Abstract
An agar well diffusion assay (AWDA) was used to isolate a high bacteriocin-producing strain with a broad spectrum of antibacterial activity, strain MXG-68, from Inner Mongolia traditional fermented koumiss. Lactobacillus plantarum MXG-68 was identified by morphological, biochemical, and physiological characteristics and 16S rDNA analysis. The production of antibacterial substance followed a growth-interrelated model, starting at the late lag phase of 4 h and arriving at a maximum value in the middle of the stationary phase at 24 h. Antibacterial activity was abolished or decreased in the presence of pepsin, chymotrypsin, trypsin, proteinase, and papain K. The results showed that antibacterial substances produced by L. plantarum MXG-68 were proteinaceous and could thus be classified as the bacteriocin, named plantaricin MXG-68. The molar mass of plantaricin MXG-68 was estimated to be 6.5 kDa, and the amino acid sequence of its N-terminal was determined to be VYGPAGIFNT. The mode of plantaricin MXG-68 action was determined to be bactericidal. Bacteriocin in cell-free supernatant (CFS) at pH 7 was stable at different temperatures (60 °C, 80 °C, 100 °C, 121 °C for 30 min; 4 °C and − 20 °C for 30 days), as well as at pH 2.0–10.0. Antibacterial activity maintained stable after treatment with organic solvents, surfactants, and detergents but increased in response to EDTA. Response surface methodology (RSM) revealed the optimum conditions of bacteriocin production in L. plantarum MXG-68, and the bacteriocin production in medium optimized by RSM was 26.10% higher than that in the basal MRS medium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Food safety is essential to human health, and food spoilage can bring about not only huge economic losses but also serious illnesses (Goyal et al. 2018). Microbial pollution caused by pathogens such as Salmonella Typhimurium, Listeria monocytogenes, Shigella flexneri, Escherichia coli, and Staphylococcus aureus is a primary cause of food spoilage (Gaspar et al. 2018; Lv et al. 2018a). Use of biopreservatives such as lactic acid bacteria (LAB) and their bacteriocins is a promising way to inhibit microbial pollution, lengthen shelf life, and ameliorate food quality (Wayah and Philip 2018; Xi et al. 2018).
Antibacterial peptides known as bacteriocins that are ribosomally synthesized by bacteria can inhibit the growth of similar or closely related bacterial strains under common circumstance (Nishie et al. 2012). Bacteriocins of LAB play a very important role in the food industry as natural preservatives, and most bacteriocins produced by LAB are generally recognized as safe (GRAS) (Kumar et al. 2016; Lan et al. 2012; Merzoug et al. 2016). Nevertheless, nisin is the only FDA (1988) and WHO (1961) certified bacteriocin in food application and the only commercially licensed lactic acid bacteriocin, being approved as a natural food additive by about 50 countries at present (Komora et al. 2017; López et al. 2017; Peres et al. 2012). Oladunjoye et al. (2016) showed that 5000 IU/mL nisin could effectively inhibit the growth of Listeria monocytogenes in fresh-cut tomato, while Na-kyoung et al. (2015) showed that the shelf life of beef jerky was prolonged and the growth of spoilage bacteria and pathogenic bacteria was inhibited by treatment with 100 IU nisin/g for 3 days and 500 IU nisin/g for 21 days respectively. Nisin also has practical applications as a preservative in foods, such as cheese, cream, pasteurized milk, canned vegetables, and alcoholic beverages (Gharsallaoui et al. 2016). However, the number of certified and commercialized bacteriocins produced by LAB is limited because of their narrow antibacterial spectrum, poor stability, comparatively low yield or unclear action mechanism. Hence, broad-spectrum bacteriocins with good stability secreted by LAB strains from different food sources are required, which is beneficial to both food safety and commercialized application.
Bacteriocin-producing strains of L. plantarum have been reported from many food sources, including milk, meat and meat products, cheese, doughs, fermented cucumber, olives, silage, suan-tsai, grapefruit juice, and pineapple (Todorov et al. 2014). Certain bacteriocins produced by L. plantarum have been reported to inhibit pathogenic bacteria and spoilage bacteria including Gram-positive bacteria and Gram-negative bacteria, as well as to have good stability. These include bacteriocin LD4, bacteriocin BH-1, plantaricin KL-1Y, plantaricin LpU4, and plantaricin MG (Botthoulath et al. 2018; Kumar et al. 2016; Man et al. 2014; Milioni et al. 2015; Rumjuankiat et al. 2015). The action mode of L. plantarum bacteriocins is usually bactericidal, although bacteriostatic effects have been reported for plantaricin C19 and plantaricin TF711 (Milioni et al. 2015). Given the broad antimicrobial spectrum against pathogenic bacteria and the stability characteristics under adverse conditions, the bacteriocin of L. plantarum has the potential for application in food safety as well as therapeutics.
