, probiotics have been demonstrated to promote growth performance at levels similar to AGPs [18] and to reduce gastrointestinal colonization by pathogens [19].
Lactobacillus
Lactobacillus species are common probiotics, and they play important roles in pathogen defense and improved immunity in piglets [20–22]. The number of described species in the genus Lactobacillus has increased considerably during the last 10–15 years. There are currently 151 described Lactobacillus species [23]. A recent study [24] using next-generation sequencing technology identified Lactobacillus as a core fecal microbiota across the growth stages of pigs. Swine fecal microbiota was found to change significantly across growth stages, but populations of the core members were stable. Furthermore, Niu et al. [25] found that Lactobacillus is one of the most dominant genera, accounting for approximately 15% of 16S rRNA gene sequences from porcine intestinal samples, regardless of age. In addition, the most important end product of fermentation by lactobacilli is lactic acid, which is crucial for piglets’ health.
Inhibition of Pathogens
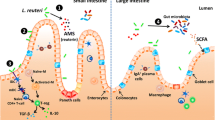
In order to cause infections in piglets, enteric pathogens need to first attach to and then breach the intestinal epithelial barrier [26]. In healthy piglets, commensal bacterial communities in the GI tract colonize intestinal mucosa and form a layer of bacteria covering the mucosal surface. By occupying a diverse array of adherence niches along the GI tract, this layer of dense and complex microbial communities can effectively block the attachment and subsequent colonization by most invading enteric pathogens [27]. This phenomenon is called competitive exclusion [28]. The ability of probiotic lactobacilli to reduce colonization of bacterial pathogens in the gastrointestinal tract is very important for piglets’ health. Probiotic lactobacilli exclude pathogens from attaching to mucosal surfaces through competition for shared binding sites [29, 30] and steric interference of protein adhesins located on the surface of pathogenic bacteria [31, 32]. In addition to preventing adhesion, an in vitro study demonstrated the varying ability of Lactobacillus (L.) acidophilus TMC 0356 and Lactobacillus rhamnosus TMC 0503 to displace Salmonella typhimurium, Cronobacter sakazakii, Clostridium difficile, and Escherichia coli which were already adherent to human epithelial cells [33]. Inhibition of pathogen adherence is also seen in a pig intestinal mucosa model [34]. These studies demonstrate probiotic strain and host-specific inhibition of pathogens, highlighting the need for case-by-case selection of probiotic cultures to reduce adherence of specific pathogens. The production of pathogen-inhibiting compounds is a well understood probiotic mechanism [35]. Neal-McKinney et al. [36] demonstrated that the production of lactic acid by Lactobacillus cultures is an important mechanism for the reduction of Campylobacter jejuni in livestock animals. Hydrogen peroxide production by Lactobacillus has also been shown to inhibit Salmonella [37].
Enhancement of Barrier Function
The intestinal epithelium is specialized to ensure optimal absorption of nutritional compounds, yet at the same time to exclude and neutralize or detoxify harmful components of the intestinal contents including microorganisms. In a healthy gut these functions are optimized and a healthy epithelium is essential to maintaining a healthy gut [38]. The epithelial lining consists of a single layer of epithelial cells covered by layers of mucus produced by specialized goblet cells. Epithelial cells are joined together by cell junctions such as tight junctions (TJs). TJs play a major role in preventing molecules from entering the epithelium between cells [39]. Various stressors may cause weakening of TJs and increase un-regulated paracellular transport of macromolecules into the mucosa. The uncontrolled diffusion of intraluminal toxins, antigens, and enteric microbiota to the underlying tissue results in local and systemic inflammation. The potential of lactobacilli to strengthen the epithelial barrier can be evaluated in vitro by determining the trans-epithelial electrical resistance (TER) in epithelial monolayer cell lines such as Caco-2 or HT29 epithelial cells. TER is dependent on the paracellular flux of ions which is regulated primarily by TJs. Stimulation of TJs may be caused by short-chain fatty acids produced by lactobacilli [40]. Fermentation products of lactobacilli from various types of prebiotic carbohydrates increased TER in Caco-2 monolayers, and the effect was strain and prebiotic dependent.
Improvement of barrier function by lactobacilli and the consequences for disease have been demonstrated in animal models [41]. Stress, especially in the early weaning stage of piglets, induces changes in gut microbiota and the gut epithelial barrier [42, 43]. Previous studies have indicated that stress greatly affects the gastrointestinal microflora, decreasing total Lactobacillus populations in severely stressed animals, and thus providing an opportunity for overgrowth of pathogens [44]. In support of this, Bateup et al. [45] found that the composition of Lactobacillus populations, especially in the stomach and caecal contents of 24-day-old pigs, showed evidence of instability during this stressful period.
