Abstract
The influence of temperature, initial pH, and carbon and nitrogen sources on bacteriocin secreted by Lactococcus lactis MM19 (MM19) and Pediococcus acidilactici MM33 (MM33) was evaluated. It was found that 30 and 45 °C were the growth temperatures for higher nisin and pediocin production by MM19 and MM33, respectively. The initial pH values for higher production of nisin and pediocin were 9 and 6, respectively. Glucose and wheat peptone E430 were found as suitable carbon and nitrogen sources, respectively, for highest nisin production by MM19 at 30 °C and initial pH of 9. In these conditions, nisin production could be increased by 6.7 times as compared to the control medium (de Man, Rogosa, and Sharpe—MRS broth). Similarly, fructose and pea peptone were suitable carbon and nitrogen sources, respectively, for highest production of pediocin by MM33 at 45 °C and initial pH of 6. In these conditions, pediocin production by MM33 was increased by three times as compared to the control medium (tryptone-glucose-yeast extract—TGE broth).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Probiotics are widely defined as «live microbial feed supplements» with beneficial effects on the host by improving its intestinal microbial balance. Lactococcus lactis subsp. lactis and Pediococcus acidilactici are microorganisms which are classified as generally recognized as safe (GRAS). These beneficial bacteria are used in pharmaceutical and food industries [1]. Probiotic bacteria are known for their production of antimicrobial metabolites such as lactic acid, diacetyl, hydrogen peroxide, and bacteriocins [2]. Lactic acid bacteria (LAB) and their bacteriocins are also used as food preservatives. Therefore, production of LAB biomass and their bacteriocins with high antibacterial activity are important in both technological and economical point of view.
Nisin is approved for food preservation by many countries, and it has been produced industrially [3]. According to Parente and Ricciardi [3] in the industrial process, yeast extract is added to pasteurized milk and the medium is treated with a protease. The treated substrate is used as a fermentation medium for nisin production under controlled pH and temperature. At the end of fermentation, a nisin extraction process is conducted, and the powder of extracted nisin is obtained by a spray-drying step. The powder is standardized and mixed with NaCl to obtain a final powder product with an activity of 1 × 106 IU g−1 [3]. Further steps for purification of nisin are very expensive, and therefore, commercial nisin is routinely available as raw powder product for food application [3].
The bacterial growth and bacteriocin production by LAB are strongly influenced by microbial strains, medium composition consisting of carbon and nitrogen sources as well as other growth factors, such as oligo elements, some minerals and vitamins, and other fermentation conditions such as initial pH, temperature, and dissolved oxygen level [3–6]. In addition, the LAB are nutritionally fastidious microorganisms. Their growth and bacteriocin production are often limited by organic nitrogen sources rather than by carbon substrates [3].
Recently, our laboratory has isolated many LAB from human intestine, and two of them were identified as Lactococcus lactis supsp. lactis (MM19) and Pediococcus acidilactici (MM33) [7]. L. lactis MM19 is a nisin-producing strain, while P. acidilactici MM33 is a pediocin-producing strain [8]. For bacteriocin production from these isolated strains in the previous research, de Man, Rogosa, and Sharpe (MRS) broth medium was used as a basic medium [7, 8]. In case of pediocin production by P. acidilactici, Biswas et al. [9] demonstrated that tryptone-glucose-yeast extract (TGE) broth was a better medium as compared to MRS broth. It is supposed that by utilization of suitable carbon and nitrogen sources as well as applying suitable temperature and pH for the growth of these isolated strains (MM19 and MM33), higher bacteriocins can be reached.
Therefore, the main objective of the present work was to study the influence of temperature, initial pH, and different sources of carbon and nitrogen on bacteriocin production by L. lactis MM19 and P. acidilactici MM33.
Materials and Methods
Bacterial Strains
L. lactis MM19 and P. acidilactici MM33 were isolated in our laboratory from human stool [7]. These organisms were used to produce nisin and pediocin, respectively. They were stored at −80 °C in MRS broth medium (Difco Laboratories, Detroit, MI, USA) containing glycerol (10 % V/V). In this study, the basal media for culturing L. lactis MM19 and P. acidilactici were MRS broth and TGE, respectively. The composition of these media is presented in Table 1. Before each experiment, stock cultures were activated by propagating through two consecutive 24-h growth cycles at 37 °C.