Koumiss is a traditionally fermented mare’s milk popular among the people of Mongolia and has been used for decades for its health-promoting potential, which includes increasing immunity and treating cardiovascular disease and tuberculosis (Rong et al. 2015). The composition of microorganisms in koumiss is very complex and includes various types of Lactobacillus, with the dominant species being L. plantarum, L. helveticus, and L. casei (Sun et al. 2010; Wu et al. 2009). Some studies have screened and characterized Lactobacillus strains from koumiss (Pan et al. 2010; Sedláček et al. 2010; Wang et al. 2011). However, only a few studies have characterized L. plantarum bacteriocin originating from koumiss, which possesses a broad spectrum of antibacterial activity (Xie et al. 2011).
Therefore, this study was conducted to screen and identify strains originating from Inner Mongolia traditional fermented koumiss for potential isolates with a broad antibacterial spectrum. The second goal was to confirm the characteristics of the antibacterial substances, and to optimize the nutrients required for the production of antibacterial substances using RSM.
Materials and methods
Samples, indicator strains, and cultivation conditions
Twenty koumiss samples were collected from the Zhongqi, Houqi, Zhaqi, Zharuteqi, and Namanqi areas of Tongliao, Inner Mongolia Autonomous Region of China. Media and chemical compounds were purchased from Oxoid and Sigma respectively. All indicator strains were preserved at − 80 °C. The indicator strains were grown as follows: Lactobacillus plantarum, Lactobacillus sakei, and Lactobacillus acidophilus were cultured at 37 °C in De man-rogosa-sharpe medium (MRS) (Oxoid, England); Lactococcus lactis was cultivated at 37 °C in M17 medium (Oxoid, England); Clostridium perfringens and Clostridium sporogenes were incubated at 37 °C in reinforced clostridial medium (RCM) (Oxoid, England); Listeria monocytogenes was cultured at 37 °C in tryptic soy broth enriched with 0.6% yeast extract (TSA-YE) (Oxoid, England); Pseudomonas aeruginosa and Enterococcus faecalis were cultivated at 37 °C in Luria-Bertani medium (LB) (Oxoid, England); all other indicator strains including Staphylococcus aureus, Bacillus subtilis, Bacillus cereus, Shigella dysenteriae, Micrococcus luteus, Escherichia coli, Pseudomonas fluorescens, Pseudomonas putida, Salmonella Typhimurium, and Salmonella enterica Typhimurium were grown at 37 °C in nutrient broth medium (NB) (Oxoid, England).
Preparation of cell-free supernatant (CFS) and determination of antibacterial activity
Cell-free supernatants were obtained by centrifuging the cultures at 12,000×g for 10 min at 4 °C, after which they were adjusted to pH 7.0 using 1 M NaOH and treated with hydrogen peroxidase to eliminate the antibacterial influence of acids and hydrogen peroxide and then filter-sterilized using a filter with a pore size of 0.22 μm.
The antibacterial activity was measured by agar well diffusion assay (AWDA). Briefly, homologous medium agar (0.7%) inoculated with indicator strain (107 CFU/mL) was overlaid onto 10 mL of 1.2% agar. Wells with a 6-mm diameter were cut out of the plate and filled with 50 μL of CFS. The plates were held at room temperature for 3 h in a laminar air flow hood, then cultivated at 37 °C for 12 h, after which the inhibition diameters on the plate were measured using a digital vernier caliper. Inhibition diameters were reported as the mean ± standard deviation (SD) in millimeter (n = 3).
Isolation and screening of antibacterial substance-producing strains
A total of 25 mL of koumiss was added to 225 mL sterilized normal saline, after which tenfold serial dilutions were performed. Next, 100 μL of suitable dilutions were plated on the corresponding medium for isolation of LAB. MRS agar medium and M17 agar medium were used to isolate lactobacilli and lactococci strains. The plates were cultured at 37 °C for 48–72 h. Gram-positive strains that were catalase negative were considered potential LAB, and needed to be isolated and purified for further characterization. Additionally, lactobacilli and lactococci strains (about 109 CFU/mL) were further cultivated in MRS medium and M17 medium, respectively, at 30 °C for 24 h. CFS samples were used to screen for antibacterial substance-producing strains by the AWDA method.
Identification of antibacterial substance-producing strains
Microscopic observation and Gram staining were applied to observe the individual morphologies of strain MXG-68. Colony morphology of strain MXG-68 was observed after cultivation on MRS agar medium for 48 h at 37 °C. Additionally, biochemical tubes were used to confirm the biochemical and physiological characteristics of the strain. Bergey’s Manual of Systematic Bacteriology was then utilized for preliminarily species identification of strain MXG-68.