Improvement of Immunity
The immune system of the intestinal tract, referred to as the gut-associated lymphoid tissues (GALT), contains the largest pool of immunocompetent cells in the human body [46]. The major function of the GALT is to control our relationship with the microbiota. A central strategy is to minimize contact between microorganisms and the epithelial cell surface, thereby limiting tissue inflammation and microbial translocation [47]. Commensal microorganisms that penetrate the epithelial barrier will be rapidly phagocytosed and destroyed by intestinal macrophages. This dialogue between the gut microbiota and the immune system allows the host to tolerate a large amount of antigens in the gut. Intestinal bacteria at the mucosal surface can create signals called microbial-associated molecular patterns (MAMPs) that stimulate pattern recognition receptors. For example, toll-like receptors (TLR), expressed on the surface of epithelial cells, trigger a cascade of immunological defense mechanisms including the production of antimicrobial peptides, pro- and anti-inflammatory cytokines, or triggers for apoptosis [48]. In turn, to protect their ecological niche, a dominant action of the healthy microbiota on the immune system is to reinforce barrier immunity and therefore their own containment.
Most of probiotic effector molecules are present in the bacterial cell envelope, which is the first side to interact with host intestinal cells [49, 50]. In lactobacilli the cytoplasmic membrane is covered by pentapeptide stem-connected layers of peptidoglycan, which has been shown to modulate immune responses [51, 52]. This layer also serves as a platform for anchoring cell surface molecules, such as wall teichoic acids, wall polysaccharides, and surface proteins [53–55]. In lactobacilli, the disaccharide unit of peptidoglycan can undergo a wide range of modifications, which have important consequences for bacterial physiology. In addition to peptidoglycan, all lactobacilli produce lipoteichoic acid, which contains di-acylated and/or tri-acylated glycolipids [56, 57] that are thought to signal via the heterodimeric TLR complexes TLR-2/6 and TLR-2/1, respectively [58].
In addition to cell wall associated molecules, bacterial genomic DNA can also interact with the host. TLR-9 recognizes bacterial genomic DNA, which, unlike eukaryotic DNA, contains a high frequency of unmethylated CpG motifs [59]. Different species of lactic acid bacteria might differ in their capacity to elicit TLR-9 signaling due to differences in C+G composition and the frequency of stimulatory motifs in the DNA. The expression of TLR-9 by immune cells is intracellular and endosomal, and in polarized epithelial cells, it is expressed on both the apical and basolateral membranes. In polarized epithelial cells, TLR-9 has been shown to have tolerogenic effects to chronic TLR challenges depending on the location of the stimulus [60].
Considering the immature microbiota composition in the GI tract and the inadequate immune system of piglets, supplementation with lactobacilli could effectively activate the body to establish immunity to defend against infection of pathogens.
Modification of the Microbiota Composition
Probiotic lactobacilli can be used in piglets to support the development of a stable microbiota, to prevent diarrheal diseases. During the weaning and post-weaning periods, lactobacilli are used in pigs to modulate the gastrointestinal microbiota to prevent post-weaning diarrhea and stimulate growth. Yang et al. [61] showed that Lactobacillus plantarum significantly decreased E. coli and aerobe counts and increased lactobacilli and anaerobe counts in the digesta and mucosa of most sections of the GI tract compared with a control group. Liu et al. [62] reported that Lactobacillus reuteri I5007 plays a positive role in gut development in piglets by modulating microbial composition and intestinal development. Denaturing gradient gel electrophoresis revealed that L. reuteri I5007 affected the colonic microbial communities on day 14, in particular, and reduced numbers of Clostridium spp. In weaning pigs, administration of L. reuteri BSA131 decreased the number of enterobacteria in the feces [63]. The mechanism that contributes to the selective stimulation of bacterial groups by lactobacilli supplementation relates to cross-feeding. Cross-feeding is the phenomenon of partial degradation products being released by primary degraders that stimulate the growth of other bacterial groups. For instance, lactate produced by lactobacilli can be converted by lactate-utilizing bacteria, such as Eubacterium and Anaerostipes, to produce butyrate [64]. Other butyrate-producing bacteria, such as Faecalibacterium prausnitzii and Roseburia spp., mostly belong to the Firmicutes phylum and convert acetate into butyrate [65, 66]. A stimulation of F. prausnitzii was observed after dietary intervention with inulin (10 g/day for 16 days) in healthy subjects [67]. This mechanism also explains the butyrogenic effect observed after Lactobacillus administration. Lactobacilli do not produce butyrate but provide lactate or acetate for cross-feeding to those other bacterial groups.