In order to estimate the bacteriocin activity, a well diffusion assay was used. Lactobacillus sakei was used an indicator strain in this assay. Stock cultures of L. sakei were stored at −80 °C in MRS broth containing glycerol (10 % V/V). L. sakei was propagated in MRS culture broth at 37 ± 1 °C. Before being used in experiments, this strain was propagated in broth twice overnight.
Bacteriocin Assay
For bacteriocin assay, the cell-free supernatant (CFS) of cultured broth of L. lactis MM19 and P. acidilactici MM33 was used. Supernatant was obtained by centrifugation of the cultured broth at 6000×g for 30 min at 4 °C followed by neutralization to pH 6.5 using 1 N NaOH solution. The neutralization of supernatant aimed at preventing the effect of organic acids produced by these cultures. The neutralized supernatant was then filtered through a 0.2-µm filter (Sarsted, Montréal, QC, Canada) to obtain cell-free supernatant [7, 10].
Bacteriocin production was confirmed by the agar well diffusion assay as described by Schillinger and Luke [11]. First, 30 ml of unsolidified MRS soft agar (MRS broth supplemented with 0.75 % agar instead of 1.5 %, w/v) was inoculated with approximately 106 CFU ml−1 of L. sakei used as the indicator strain. The mixture was then poured into 100 × 15-mm standard Petri dishes and allowed to solidify for 30 min at room temperature. Wells of 6 mm diameter were cut from the gel, and 80 µl of CFS was placed into each well. All plates were then incubated at 37 ± 1 °C for 24 h, and inhibition zone of each well was examined. The inhibition was scored positive if the width of the clear zone around the well was ≥0.5 mm. Antimicrobial activity was expressed as arbitrary units (AU) per ml. The AU was defined as the reciprocal of the highest dilution showing a clear zone of growth inhibition [12].
Effect of Temperature on Bacteriocin Production by L. lactis MM19 and P. acidilactici MM33
Effect of temperature on production of nisin and pediocin by L. lactis MM19 and P. acidilactici MM33, respectively, was evaluated. These cultures were grown in flasks of 150 ml containing 75 ml of medium at different temperatures of 25, 30, 37, and 45 °C for 16 h. The flasks were inoculated with 2 % inoculum (v/v), and samples were taken every 2 h. The antimicrobial activity (AU/ml) of the CFS of these strains against L. sakei was determined using well diffusion assay method as described above. The suitable temperatures for higher production of bacteriocins by L. lactis MM19 and P. acidilactici MM33 were used for further experiments.
Effect of Initial pH on Bacteriocin Production by L. lactis MM19 and P. acidilactici MM33
Effects of initial pH of the media on production of nisin and pediocin by L. lactis MM19 and P. acidilactici MM33, respectively, was evaluated. These bacteria were grown in flasks of 150 ml containing 75 ml of medium conditioned at various pH (from 4 to 11) for 16 h. The flasks were inoculated with 2 % inoculum (v/v), and samples were taken every 2 h. The antimicrobial activity (AU/ml) of the CFS of these strains against L. sakei was determined using well diffusion assay method as described above. The final pH of the fermented broth was also determined. The initial pH for higher production of bacteriocins by L. lactis MM19 and P. acidilactici MM33 was used for further experiments.
Effect of Different Carbon Sources on Bacteriocin Production by L. lactis MM19 and P. acidilactici MM33
Effect of many carbon sources on nisin and pediocin production by L. lactis MM19 and P. acidilactici MM33, respectively, was tested at initial pH 9 for MM19 and at initial pH 6 for MM33. The carbon sources tested were glucose, lactose, galactose, sucrose, fructose, and maltose (Laboratoire MAT, Beauport, QC, Canada) (20 g/l), the basal medium without sugar served as a control media. The incubation temperature tested were 30 °C for L. lactis MM19 and 45 °C for P. acidilactici MM33, in flask of 150 ml containing 75 ml of medium, for 16 h. The flasks were inoculated with 2 % inoculum (v/v), and samples were taken every 2 h. The antimicrobial activity (AU/ml) of the CFS of these strains against L. sakei was determined using well diffusion assay method as described above. The carbon sources that caused highest production of bacteriocins by L. lactis MM19 or P. acidilactici MM33 were used for later experiment.