Molecular identification was conducted by amplification and sequencing of the 16S rDNA gene of strain MXG-68. The total genomic DNA of strain MXG-68 was obtained using a TIANamp Bacteria DNA Kit (Tiangen, China). The MXG-68-F and R primers (5′-GACGAACGCTGSCGGCGTGCCTAAT-3′ and 5′-GGTGATCCAAC CGCAGGTTCTCCTA-3′) based on known sequences of 16S rDNA in L. plantarum (GenBank accession numbers KC429782.1, NR_115605.1, NR_113338.1, NR_104573.1, NR_042394.1) were utilized to amplify the 16S rDNA by PCR using the following program: 94 °C for 5 min followed by 30 cycles of 94 °C for 30 s, 52 °C for 30 s, and 72 °C for 1 min, 30 cycles, and then final extension at 72 °C for 10 min, after which the samples were preserved at 4 °C. The PCR products were identified by agarose gel electrophoresis at 100 V and then sequenced. The sequences were subsequently utilized for nucleotide BLAST searches of the NCBI database, and the neighbor-joining method was used to construct a phylogenic tree.
Characterization of antibacterial substances produced by L. plantarum MXG-68
Determination of growth kinetics and antibacterial substance production
One percent of L. plantarum MXG-68 (about 109 CFU/mL) inoculated in MRS medium was cultivated at 30 °C for 32 h, during which time samples were taken at 2 h intervals for enumeration (CFU/mL) by the plate colony counting method. The CFS of cultures was used to measure antibacterial activity by the AWDA method, with S. Typhimurium ATCC14028 as the indicator strain.
Determination the nature of antibacterial substance
One percent of L. plantarum MXG-68 (about 109 CFU/mL) inoculated in MRS medium (pH 6.5) was incubated at 30 °C for 24 h, after which the CFS was gathered for use in future experiments.
To ascertain the nature of the antibacterial substance, CFS was amended with pepsin, trypsin, chymotrypsin, papain, proteinase K, α-amylase, or lipase to a final concentration 1 mg/mL, and was then cultivated at 37 °C for 2 h. A portion of untreated CFS was cultured for 2 h at 37 °C as a control. The inhibition diameters were determined by the AWDA method using S. typhimurium ATCC14028 as an indicator strain.
Measurement of molar mass and N-terminal amino acid sequence of antibacterial substance
Tricine-SDS-PAGE was used to test the molar mass of the antibacterial compound. Briefly, CFS was subjected to ammonium sulfate precipitation to 50% saturation, resuspended in sterile water, and dialyzed at 4 °C for 24 h against potassium-sodium phosphate buffer (20 mM, pH 6.0). The partially purified bacteriocins with antibacterial activity were analyzed by Tricine-SDS-PAGE along with protein molar mass marker at 100 V for about 5 h. The gel was then divided into two parts after electrophoresis, one that was dyed with Coomassie Brilliant Blue R250 (CBB R250) and another that was washed with sterile water and overlaid with indicator S. typhimurium ATCC14028 (107 CFU/mL) in NB semisolid medium to determine the antibacterial activity. The bacteriocin was further purified by ion exchange chromatography, gel filtration chromatography, and reverse-phase chromatography, after which the purified bacteriocin was used to detect the N-terminal amino acid sequence by the Edman degradation method utilizing a 494cLC automated protein sequencer (Applied Biosystems, USA). The N-terminal amino acid sequence that we acquired was compared with sequences available in the NCBI database (https://blast.ncbi. nlm.nih.gov/Blast) to determine the homology with sequences that were previously reported.
Determination of action mode of antibacterial substance
Cell-free supernatants (20 mL) were mixed with 4-h-old cultures of S. typhimurium ATCC14028, and then added to 80 mL of NB medium. Additionally, 20 mL of MRS medium was used in place of the CFS as a control. The samples were then incubated at 37 °C for 14 h, during which time, samples were taken at regular intervals of 1 h for enumeration of viable cells and determination of the cell densities (OD600) of S. typhimurium ATCC14028 by the plate colony counting method and turbidimetric assay at 600 nm, respectively.
Influence of different temperatures, pH, organic solvents, surfactants, and detergents on antibacterial activity
To identify the temperature stability of the antibacterial substances, CFS samples were treated at 60 °C, 80 °C, or 100 °C for 30 min in a water bath, autoclaved at 121 °C for 30 min, or refrigerated at 4 °C and − 20 °C for 30 days. Untreated CFS at room temperature was used as a control.
To determine the pH stability of the antibacterial substance, CFS was adjusted to various pH values between 2.0 and 10.0 with 1 M HCl or 1 M NaOH, respectively, after which samples were incubated at 37 °C for 2 h and the pH was readjusted to pH 7.0. To ascertain the influence of organic solvents on antibacterial substance, CFS was supplemented with 1% (v/v) ethanol, isopropanol, methanol, acetonitrile, acetone, ethyl acetate, or surfactants. The impact of surfactants on the antibacterial substance was measured by adding 1 mg/mL of Tween-80, Tween-20, and Triton X-100 and 0.1 mg/mL, 1.0 mg/mL, and 5.0 mg/mL of EDTA into CFS. CFS supplemented with 1 mg/mL of urea and SDS was used to determine the impact of the detergents on antibacterial substances. All samples were cultivated at 37 °C for 2 h. In addition, untreated CFS incubated at 37 °C for 2 h was used as a control. The inhibition diameters against S. typhimurium ATCC14028 were then measured by AWDA.