Function of Short-Chain Fatty Acids
Short-chain fatty acids (SCFA) are the major anions within the intestinal lumen and are mainly produced by anaerobic fermentation of undigested carbohydrates and, to a lesser extent, proteins [68, 69]. Most of the SCFA formed by intestinal bacteria are rapidly absorbed and used to some degree as energy substrates by mucosal epithelial cells. In this way, SCFA provide about 10% of the daily caloric requirements in humans [70], with butyrate being the preferred energy source for the colonocytes. Readily fermentable dietary fiber has been shown to stimulate epithelial cell proliferation in the intestine only in the presence of gut bacteria, suggesting that the end products of fermentation are responsible for this effect [71]. Increased SCFA synthesis also contributes to host homeostasis by acidifying the luminal pH, which inhibits the growth of pathogens [72], reduces the formation of secondary bile acids [73], and impairs the activity of specific enzymes such as proteases. Furthermore, SCFA have been shown to possess anti-inflammatory capacities, affect satiety hormones, and play a role in insulin resistance [74]. SCFA are speculated to have a role in prevention of some human pathological conditions such as ulcerative colitis and colon carcinogenesis. Diversion colitis, which occurs in diverted segments of the large bowel excluded from fecal transit, improves after treatment with a local perfusion of SCFA [75].
Many health benefits in and outside the gut have been attributed to increased production of SCFA by stimulated beneficial bacteria. Simple acidification of the colonic lumen by the production of SCFA can explain some of the observed benefits of prebiotics. In addition, SCFA are considered as a class of bacterial products that mediate the interactions between the diet, the intestinal microbiota, and the host. Two major SCFA signaling mechanisms have been identified: the inhibition of histone deacetylase and the activation of G-protein coupled receptors [76]. In addition, SCFA easily enter the cells through passive diffusion or receptor-mediated transport and can internally act at other sites [77, 78]. The transduction pathway of butyrate-induced apoptosis has been shown to involve the activation of the caspase cascade. Butyrate activates p38 mitogen-activated protein kinase (p38 MAPK), which in turn up-regulates expression and receptor activity of the peroxisome proliferator activated receptor gamma (PPARγ). PPARγ activates caspase-8 and caspase-9 leading to increased caspase-3 activity which will eventually result in cell death [79]. Activation of PPARγ has been effective in the prophylaxis, and to a lesser extent, in the treatment of several animal models of acute or chronic colitis [80, 81]. PPARγ plays a fundamentally important role in the immune response through its ability to inhibit the expression of inflammatory cytokines and to direct the differentiation of immune cells towards anti-inflammatory phenotypes [82, 83].
Protective Function of Other Metabolites from Lactobacilli
The intestinal mucosa is the interface between the internal and external environments and forms a crucial line of defense to prevent luminal pathogens and harmful substances from entering into the internal milieu. This barrier function is ensured by protection mechanisms at multiple levels [84]. Certain probiotic lactobacilli have the ability to secrete antimicrobial substances, such as bacteriocins and organic acids, which inhibit the growth of other bacteria. The production of bacteriocins by probiotic lactobacilli has the potential to prevent gastrointestinal infection in humans. A direct challenge study in mice demonstrated that bacteriocin production by Lactobacillus salivarius UCC118 reduced counts of Listeria monocytogenes by 80% in the liver and spleen of infected mice relative to a negative control [85]. L. salivarius UCC118 also protected mice from infection by S typhimurium, but the protection was not bacteriocin mediated. While bacteriocin production by probiotic cultures is hoped to be an important alternative to antibiotics in the treatment of bacterial infections, the effectiveness of this mechanism has not yet been evaluated in humans.
Supplementation of lactobacilli results in a decrease of the colonic luminal pH due to the production of lacto acid, which affects the composition of the microbiota due to the differential sensitivity of bacterial species to acidic pH. Bacteroides spp. are relatively sensitive to mildly acidic pH, whereas Firmicutes spp. and bifidobacteria are more acid tolerant and are therefore less affected by a decrease in pH [86]. Because of special physiological characteristics, piglet guts lack enough acid to help food metabolism. Hence, supplementation with lactobacilli could effectively promote metabolism and nutrition absorption.
The secretion of mucus and immunoglobulin A by different epithelial cells minimizes the chances for direct contact of bacteria with epithelial cells. Commensal species have been shown to limit pathogen colonization through competition for nutrients and adhesion sites, a process called colonization resistance [87]. Lactobacillus GG could prevent cytokine-induced apoptosis and inhibit pro-apoptotic p38/MAPK activation [88, 89]. These factors are also able to modulate hydrogen peroxide induced damage in Caco-2 cells [90]. Other molecules produced by lactobacilli have been found to have important characteristics. An analysis of genomic sequences from Lactobacillus strains predicts a broad group of bacteriocins that are active against Gram-positive bacteria such as L. salivarius UCC118. A class II bacteriocin produced by Lactobacillus strains also has the ability to protect mice against infection with L. monocytogenes [85]. Several other lactobacilli have been tested in different in vivo and in vitro tests with positive results [91, 92].