Effect of Different Nitrogen Sources on Bacteriocin Production by L. lactis MM19 and P. acidilactici MM33
Effect of multiple nitrogen sources on production of nisin and pediocin by L. lactis MM19 and P. acidilactici MM33, respectively, was tested at initial pH 9 for MM19 and at initial pH 6 for MM33. The carbon sources were glucose for L. lactis MM19 and fructose for P. acidilactici MM33 at the concentration of 20 g/l as determined in previous experiment. The nitrogen sources tested were casein peptone E1, kosher casein peptone, tryptone plus, tryptone N1, casein hydrolysate C2, meat peptone N2, casein-meat peptone E2, meat peptone S2, meat extract N1, gelatine peptone N2, gelatine peptone N3, malt extract R2, yeast extract, malt extract R3, pea peptone, wheat peptone E430, soy peptone AM41, wheat peptone E1, vegetable peptone, vegetable peptone ET1, soy peptone A3SC, and casein peptone plus. The basal media of MRS and TGE without nitrogen served as control media. All nitrogen sources were from Organotechnie® S.A.S (La Courneuve, France). The incubation temperature tested was 30 °C for L. lactis MM19 and 45 °C for P. acidilactici MM33, in flask of 150 ml containing 75 ml of medium. The flasks were inoculated with 2 % inoculum (v/v), and samples were taken every 2 h during 16 h. The antimicrobial activity (AU/ml) of the CFS of these strains against L. sakei was determined using well diffusion assay method as described above.
Statistical Analysis
The experiments were conducted in duplicate, and each parameter was analyzed in triplicate. The results were reported as mean ± standard error.
Results and Discussion
The production of biomass and bacteriocin by LAB is dependent on the culture media compositions, environmental and pH conditions, and physical conditions (temperature, oxygen tension, agitation, and more). The culture conditions for the bacteriocin production were determined for some LAB strains isolated from different environments, mainly from foods [13, 14].
Effect of Temperature on Bacteriocin Production
Figure 1 shows the influence of temperature on nisin production [antimicrobial activity (AU/ml)] of L. lactis MM19. The highest nisin production was obtained when the fermentation temperature was carried out at 30 and 37 °C. From 25 to 37 °C, the bacteriocin activity increased from 500 to 6200 AU/ml, representing an activity increase by more than ten times. There was no significant difference (P > 0.05) in antibacterial activity between 30 and 37 °C. Therefore, 30 °C was selected as incubation temperature of L. lactis MM19 for further experiments.
Profile of pediocin production by P. acidilactici MM33 is presented in Fig. 2. It can be observed that highest pediocin production was obtained at 45 °C as compared to other growth temperature. An antibacterial activity of 25,000 AU/ml was reached after 14 h at 45 °C as compared to 20,000, 1500, and 0 AU/ml at 37, 30, and 25 °C, respectively. Therefore, 45 °C was selected as the incubation temperature for further experiments for MM33.
Cheigh et al. [14] investigated the influence of growth parameters on the production of a nisin-like bacteriocin by Lactococcus lactis subsp. lactis A164 isolated from kimchi. The author found that preferred temperature for the bacteriocin production was at 30 °C which is similar to our bacterial strain MM19 [14]. Biswas et al. [9] found that the bacterial dry mass, organic acid, and pediocin AcH production by P. acidilactici H were maximum in TGE broth with an initial pH of 6.5 after 16 h of incubation at 30 or 37 °C; however, a reduction in both bacterial dry mass and pediocin AcH production was observed at 40 °C. Thus, depending on bacterial strain, the suitable temperature for growth or for bacteriocin production can vary significantly.