Optimization of nutrients for the bacteriocin production using response surface methodology (RSM)
The Box-Behnken design (BBD) of RSM was applied to investigate the influence of modified MRS media on bacteriocin production. The design was consisted of 29 runs including five replications at the center points for appraising the purely experimental indeterminacy variance. The modified MRS media were consisted of the basal MRS media and lactose, tryptone, ascorbic acid, and EDTA at different levels (Table 4). Twenty-nine types of modified MRS media and basal MRS medium were used to cultivate L. plantarum MXG-68 for 24 h at 30 °C separately. The supernatants were then acquired, and their inhibition diameters (the response) against S. typhimurium ATCC14028 were determined by the AWDA method.
Statistical analysis
All experiments were operated in triplicate and the results were reported as the mean ± standard deviation (SD). SPSS 17.0 was used to conduct statistical analysis of the experimental results, while Design Expert 8.0.6.1 was used to identify the nutrients required for bacteriocin production by L. plantarum MXG-68. Additionally, Fisher’s test for analysis of variance (ANOVA) was used to determine the statistical significance of the model.
Results
Isolation and screening of antibacterial substance-producing strains
Eighty-six lactobacilli and lactococci strains were isolated from Inner Mongolia traditional koumiss samples, of which 41 demonstrated antibacterial activity against L. monocytogenes ATCC15313, B. cereus ATCC11788, E. coli ATCC25922, and S. typhimurium ATCC14028. The inhibition diameters of strain MXG-68 were significantly higher than those of the other 40 strains (P < 0.01), hence, it was selected for further experiments. Strain MXG-68 demonstrated a broad spectrum of antibacterial activity against both Gram-negative bacteria and Gram-positive bacteria as shown in Table 1.
Identification of strain MXG-68
Strain MXG-68 was identified by morphological, biochemical, physiological, and molecular identification methods. The strain was a rod-shaped and Gram-positive bacillus that produced milky-white, small, dome-shaped colonies with protrusions and opacification. Strain MXG-68 was negative for the gelatin liquefaction, nitrate reduction, H2S, indole, catalase, ammonia production, starch hydrolysis, V. P, arginine hydrolysis, and rhamnose fermentation tests, while it was positive for gas production from glucose fermentation. Additionally, strain MXG-68 had the ability to ferment many carbohydrates including glucose, sucrose, esculin, fructose, maltose, galactose, lactose, mannitol, raffinose, sorbitol, ribose, pectinose, xylose, melibiose, and cellulose. The growth of strain MXG-68 was not evidently affected by 6.5% NaCl or 10% NaCl (P > 0.05). Based on the comparison of the morphological, biochemical, and physiological features to those of reference strains in Bergey’s Manual of Systematic Bacteriology, strain MXG-68 was preliminarily identified as L. plantarum.
Molecular identification was conducted by amplification and sequencing of the 16S rDNA in strain MXG-68. The band produced by PCR method was approximately 1500 bp, and sequencing confirmed that the band was 1534 bp (Fig. 1a). The sequence was submitted to GenBank (NCBI) under accession number KY750314. To further determine if strain MXG-68 belonged to L. plantarum, a phylogenetic tree was generated to compare its 16S rDNA to that of other lactobacilli strains. Upon analysis, 16S rDNA of strain MXG-68 (KY750314), L. plantarum JCM1149 (NR_115605.1), L. plantarum NBRC15891 (NR_113338.1), L. plantarum CIP103151 (NR_104573.1), and L. plantarum NRRL B-14768 (NR_042394.1) were clustered into one group (Fig. 1b). Additionally, the 16S rDNA of strain MXG-68 exhibited high homology of 99.47–99.93% with other members of this group. These results further demonstrated that strain MXG-68 was L. plantarum.
a Agarose gel electrophoresis of PCR amplification products with primers MXG-68-F and MXG-68-R designed to amplify the 16S rDNA of strain MXG-68. M, marker III. Lane 1: amplicon of 16S rDNA in strain MXG-68. b Phylogenetic trees derived from the 16S rDNA sequence of L. plantarum MXG-68. All sequences were from lactobacilli strains
Characterization of antibacterial substance produced by L. plantarum MXG-68
Growth kinetics and production of antibacterial substance
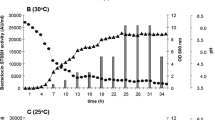
As shown in Fig. 2, L. plantarum MXG-68 exhibited a presentative sigmoidal growth curve composing of an ephemeral lag phase of 4 h, arriving late log phase at 14 h and afterward stationary phase. The production of the antibacterial substance followed a growth-interrelated model, beginning at the late lag phase of 4 h and arriving at the maximum value in the middle of the stationary phase (24 h). Antibacterial activity exhibited a rapid upward trend from 4 to 24 h, as well as a slow downward trend from 24 to 32 h.