Need for Caution
Because of special physiological characteristics of piglets such as a scarcity of acids secreted by the stomach, as well as immature microbiomes and immune systems, supplementation of lactobacilli could effectively enhance the GI tract environment by inducing an optimal composition of microbiota, improving intestinal barrier function, and improving immunity for defense against pathogen infection. However, the results of feeding Lactobacillus spp. to pigs are inconsistent, with some reports showing no difference in growth performance of weaning pigs fed diets with and without lactobacilli [93]. It is known that lactobacilli have strain-specific characteristics, and that these may affect specific interactions between bacterial populations and the host. First, the strain was non-indigenous to the farm and may not have possessed the ecological attributes necessary for long-term association with these pigs. Second, the strain was initially predominant in the GI of piglets and may not have been suitably adapted for life in the gastrointestinal milieu of older pigs. Hence, when isolating probiotic bacteria, we should consider the animal species. The probiotic bacteria isolated from an animal species should be use in the certain species in application of the probiotic production. Also, the probiotic bacteria isolated from animals in certain growth period should used in such growth period of animal, which could play efficient roles.
The microbiome in the GI tract of piglets is immature, especially in neonatal animals. Providing probiotic bacteria to neonatal animals to establish a healthy gastrointestinal microbiome would be helpful [8]. Supplementation of probiotic bacteria for animals with disorders of the GI tract occurred caused by stress or pathogen infection also could be important, to reconstitute healthy microbiomes. However, the doses for supplementation are unknown and require further research.
For special human group such as premature infants and hypoimmunity persons, usage of probiotic product needs attention for their adverse reactions, for generalized infection, excessive immune stimulation, gene transfer, or untoward effect of gastrointestinal [94, 95].
Future Prospects
Other strains of probiotics also have been used in neonatal animals including species of Bifidobacterium, Lactobacillus, Streptococcus, Saccharomyces, Aspergillus, and Bacillus. Bifidobacterium is the predominant genus of the gut microbiota of infants [96]. Owing to their recognized benefits to human health, bifidobacteria also play an important role in the health of neonatal piglets. Therefore, combined use of multiple strains of probiotic bacteria may lead to larger improvements compared to single strains [97].
Considering the beneficial effects of prebiotics such as oligosaccharides, active peptides, and other biologically essential microelements, combined use of probiotic bacteria and prebiotics in piglets may enhance the useful effects. Combined use of organic Chromium and probiotic Bacillus subtilis KT260179 might have a greater effect on regulating animal model mouse body metabolism [98]. Use of probiotic cheese, flaxseed, and other prebiotics is a good dietary supplement for piglets before weaning, helping them to adapt to changes in diet more easily and reducing the likelihood of chronic diseases [99, 100].
Culture-dependent methods combined with meta-omics approaches can link proteins and metabolic pathways to functions and probiotic properties of selected strains, which consist of the application of high-throughput culture conditions to the study of the body microbiota and uses matrix-assisted laser desorption/ionization–time of flight or 16S rRNA amplification and sequencing for the identification of growing colonies [101]. Culturomics revolutionized the understanding of the relationships among the human microbiome, health, and diseases and generated a number of sequences that can be assigned to a known microorganism.
Conclusion
The production of piglets has entered an era when the use of antibiotics is increasingly banned. Probiotics, which are a potential alternative to in feed antibiotics, can expect a promising future. Besides, the selection of excellent strains and improved processing techniques, more research, especially in the form of well-designed animal trials, is needed to evaluate the efficacy. More studies are also needed to explore the mechanisms of action of lactobacilli in piglets. With evolving knowledge, effective use of lactobacilli will be possible in the future.
GI, gastrointestinal; TJs, tight junctions; TER, trans-epithelial electrical resistance; GALT, gut-associated lymphoid tissues; MAMPs, microbial-associated molecular patterns; TLR, toll-like receptors; SCFA, short-chain fatty acids.