Effect of Initial pH on Bacteriocin Production
The MRS medium and the incubation temperature of 30 °C were used to evaluate the effects of initial pH of medium on nisin production by L. lactis MM19, and the results are presented in Fig. 3a. It can be observed that nisin activity was highest at pH 8 where an antibacterial activity of 21,333 AU was obtained. However, there was no significant difference (P > 0.05) in antibacterial activity among initial pH 7, 8, and 9 (Fig. 3a). Further, pH 9 was found to be as preferred initial pH for the growth of MM19 (data not shown). Therefore, pH 9 was chosen as the initial pH in further experiments for L. lactis MM19.
The TGE medium and the incubation temperature of 45 °C were used to evaluate the effects of initial pH of medium on pediocin production by P. acidilactici MM33, and the results are presented in Fig. 3b. Results showed that a higher bacteriocin production was obtained at pH 5 and 6 as compared to other pH, with a maximum bacteriocin activity of 25,600 AU/ml. Therefore, pH 6 was chosen as the initial pH in further experiments for P. acidilactici MM33.
These results showed that the production of bacteriocin was dependant on initial medium pH. The final pH values of all cultures ranged between 4.21 and 5.53 for the nisin production by L. lactis MM19. The pH at the end of the growth was stable with the initial pH from 4 to 11. In case of pediocin production by P. acidilactici MM33, the final pH of the culture varied between 3.63 and 4.23 with the initial pH of 4–9, but it was reduced to 7.99 and 8.25 for initial pH 10 and 11, respectively. This suggests that pediocin production is not affected by low pH values.
Similar results were reported for other bacteriocins such as plantaricin A [15], plantaricin S and T [16], plantaricin 149 [17], and plantaricin ST31 [18] where the production of bacteriocins was affected by pH. The optimal pH for bacteriocin production was usually about 5.5–6.0, for example, nisin Z [19] and lactococcin 140 [5], and these pH values were lower than the optimal pH for bacterial growth. In case of lactococcin 140 that was produced by L. lactis subsp. lactis 140NWC, it was found that a maximum activity of 15.4 × 106 AU/ml was obtained after 7 h at pH 5.5 [5]. Cheigh et al. [14] also found that the bacteriocin production by P. acidilactici isolated from kimchi was also affected by pH of the culture broth. The authors found that although the cell growth at pH 6.0 was also most at same level at pH 5.5 and 6.5, higher bacteriocin activity was obtained at pH 6.0, and therefore, the optimal pH for growth and bacteriocin production was 6.0. [14]. Thus, it can be concluded that optimal pH for the growth or bacteriocin production depends significantly on the characteristics of microbial strains used.
Effect of Carbon Sources on Bacteriocin Production
Effects of various carbon sources (glucose, lactose, galactose, sucrose, and fructose) on nisin and pediocin production by L. lactis MM19 and P. acidilactici MM33, respectively, are presented in Fig. 4. The utilization of glucose or lactose has led to higher nisin production with an antimicrobial activity of 6400 AU/ml as compared to 2667, 2133, and 1600 AU/ml in the presence of fructose, maltose, and galactose, respectively. Sucrose did not induce bacteriocin production by MM19. The present research showed that glucose and lactose are the most suitable carbon source for nisin production. Therefore, glucose was selected as the carbon sources in further experiments for L. lactis MM19.
In case of P. acidilactici MM33, galactose and fructose resulted in a higher production of pediocin with an antimicrobial activity of 12,800 and 17,067 AU/ml, respectively (Fig. 4). The other carbon sources produced less pediocin, which caused lower antimicrobial activity. Based on these results, pediocin production is found to be stimulated mostly in the presence of galactose and fructose. Since no significant difference (P > 0.05) was observed between fructose and galactose, fructose was chosen as the carbon source for future experiments for P. acidilactici MM33.