Nature of antibacterial substance
Entire inactivation was discovered after treatment of CFS with pepsin and trypsin, and antibacterial activity was obviously reduced after treatment of CFS with chymotrypsin, papain and proteinase K (P < 0.01); however, α-amylase and lipase did not impact its antibacterial activity (P > 0.05) (Table 2). These findings indicated that the antibacterial substance was of a proteinaceous nature and could therefore be grouped in the bacteriocin family. The bacteriocin produced by L. plantarum MXG-68 was named plantaricin MXG-68.
Molar mass and N-terminal amino acid sequence of bacteriocin
Upon Tricine-SDS-PAGE, the partially purified bacteriocin generated a protein band with a molar mass of about 6.5 kDa in the stained part of the gel, while the other part overlaid with S. typhimurium ATCC14028 generated an inhibition zone corresponding to the protein band (Fig. 3).
The N-terminal amino acid sequence of plantaricin MXG-68 was VYGPAGIFNT which showed no apparent homology with other known bacteriocins produced by L. plantarum upon a BLAST search of the GenBank database.
Action mode of plantaricin MXG-68
As shown in Fig. 4, the growth of S. typhimurium ATCC14028 was restrained in response to the addition of CFS, but there was no entire loss in viable cell numbers of indicator strain S. typhimurium ATCC14028. The presence of CFS brought about 99.7% decrease in the viable cell numbers of S. typhimurium ATCC14028 in the late logarithmic phase (10 h). Moreover, the OD600 of S. typhimurium ATCC14028 which included both dead and viable cells, showed no significant changes during co-cultivation with CFS from 4 h to 14 h. These results demonstrated that plantaricin MXG-68 produced by L. plantarum MXG-68 exerted bactericidal action rather than bacteriostatic action.
Influence of antibacterial substances on the growth of S. typhimurium ATCC14028. Viable cell numbers (CFU/mL) were observed in the absence (black circle) and presence (white circle) of CFS. Cell densities at 600 nm (OD600) were observed in the absence (black square) and presence (white square) of CFS. Viable cell numbers and cell densities are expressed as the mean ± SD of Log10 CFU/mL (n = 3) and OD600 (n = 3)
Effects of temperature, pH, organic solvents, surfactants, and detergents on plantaricin MXG-68 stability
As shown in Table 3, the antibacterial activity of plantaricin MXG-68 was not obviously affected by treatment at 60 °C, 80 °C, or 100 °C for 30 min. Moreover, 97.69% of its antibacterial activity remained after sterilization at 121 °C for 30 min. These results demonstrated that plantaricin MXG-68 was highly thermostable. Moreover, plantaricin MXG-68 kept nearly 100% of its antibacterial activity after refrigeration at 4 °C and − 20 °C for 30 days. The activity of plantaricin MXG-68 was stable at pH values between 2.0 and 10.0, with the optimum value occurring at pH 6.0, and activities of 95.95 and 97.45% at pH 2.0 and pH 10.0, respectively.
The antibacterial activity of plantaricin MXG-68 did not obviously decrease after treatment with organic solvents, detergents, or partial surfactants, including Tween-80, Tween-20, and Triton X-100, relative to the control (P > 0.05). However, treatment with 0.1 mg/mL, 1 mg/mL, and 5 mg/mL EDTA greatly increased the antibacterial activity of plantaricin MXG-68 relative to the control (P < 0.01).
Nutritional optimization for bacteriocin production
The inhibition diameters of 29 runs using the BBD design are shown in Table 4. The final equation generated by the analysis, which is as follows, could be applied to make predictions about the responses for given levels of each factor. Y = − 26.63083 + 7.97133*A + 18.68633*B + 3.50817*C + 13.15000*D−0.49000*A*B + 0.21500*A*C + 0.020000*A*D−0.020000*B*C−0.060000*B*D−0.010000*C*D-4.74067*A2–8.86567*B2–1.08892*C2–1.31517*D2. The results of ANOVA of the model are shown in Table 5. The significance of the model (P < 0.01) and the non-significance of lack-of-fit (P > 0.05) were obviously advantageous, indicating high predictability of the model. All linear coefficients and quadratic coefficients, as well as a portion of two interaction coefficients (AB and AC), were found to be significant. The R2 value, which is the proportion of the interpretable variation to total variation, was 0.9977, further supporting that there was an accurate correlation between the predicted and actual values. The adjusted R2 of 0.9953 demonstrated that the model could coincide with the process of bacteriocin production. Additionally, the coefficient of variation was low (CV% = 0.50), demonstrating that the model was exact and dependable. The Pred R2 of 0.9876 was reasonably consistent with the adjusted R2. The high value for the adeq precision (61.543) suggested that the polynomial quadratic model could be applied to navigate the design space.
The normal probability versus residuals was drawn on a graph demonstrating that the data were extremely close to a straight line and located on either side of the line, indicating that the model was reasonably good (Fig. 5). Three-dimensional plots expressing the influence of two variables on the bacteriocin production while other variables were kept at zero indicated the bacteriocin production increased and then decreased as one variable increased when the other variable was fixed (Fig. 6).