References
Clemente JCC, Ursell LKK, Parfrey LWW, Knight R (2012) The impact of the gut microbiota on human health: an integrative view. Cell 148:1258–1270
Cabreiro F, Gems D (2013) Worms need microbes too: microbiota, health and aging in Caenorhabditis elegans. EMBO Mol Med 5:1300–1310
Erkosar B, Storelli G, Defaye A, Leulier F (2013) Host-intestinal microbiota mutualism: “learning on the fly”. Cell Host Microbe 13:8–14
Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T et al (2010) A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464:59–65
Human Microbiome Project Consortium (2012) Structure, function and diversity of the healthy human microbiome. Nature 486:207–214
Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, Nielsen J, Bäckhed F (2013) Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 498:99–103
Li J, Jia H, Cai X, Zhong H, Feng Q, Sunagawa S, Arumugam M, Kultima JR, Prifti E, Nielsen T et al (2014) MetaHIT consortium; MetaHIT consortium an integrated catalog of reference genes in the human gut microbiome. Nat Biotechnol 32:834–841
Brown RF, Jugg BJ, Harbanm FM, Ashley Z, Kenward CE, Platt J, Hill A, Rice P, Watkins PE (2002) Pathophysiological responses following phosgene exposure in the anaesthetized pig. J Appl Toxicol 22:263–269
Hooper LV, Gordon JI (2001) Commensal host-bacterial relationships in the gut. Science 292:1115–1158
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME (2014) Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11:506–514
Hoyos AB (1999) Reduced incidence of necrotizing enterocolitis associated with enteral administration of Lactobacillus acidophilus and Bifidobacterium infantis to neonates in an intensive care unit. Int J Infect Dis 3:197–202
Sgouras D, Maragkoudakis P, Petraki K, Martinez-Gonzalez B, Eriotou E, Michopoulos S, Kalantzopoulos G, Tsakalidou E, Mentis A (2004) In vitro and in vivo inhibition of Helicobacter pylori by Lactobacillus casei strain Shirota. Appl Environ Microbiol 70:518–526
Parvez S, Malik KA, Kang SA, Kim HY (2006) Probiotics and their fermented food products are beneficial for health. J Appl Microbiol 100:1171–1185
Rosenfeldt V, Benfeldt E, Nielsen SD, Michaelsen KF, Jeppesen DL, Valerius NH, Paerregaard A (2003) Effect of probiotic Lactobacillus strains in children with atopic dermatitis. J Allergy Clin Immun 11:389–395
Sartor RB (2004) Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology 126:1620–1633
Huyghebaert G, Ducatelle R, Van IF (2011) An update on alternatives to antimicrobial growth promoters for broilers. Vet J 187:82–188
Yang Y, Iji PA, Choct M (2009) Dietary modulation of gut microflora in broiler chickens: a review of the role of six kinds of alternatives to in-feed antibiotics. World Poultry Sci J 65:97–114
Shen YB, Piao XS, Kim SW, Wang L, Liu P, Yoon I, Zhen YG (2009) Effects of yeast culture supplementation on growth performance, intestinal health, and immune response of nursery pigs. J Anim Sci 87:2614–2624
Molbak L, Thomsen LE, Jensen TK, Knudsen KEB, Boye M (2007) Increased amount of Bifidobacterium thermacidophilum and Megasphaera elsdenii in the colonic microbiota of pigs fed a swine dysentery preventive diet containing chicory roots and sweet lupine. J Appl Microbiol 103:1853–1867
Wegmann U, MacKenzie DA, Zheng J, Goesmann A, Roos S, Swarbreck D, Walter J, Crossman LC, Juge N (2015) The pan-genome of Lactobacillus reuteri strains originating from the pig gastrointestinal tract. BMC Genomics 6:1023
Lamas B, Richard ML, Leducq V, Pham HP, Michel ML, Da CG et al (2016) Card9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med 22:598–605
Li H, Lei Z, Chen L, Qi Z, Wang W, Qiao J (2016) Lactobacillus acidophilus, alleviates the inflammatory response to enterotoxigenic Escherichia coli, k88 via inhibition of the nf-κb and p38 mitogen-activated protein kinase signaling pathways in piglets. BMC Microbiol 16:273
Vandamme P, De Bruyne K, Pot B (2014) Phylogenetics and systematics. In: Holzapfel WH, Wood BJB (eds) Lactic acid bacteria: biodiversity and taxonomy. Wiley Blackwell, Chichester, pp 31–44
Kim J, Nguyen SG, Guevarra RB, Lee I, Unno T (2015) Analysis of swine fecal microbiota at various growth stages. Arch Microbiol 197:753–759
Niu Q, Li P, Hao S, Zhang Y, Kim SW, Li H et al (2015) Dynamic distribution of the gut microbiota and the relationship with apparent crude fiber digestibility and growth stages in pigs. Sci Rep 5:9938
Roselli M, Finamore A, Britt MS, Mengheri E (2006) The probiotic bacteria Bifidobacterium animalis MB5 and Lactobacillus rhamnosus GG protect the intestinal Caco-2 cells from inflammation-associated response induced by enterotoxigenic Escherichia coli K88. Br J Nutr 95:1177–1184