It has been observed that carbon sources have important impact on bacteriocin production by LAB. For example, Todorov and Dicks [20] evaluated the effect of MRS broth without glucose, and supplemented with 20 g/l of fructose, sucrose, lactose, mannose, or maltose on bacteriocin production by Lactobacillus plantarum ST194BZ. The authors found that in the presence of 10 or 20 g/l of d-mannose, strain ST194BZ produced bacteriocin levels of 12,800 AU/ml, and in the presence of 30 or 40 g/l of mannose, the activity levels were increased to 25,600 AU/m, while other sugars such as fructose, saccharose, or lactose did not improve bacteriocin production [20]. Thus, suitable carbon source for high production of bacteriocin should be evaluated since it also depends on properties of LAB strains as observed in current study.
Effect of Nitrogen Sources on Bacteriocin Production
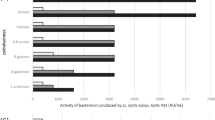
The effects of various nitrogen sources on nisin and pediocin production by L. lactis MM19 and P. acidilactici MM33 are presented in Fig. 5. L. lactis MM19 was inoculated in MRS medium in the presence of twenty-two different sources of nitrogen, and the fermentation was performed at 30 °C for 16 h. Nisin production was significantly increased by the presence of wheat peptone E430, soy peptone AM41, and wheat peptone E1 (Fig. 5). The highest production of nisin was observed in the presence of wheat peptone E430 with an activity of 42,691 AU/ml as compared to the MRS broth with an activity of 6400 AU/ml. This showed an increase by 6.7-fold the production of nisin. In case of P. acidilactici MM33, the highest pediocin production was observed in the presence of pea peptone with an activity of 51,237 AU/ml as compared to 17,067 AU/ml in the TGE medium (Fig. 5). This showed an increased by threefold production of pediocin.
The obtained results are of interest since it demonstrated that utilization of suitable nitrogen source for target LAB strains, in this case MM19 and MM33, could improve significantly their antibacterial activity. In other study, De Carvalho et al. [21] evaluated the effect of nitrogen sources (yeast extract, trypticase, meat extract, soy peptone, meat peptone, casein peptone, and ammonium sulfate) on bovicin HC5 produced by Streptococcus bovis HC5; the author found that when adding yeast extract (0.5 g/l) plus trypticase (1 g/l) into basic medium, highest bovicin HC5 specific activity and dry cell mass were obtained. With this mixed nitrogen source, bacteriocin could be produced at least three times higher than other added nitrogen source (1.5 g/l). The results confirmed that selection of suitable nitrogen source may improve bacteriocin production by specific LAB strains [21].
Conclusions
The culture medium that improved the production of nisin by L. lactis MM19 was the MRS broth containing glucose as a source of carbon, and wheat peptone E430 as a source of nitrogen, with an initial pH of 9 and a growth temperature of 30 °C. This formulated medium increased nisin activity by more than 6.7 times as compared to the basal MRS broth. The culture medium that improved the production of pediocin by P. acidilactici MM33 was the MRS broth containing fructose as a carbon source, and pea peptone as nitrogen source, with an initial pH of 6 and temperature maintaining at 45 °C. The formulated medium increased the pediocin production by three times as compared to the TGE broth.