The maximum inhibition diameter of 21.79 mm was achieved at a lactose concentration of 0.84%, tryptone concentration of 1.01%, ascorbic acid concentration of 1.66 ppm, and EDTA concentration of 4.98 mg/mL in basal MRS medium. Under these conditions, the bacteriocin production was 26.10% higher than that in the basal MRS medium. Verification experiments conducted in triplicate to check the optimization findings and confirm the precision of the model revealed an inhibition diameter from L. plantarum MXG-68 of 21.77 mm. The applicability of the model was effectively confirmed by the consistency between the predicted and verification values.
Discussion
In our study, antibacterial substance-producing strains were separated from Inner Mongolia traditional koumiss, which is known to be a good source of lactic acid bacteria. In previous studies, antibacterial substance-producing strains have been isolated from other foods, such as E. faecium and S. thermophilus from cheeses and yogurts (Yang et al. 2012), L. paracasei ST11BR and L. pentosus ST151BR from fermented yak milk (Luo et al. 2011), L. plantarum ST13BR and L. lactis ST34BR from South African barley beer (Todorov and Dicks 2004), L. plantarum JJ18 and L. plantarum JJ60 from idli batter (Agaliya and Jeevaratnam 2013), L. plantarum from Chinese and Mongolian traditionally fermented foods (Yu et al. 2015), L. plantarum LD1 from dosa batter (Gupta and Tiwari 2014), L. plantarum ZJ008 from fresh milk (Zhu et al. 2014), L. plantarum H5 from Persian sturgeon, L. plantarum LD4 from fermented dosa of southern India (Ghanbari et al. 2013), and L. plantarum from Chinese traditional low salt fermented whole fish (Zeng et al. 2014). Two categories of media were selected for the separation of LAB, MRS to isolate a general range of LAB from the samples, followed by M17 medium to separate lactococci strains. Therefore, the selection of food sources and media played an important role in successfully isolating antibacterial substance-producing strains (Yang et al. 2012).
Most bacteriocins produced by different LAB possess a narrow antibacterial spectrum and ineffectively inhibit Gram-negative bacteria. This is because the outer membrane of these LAB blocks the locus for bacteriocin action, for example, plantaricin W from L. plantarum LMG 2379, plantaricin C from L. plantarum LL441, plantaricin D from L. plantarum BFE905, and plantaricin T from L. plantarum LPCO10 (Gong et al. 2010). Investigations of bacteriocins with broad antibacterial spectra have been important to human health and the food industry. To date, only a few bacteriocins have been reported to inhibit Gram-negative strains (Perez et al. 2014). As a result of its broad antibacterial spectrum, plantaricin MXG-68 produced by L. plantarum MXG-68 strain isolated in the present study may be of interest as a food preservative. Antibacterial substance produced by L. plantarum MXG-68 is able to constrain not only Gram-positive strains but also Gram-negative strains. Similar findings have been described for plantaricin from L. plantarum TF711, plantaricin MG, L. plantarum MF6, and L. plantarum MF13 (Agaliya and Jeevaratnam 2013; Gupta and Tiwari 2014; Hu et al. 2013; Man et al. 2012; Yu et al. 2015; Zhu et al. 2014). Our results combined with those of previous studies indicated that the antibacterial spectrum of LAB was strain-specific. Strain MXG-68 was preliminarily identified as L. plantarum based on morphological, biochemical, and physiological characteristics and confirmed based on the nucleotide sequence of its 16S rDNA.
The results of growth kinetics and antibacterial substance production indicated that the secretion of bacteriocin was closely associated with the growth of L. plantarum MXG-68. The highest growth and antibacterial substance production occurred in the middle of the stationary phase and at 24 h simultaneously, which is different from that observed for plantaricin BM-1 and bacteriocin LD4 (Kumar et al. 2016). Antibacterial substance from L. plantarum MXG-68 has a bactericidal impact on the impressible strain. Similar results have been reported for other bacteriocins from LAB, such as Lactococcin MMT24 from L. lactis MMT24 (Ghrairi et al. 2006) and plantaricin 423 from L. plantarum 423 (Gong et al. 2010).
Notably, the antibacterial activity against S. typhimurium ATCC14028 was obviously reduced or completely lost after treatment with pepsin, trypsin, chymotrypsin, papain, and proteinase K. However, lipase and α-amylase did not influence the antibacterial activity, carbohydrates, and lipids played no role in antibacterial activity (Todorov and Dicks 2004). These results demonstrated that antibacterial substances produced by L. plantarum MXG-68 were proteinaceous in nature, and could therefore be divided into various types of bacteriocin. The bacteriocin produced by L. plantarum MXG-68 was named plantaricin MXG-68. Different bacteriocins have shown diverse reactions to proteolytic enzymes, for example, bacteriocin JJ18, plantaricin MG, and brevicin 37 (Agaliya and Jeevaratnam 2013; Gong et al. 2010). The molar mass of plantaricin MXG-68 was about 6.5 kDa based on Tricine-SDS-PAGE electrophoresis, which is relatively small for a polypeptide (Kumar et al. 2016).