Turner MS, Waldherr F, Loessner MJ, Giffard PM (2007) Antimicrobial activity of lysostaphin and a Listeria monocytogenes bacteriophage endolysin produced and secreted by lactic acid bacteria. Syst Appl Microbiol 30:58–67
Lawley TD, Walker AW (2013) Intestinal colonization resistance. Immunology 138:1–11
Lu L, Walker WA (2001) Pathologic and physiologic interactions of bacteria with the gastrointestinal epithelium. Am J Clin Nutr 73:1124s–1130s
Markowicz C, Olejnik-Schmidt A, Borkowska M, Schmidt MT (2014) SpaCBA sequence instability and its relationship to the adhesion efficiency of Lactobacillus casei group isolates to Caco-2 cells. Acta Biochim Pol 61:341–347
Bernet MF, Brassart D, Neeser JR, Servin AL (1994) Lactobacillus acidophilus La-1 binds to cultured human intestinal-cell lines and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut 35:483–489
Lee YK, Puong KY (2002) Competition for adhesion between probiotics and human gastrointestinal pathogens in the presence of carbohydrate. Br J Nutr 88:S101–S108
Gueimonde M, Jalonen L, He F, Hiramatsu M, Salminen S (2006) Adhesion and competitive inhibition and displacement of human enteropathogens by selected lactobacilli. Food Res Int 39:467–471
Collado MC, Grzeskowiak L, Salminen S (2007) Probiotic strains and their combination inhibit in vitro adhesion of pathogens to pig intestinal mucosa. Curr Microbiol 55:260–265
Oelschlaeger TA (2010) Mechanisms of probiotic actions—a review. Int J Med Microbiol 300:57–62
Neal-McKinney JM, Lu X, Duong T, Larson CL, Call DR, Shah DH, Konkel ME (2012) Production of organic acids by probiotic lactobacilli can be used to reduce pathogen load in poultry. PLoS One 7:e43928
Pridmore RD, Pittet AC, Praplan F, Cavadini C (2008) Hydrogen peroxide production by Lactobacillus johnsonii NCC 533 and its role in anti-Salmonella activity. FEMS Microbiol Lett 283:210–215
Lopez P, Gonzalez-Rodriguez I, Sanchez B, Ruas-Madiedo P, Suarez A, Margolles A, Gueimonde M (2012) Interaction of Bifidobacterium bifidum LMG13195 with HT29 cells influences regulatory-Tcellassociated chemokine receptor expression. Appl Environ Microbiol 78:850–2857
Chassaing B, Gewirtz AT (2016) Has provoking microbiota aggression driven the obesity epidemic? BioEssays 38:122–128
Sultana R, McBain AJ, O’Neill CA (2013) Strain-dependent augmentation of tight–junction barrier function in human primary epidermal keratinocytes by Lactobacillus and Bifidobacterium lysates. Appl Environ Microbiol 79:4887–4894
Wlodarska M, Willing B, Keeney KM, Menendez A, Bergstrom KS, Gill N, Russell SL, Vallance BA, Finlay BB (2011) Antibiotic treatment alters the colonic mucus layer and predisposes the host to exacerbated Citrobacter rodentium-induced colitis. Infect Immun 79:1536–1545
Isaacson R, Kim HB (2012) The intestinal microbiome of the pig. Anim Health Res Rev 13:100–109
Weese JS, Slifierz M, Jalali M, Friendship R (2014) Evaluation of the nasal microbiota in slaughter-age pigs and the impact on nasal methicillin-resistant Staphylococcus aureus (MRSA) carriage. BMC Vet Res 15:69
Konstantinov SR, Awati AA, Williams BA, Miller BG, Jones P, Stokes CR et al (2006) Post-natal development of the porcine microbiota composition and activities. Environ Microbiol 8:1191–1199
Bateup JM, Dobbinson S, McConnell MA, Munro K, Tannock GW (1998) Molecular analysis of the composition of Lactobacillus populations inhabiting the stomach and caecum of pigs. Microb Ecol Health Dis 10:95–102
Pabst R, Russell MW, Brandtzaeg P (2008) Tissue distribution of lymphocytes and plasma cells and the role of the gut. Trends Immunol 29:206–208
Belkaid Y, Hand TW (2014) Role of the microbiota in immunity and inflammation. Cell 157:121–141
Sharma R, Young C, Neu J (2010) Molecular modulation of intestinal epithelial barrier: contribution of microbiota. J Biomed Biotechnol 4:305879
Remus DM, Kleerebezem M, Bron PA (2011) An intimate tetea-tete–how probiotic lactobacilli communicate with the host. Eur J Pharmacol 668:S33–S42
Wells JM, Rossi O, Meijerink M, van Baarlen P (2011) Epithelial crosstalk at the microbiota–mucosal interface. Proc Natl Acad Sci U S A 108:4607–4614
Asong J, Wolfert MA, Maiti KK, Miller D, Boons GJ (2009) Binding and cellular activation studies reveal that toll-like receptor 2 can differentially recognize peptidoglycan from Gram-positive and Gram-negative bacteria. J Biol Chem 284:8643–8653
Dziarski R (2003) Recognition of bacterial peptidoglycan by the innate immune system. Cell Mol Life Sci 60:1793–1804
Delcour J, Ferain T, Deghorain M, Palumbo E, Hols P (1999) The biosynthesis and functionality of the cell-wall of lactic acid bacteria. Antonie Van Leeuwenhoek 76:159–184
Kleerebezem M, Hols P, Bernard E, Rolain T, Zhou M, Siezen RJ, Bron PA (2010) The extracellular biology of the lactobacilli. FEMS Microbiol Rev 34:199–230