References
Macfarlane GT, Cummings JH (1999) Probiotics and prebiotics: Can regulating the activities of intestinal bacteria benefit health? West J Med 171:187–191
Lindgren SE, Dobrogosz WJ (1990) Antagonistic activities of lactic acid bacteria in food and feed fermentations. FEMS Microbiol Rev 7:149–163
Parente E, Ricciardi A (1999) Production, recovery and purification of bacteriocins from lactic acid bacteria. Appl Microbiol Biotechnol 52:628–638
Parente E, Ricciardi A (1994) Influence of pH on the production of enterocin 1146 during batch fermentation. Lett Appl Microbiol 19:12–15
Parente E, Ricciardi A, Addario G (1994) Influence of pH on growth and bacteriocin production by Lactococcus lactis subsp, lactis 140NWC during batch fermentation. Appl Microbiol Biotechnol 41:388–394
Leroy F, De Vuyst L (2001) Growth of the bacteriocin-producing Lactobacillus sakei strain CTC 494 in MRS broth is strongly reduced due to nutrient exhaustion: a nutrient depletion model for the growth of lactic acid bacteria. Appl Environ Microbiol 67:4407–4413
Millette M, Dupont C, Archambault D, Lacroix M (2007) Partial characterization of bacteriocins produced by human Lactococcus lactis and Pediococccus acidilactici isolates. J Appl Microbiol 102:274–282
Millette M, Dupont C, Shareck F, Ruiz MT, Archambault D, Lacroix M (2008) Purification and identification of the pediocin produced by Pediococcus acidilactici MM33, a new human intestinal strain. J Appl Microbiol 104:269–275
Biswas SR, Ray P, Johnson MC, Ray B (1991) Influence of growth conditions on the production of a bacteriocin, pediocin AcH, by Pediococcus acidilactici H. Appl Environ Microbiol 57:1265–1267
Turgis M, Vu KD, Lacroix M (2013) Partial characterization of bacteriocins produced by two new Enterococcus faecium isolated from human intestine. Probiotics Antimicrob Proteins 5:110–120
Schillinger U, Lucke FK (1989) Antibacterial activity of Lactobacillus sake isolated from meat. Appl Environ Microbiol 55:1901–1906
van Reenen CA, Dicks LM, Chikindas ML (1998) Isolation, purification and partial characterization of plantaricin 423, a bacteriocin produced by Lactobacillus plantarum. J Appl Microbiol 84:1131–1137
Kayalvizhi N, Gunasekaran P (2008) Production and characterization of a low-molecular-weight bacteriocin from Bacillus licheniformis MKU3. Lett Appl Microbiol 47:600–607
Cheigh CI, Choi HJ, Park H et al (2002) Influence of growth conditions on the production of a nisin-like bacteriocin by Lactococcus lactis subsp. lactis A164 isolated from kimchi. J Biotechnol 95:225–235
Daeschel MA, Mckeney MC, McDonald LC (1990) Bacteriocidal activity of Lactobacillus plantarum C-11. Food Microbiol 7:91–98
Jimenez-Diaz R, Rios-Sanchez RM, Desmazeaud M, Ruiz-Barba JL, Piard JC (1993) Plantaricins S and T, two new bacteriocins produced by Lactobacillus plantarum LPCO10 isolated from a green olive fermentation. Appl Environ Microbiol 59:1416–1424
Kato T, Matsuda T, Ogawa E, Ogawa H, Kato H, Doi U, Nakamura U (1994) Plantaricin-149, a bacteriocin produced by Lactobacillus plantarum NRIC 149. J Ferment Bioeng 77:277–282
Todorov S, Onno B, Sorokine O, Chobert JM, Ivanova I, Dousset X (1999) Detection and characterization of a novel antibacterial substance produced by Lactobacillus plantarum ST 31 isolated from sourdough. Int J Food Microbiol 48:167–177
Matsusaki H, Endo N, Sonomoto K, Ishizaki A (1996) Lantibiotic nisin Z fermentative production by Lactococcus lactis IO-1: relationship between production of the lantibiotic and lactate and cell growth. Appl Microbiol Biotechnol 45:36–40
Todorov SD, Dicks LMT (2005) Effect of growth medium on bacteriocin production by Lactobacillus plantarum ST194BZ, a strain isolated from Boza. Food Technol Biotechnol 43:165–173
De Carvalho AAT, Mantovani HC, Paiva AD, De Melo MR (2009) The effect of carbon and nitrogen sources on bovicin HC5 production by Streptococcus bovis HC5. J Appl Microbiol. doi:10.1111/j.1365-2672.2009.04212.x
Acknowledgments
M. Turgis is a scholarship recipient of the Foundation Armand-Frappier. The authors thank Organotechnie® S.A.S (La Courneuve, France) for providing different nitrogen sources.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Mélanie Turgis, Khanh Dang Vu, Mathieu Millette, Claude Dupont and Monique Lacroix declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Turgis, M., Vu, K.D., Millette, M. et al. Influence of Environmental Factors on Bacteriocin Production by Human Isolates of Lactococcus lactis MM19 and Pediococcus acidilactici MM33. Probiotics & Antimicro. Prot. 8, 53–59 (2016). https://doi.org/10.1007/s12602-015-9204-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-015-9204-8