The N-terminal amino acid sequence of plantaricin MXG-68 was compared to the known sequences of other bacteriocins in the NCBI database based on a BLAST search. The results indicated that the sequence VYGPAGIFNT differed from that of any known bacteriocins (Lv et al. 2018b; Tiwari et al. 2008). This apparent lack of similarities indicates that plantaricin MXG-68 may be a novel and unique bacteriocin. To obtain more information regarding the N-terminal amino acid sequence of plantaricin MXG-68, additional mass spectrometry techniques should be conducted in the future.
Hot and cold treatments are essential to food processing and storage, hence, it is necessary for bacteriocin to be stable under various temperatures to enable its use as a biological preservative. Similar to bacteriocins secreted by L. brevis OG1 and L. plantarum F1, plantaricin MXG-68 was thermostable under different temperatures. Unlike paracaseicin A and bacteriocin ST44AM, plantaricin MXG-68 showed obvious heat stability at 121 °C for 30 min, indicating it can be used during food processing (Todorov and Dicks 2009). Additionally, plantaricin MXG-68 was stable at a wide range of pH (2.0–10.0), with an optimum value at 6.0. These findings differ from those of previous reports, in which bacteriocins synthesized by LAB have been found to be generally stable under acidic conditions but deactivated under alkaline and neutral conditions. For example, paracaseicin A and bacteriocin ST31 were found to have high activity under acidic conditions but remarkably decreased activity at pH 6.0 and no activity at pH 7.0–9.0 (Bendjeddou et al. 2012).
Similar to plantaricin LC74, bacteriocin BacTN635 and lactocin RN78, plantaricin MXG-68 was stable after treatment with different organic solvents, indicating its soluble and proteinaceous nature (Perin et al. 2012; Rushdy and Gomaa 2013). Nearly 100% of the antibacterial activity remained after treatment with different organic solvents, detergents, and surfactants, except for EDTA. The chemical stability supported the potential for widespread application of bacteriocin, suggesting that it could maintain its function and structure during different stages of purification. The antibacterial activity of plantaricin MXG-68 was markedly increased after treatment with EDTA, indicating that EDTA chelated divalent cations from the protective external cell membrane of bacteria, making them susceptible to hydrophobic peptides such as bacteriocins.
RSM, which is a highly effective strategy for optimizing microbial metabolite production, is a design method for collecting statistical data, appraising the influences of factors, and determining the optimum conditions to fulfill a desirable purpose (Körbahti et al. 2007). This method has been successfully applied to optimization of the bacteriocin production of lactic acid bacteria, including L. paracasei NTU 101, L. plantarum NTU 102, E. faecium MTCC 5695, L. brevis DF01 (Lee et al. 2012), and L. casei LA-1 (Kumar et al. 2012). The results revealed that lactose, tryptone, ascorbic acid, and EDTA had positive effects on bacteriocin production of L. plantarum MXG-68 (data are not shown), thus, RSM was applied to further optimize the amount of these four factors to maximize bacteriocin production. An empirical model was established through RSM to analyze the linkages between variables (lactose, tryptone, ascorbic acid, and EDTA). The goodness of the model can be determined by the corresponding coefficient, including R2 (0.9977), adjusted R2 (0.9953), and Pred R2 (0.9876). A coefficient value closer to 1 indicates a better connection between the predicted and actual values. The signal-to-noise ratio is evaluated by adeq precision, with a ratio greater than 4 considered very good. The ratio of 61.543 indicates a sufficient signal and that the model is suitable for analysis of the design space. The results of ANOVA indicate that the effects of the investigated factors on bacteriocin production occurred in the order lactose > ascorbic acid > tryptone > EDTA. Overall, the results indicated that the model fits the bacteriocin production of L. plantarum MXG-68 very well.
In this study, lactose, tryptone, ascorbic acid, and EDTA were found to be beneficial to the bacteriocin production of L. plantarum MXG-68. Previous studies have shown that the carbon sources required for the production of bacteriocin by lactic acid bacteria differed obviously, possibly because of different energy types and carbon skeletons of bacteriocins. For instance, the carbon sources for bacteriocin production of L. plantarum LB-B1, Pediococcus acidilactici C20, L. plantarum ST23LD, and L. pentosus 31-1 are glucose, maltose, sorbitol, and lactose, respectively (Halami and Chandrashekar 2005; Todorov and Dicks 2006). Tryptone, as an organic nitrogen source, can significantly promote bacteriocin production, which may be related to the mechanism of bacteriocin production in bacterial growth. Some components of organic nitrogen can induce the initiation and expression of bacteriocin synthetic genes. Bacteriocin itself is a protein, and tryptone contains more unique amino acids that can provide materials for bacteriocin production. Ascorbic acid was found to increase the bacteriocin production by L. plantarum MXG-68 in our study, similar to the results observed for L. plantarum KC21 in a study conducted by Lim (2010). These findings differ from those of another study (Aasen et al. 2000), in which vitamin supplementation had no effect on sakacin P production by L. sakei CCUG 42687. As previously shown, EDTA could enhance the antibacterial activity of bacteriocin secreted by L. casei AP8 and plantaricin MG (Castellano et al. 2011).