Vollmer W, Blanot D, de Pedro MA (2008) Peptidoglycan structure and architecture. FEMS Microbiol Rev 32:149–167
Claes IJ, Segers ME, Verhoeven TL, Dusselier M, Sels BF, De Keersmaecker SC, Vanderleyden J, Lebeer S (2012) Lipoteichoic acid is an important microbe-associated molecular pattern of Lactobacillus rhamnosus GG. Microb Cell Factories 11:161
Jang KS, Baik JE, Han SH, Chung DK, Kim BG (2011) Multi-spectrometric analyses of lipoteichoic acids isolated from Lactobacillus plantarum. Biochem Biophys Res Commun 407:823–830
Wells JM, Mercenier A (2008) Mucosal delivery of therapeutic and prophylactic molecules using lactic acid bacteria. Nat Rev Microbiol 6:349–362
Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K et al (2000) A toll-like receptor recognizes bacterial DNA. Nature 408:740–745
Lee J, Mo JH, Katakura K, Alkalay I, Rucker AN, Liu YT, Lee HK, Shen C, Cojocaru G, Shenouda S et al (2006) Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol 8:1327–1336
Yang KM, Jiang ZY, Zheng CT, Wang L, Yang XF (2014) Effect of Lactobacillus plantarum on diarrhea and intestinal barrier function of young piglets challenged with enterotoxigenic Escherichia coli K88. J Anim Sci 4:1496–1503
Liu H, Zhang J, Zhang S, Yang F, Thacker PA, Zhang G et al (2014) Oral administration of Lactobacillus fermentum I5007 favors intestinal development and alters the intestinal microbiota in formula-fed piglets. J Agric Food Chem 62:860–866
Chang YH, Kim JK, Kim HJ, Kim WY, Kim YB, Park YH (2001) Selection of a potential probiotic Lactobacillus strain and subsequent in vivo studies. Antonie Van Leeuwenhoek 80:193–199
Munoz-Tamayo R, Laroche B, Walter E, Doré J, Duncan SH, Flint HJ, Leclerc M (2011) Kinetic modeling of lactate utilization and butyrate production by key human colonic bacterial species. FEMS Microbiol Ecol 76:615–624
Duncan SH, Holtrop G, Lobley GE, Calder AG, Stewart CS, Flint HJ (2004) Contribution of acetate to butyrate formation by human faecal bacteria. Br J Nutr 91:915–923
Falony G, Vlachou A, Verbrugghe K, De Vuyst L (2006) Cross feeding between Bifidobacterium longum BB536 and acetate-converting, butyrate-producing colon bacteria during growth on oligofructose. Appl Environ Microbiol 72:7835–7841
Ramirez-Farias C, Slezak K, Fuller Z, Duncan A, Holtrop G, Louis P (2009) Effect of inulin on the human gut microbiota: stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br J Nutr 101:541–550
Topping DL, Clifton PM (2001) Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev 81:1031–1064
Rycroft CE, Jones RM, Gibson GR, Rastall RA (2001) A comparative in vitro evaluation of the fermentation properties of prebiotic oligosaccharides. J Appl Microbiol 91:878–887
Bergman EN (1990) Energy contributions of volatile fatty-acids from the gastrointestinal tract in various species. Physiol Rev 70:567–590
Sakata T (1987) Stimulatory effect of short-chain fatty-acids on epithelialcell proliferation in the rat intestine—a possible explanation for trophic effects of fermentable fibre, gut microbes and luminal trophic factors. Br J Nutr 58:95–103
Scheppach W, Lueh H, Menzel T (2001) Beneficial health effects of low-digestible carbohydrate consumption. Br J Nutr 85:S23–S30
Zampa A, Silvi S, Fabiani R, Morozzi G, Orpianesi C, Cresci A (2004) Effects of different digestible carbohydrates on bile acid metabolism and SCFA production by human gut micro-flora grown in an in vitro semi-continuous culture. Anaerobe 10:19–26
Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ (2008) Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther 27:104–119
Kiely EM, Ajayi NA, Wheeler RA, Malone M (2001) Diversion procto-colitis: response to treatment with short-chain fatty acids. J Pediatr Surg 36:1514–1517
Schilderink R, Verseijden C, de Jonge WJ (2013) Dietary inhibitors of histone deacetylases in intestinal immunity and homeostasis. Front Immunol 4:226
Layden BT, Angueira AR, Brodsky M, Durai V, Lowe WL (2013) Short chain fatty acids and their receptors: new metabolic targets. Transl Res 161:131–140
Santini V, Gozzini A, Ferrari G (2007) Histone deacetylase inhibitors: molecular and biological activity as clinical application. Curr Drug Metab 8:383–394
Schwab M, Reynders V, Ulrich S, Zahn N, Stein J, Schroder O (2006) PPAR gamma is a key target of butyrate-induced caspase-3 activation in the colorectal cancer cell line Caco-2. Apoptosis 11:801–1811
Viladomiu M, Hontecillas R, Yuan LJ, Lu PY, Bassaganya-Riera J (2013) Nutritional protective mechanisms against gut inflammation. J Nutr Biochem 24:929–939
Annese V, Rogai F, Settesoldi A, Bagnoli S (2012) PPAR gamma in inflammatory bowel disease. PPAR Res 2012:620839
Martin H (2009) Role of PPAR-gamma in inflammation. Prospects for therapeutic intervention by food components. Mutat Res 669:1–7
Vanhoutvin SA, Troost FJ, Hamer HM, Lindsey PJ, Koek GH, Jonkers DM, Kodde A, Venema K, Brummer RJ (2009) Butyrate-induced transcriptional changes in human colonic mucosa. PLoS One 4:e6759
Koboziev I, Webb CR, Furr KL, Grisham MB (2014) Role of the enteric microbiota in intestinal homeostasis and inflammation. Free Radic Biol Med 68:122–133
Corr SC, Li Y, Riedel CU, O’Toole PW, Hill C, Gahan CG (2007) Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc Natl Acad Sci U S A 104:7617–7621
Duncan SH, Louis P, Thomson JM, Flint HJ (2009) The role of pH in determining the species composition of the human colonic microbiota. Environ Microbiol 11:2112–2122
Kamada N, Chen GY, Inohara N, Nunez G (2013) Control of pathogens and pathobionts by the gut microbiota. Nat Immunol 14:685–690
Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB (2007) Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 132:562–575
Tao Y, Drabik KA, Waypa TS, Musch MW, Alverdy JC, Schneewind O, Chang EB, Petrof EO (2006) Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells. Am J Physiol Cell Physiol 291:C1018–C11030
Seth A, Yan F, Polk DB, Rao RK (2008) Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC- and MAP kinase-dependent mechanism. American journal of physiology Gastrointest Liver Physiol 294:G1060–G1069
Macho Fernandez E, Pot B, Grangette C (2011a) Beneficial effect of probiotics in IBD: are peptidogycan and NOD2 the molecular key effectors? Gut Microbes 2:280–286
Macho Fernandez E, Valenti V, Rockel C, Hermann C, Pot B, Boneca IG, Grangette C (2011b) Anti-inflammatory capacity of selected lactobacilli in experimental colitis is driven by NOD2-mediated recognition of a specific peptidoglycan-derived muropeptide. Gut 60:1050–1059
Harper AF, Kornegay ET, Bryant KL, Thomas HR (1983) Efficacy of virginiamycin and a commercially-available Lactobacillus probiotic in swine diets. Anim Feed Sci Technol 8:69–76
Cruchet S, Furnes R, Maruy A, Hebel E, Palacios J, Medina F, Ramirez N, Orsi M, Rondon L, Sdepanian V, Xóchihua L, Ybarra M, Zablah RA (2015) The use of probiotics in pediatric gastroenterology: a review of the literature and recommendations by Latin-American experts. Paediatr Drugs 17:199–216
Chapman CM, Gibson GR, Rowland I (2011) Health benefits of probiotics: are mixtures more effective than single strains? Eur J Nutr 50:1–17
Whelan K, Myers CE (2010) Safety of probiotics in patients receiving nutritional support: a systematic review of case reports, randomized controlled trials, and nonrandomized trials. Am J Clin Nutr 91:687–703
Andrejčáková Z, Sopková D, Vlčková R, Kulichová L, Gancarčíková S, Almášiová V, Holovská K, Petrilla V, Krešáková L (2016) Synbiotics suppress the release of lactate dehydrogenase, promote non-specific immunity and integrity of jejunum mucosa in piglets. Anim Sci J 7:1157–1166
Sopková D, Hertelyová Z, Andrejčáková Z, Vlčková R, Gancarčíková S, Petrilla V, Ondrašovičová S, Krešáková L (2017) The application of probiotics and flaxseed promotes metabolism of n-3 polyunsaturated fatty acids in pigs. J Appl Anim Res 45:93–98
Yang J, Xu Y, Qian K, Zhang W, Wu D, Wang C (2016) Effects of chromium-enriched Bacillus subtilis KT260179 supplementation on growth performance, caecal microbiology, tissue chromium level, insulin receptor expression and plasma biochemical profile of mice under heat stress. Br J Nutr 115:774–781
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME (2014) Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11:506–514
Lagier JC, Khelaifia S, Alou MT, Ndongo S, Dione N, Hugon P, Caputo A et al (2016) Culture of previously uncultured members of the human gut microbiota by culturomics. Nat Microbiol. doi:10.1038/NMICROBIOL2016203
Acknowledgements
This work was sponsored by the fund of Construction of research field in Anhui Academy of Agricultural Sciences of China (No.: 16A0410) and Anhui Modern Agricultural Project for Pig Industry. The funders had no role in the design of the study and collection, analysis, and interpretation of data and in writing of the manuscript.
Authors’ Contributions
JY carried out the literature study and drafted the manuscript. KW, CW, and YW critically evaluated the manuscript. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Yang, J., Qian, K., Wang, C. et al. Roles of Probiotic Lactobacilli Inclusion in Helping Piglets Establish Healthy Intestinal Inter-environment for Pathogen Defense. Probiotics & Antimicro. Prot. 10, 243–250 (2018). https://doi.org/10.1007/s12602-017-9273-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-017-9273-y