References
Aasen IM, Møretrø T, Katla T, Axelsson L, Storrø I (2000) Influence of complex nutrients, temperature, and pH on bacteriocin production by Lactobacillus sakei CCUG 42687. Appl Microbiol Biot 53:159–166
Agaliya PJ, Jeevaratnam K (2013) Characterization of the bacteriocins produced by two probiotic Lactobacillus isolates from idli batter. Ann Microbiol 63:1525–1535
Bendjeddou K, Fons M, Strocker P, Sadoun D (2012) Characterization and purification of a bacteriocin from Lactobacillus paracasei subsp. paracasei BMK2005, an intestinal isolate active against multidrug-resisitant pathogens. World J Microbiol Biotechnol 28:1543–1552
Botthoulath V, Upaichit A, Thumarat U (2018) Identification and in vitro assessment of potential probiotic characteristics and antibacterial effects of Lactobacillus plantarum subsp. plantarum SKI19, a bacteriocinogenic strain isolated from Thai fermented pork sausage. J Food Sci Tech 55:2774–2785
Castellano P, Belfiore C, Vignolo G (2011) Combination of bioprotective cultures with EDTA to reduce Escherichia coli O157: H7 in frozen ground-beef patties. Food Control 22:1461–1465
Gaspar C, Donders GG, Palmeira-de-Oliveira R, Queiroz JA, Tomaz C, Martinez-de-Oliveira J, Palmeira-de-Oliveira A (2018) Bacteriocin production of the probiotic Lactobacillus acidophilus KS400. AMB Express 8:153
Ghanbari M, Jami M, Kneifel W, Domig KJ (2013) Antimicrobial activity and partial characterization of bacteriocins produced by lactobacilli isolated from sturgeon fish. Food Control 32:379–385
Gharsallaoui A, Oulahal N, Joly C, Degraeve P (2016) Nisin as a food preservative: part 1: physicochemical properties, antimicrobial activity, and main uses. Crit Rev Food Sci 56:1262–1274
Ghrairi T, Frère J, Berjeaud JM, Manai M (2006) Lactococcin MMT24, a novel two-peptide bacteriocin produced by Lactococcus lactis isolated from rigouta cheese. Int J Food Microbiol 105:389–398
Gong HS, Meng XC, Wang H (2010) Plantaricin MG active against Gram-negative bacteria produced by Lactobacillus plantarum KLDS1.0391 isolated from “Jiaoke”, a traditional fermented cream from China. Food Control 21:89–96
Goyal C, Malik RK, Pradhan D (2018) Purification and characterization of a broad spectrum bacteriocin produced by a selected Lactococcus lactisstrain 63 isolated from Indian dairy products. J Food Sci Tech 55:3683–3692
Gupta A, Tiwari SK (2014) Plantaricin LD1: a bacteriocin produced by food isolate of Lactobacillus plantarum LD1. Appl Biochem Biotech 172:3354–3362
Halami PM, Chandrashekar A (2005) Enhanced production of pediocin C20 by a native strain of Pediococcus acidilactici C20 in an optimized food-grade medium. Process Biochem 40:1835–1840
Hu M, Zhao H, Zhang C, Yu J, Lu Z (2013) Purification and characterization of plantaricin 163, a novel bacteriocin produced by Lactobacillus plantarum 163 isolated from traditional Chinese fermented vegetables. J Agr Food Chem 61:11676–11682
Komora N, Bruschi C, Magalhães R, Ferreira V, Teixeira P (2017) Survival of Listeria monocytogenes with different antibiotic resistance patterns to food-associated stresses. 245:79–87
Körbahti BK, Aktaş N, Tanyolaç A (2007) Optimization of electrochemical treatment of industrial paint wastewater with response surface methodology. J Hazard Mater 148:83–90
Kumar M, Jain AK, Ghosh M, Ganguli A (2012) Statistical optimization of physical parameters for enhanced bacteriocin production by L. casei. Biotechnol Bioproc E 17:606–616
Kumar V, Sheoran P, Gupta A, Yadav J, Tiwari SK (2016) Antibacterial property of bacteriocin produced by Lactobacillus plantarum LD4 isolated from a fermented food. Ann Microbiol 66:1431–1440
Lan WT, Chen YS, Wu HC, Yanagida F (2012) Bio-protective potential of lactic acid bacteria isolated from fermented wax gourd. Folia Microbiol 57:99–105
Lee YM, Kim JS, Kim WJ (2012) Optimization for the maximum bacteriocin production of Lactobacillus brevis DF01 using response surface methodology. Food Sci Biotechnol 21:653–659
Lim SM (2010) Cultural conditions and nutritional components affecting the growth and bacteriocin production of Lactobacillus plantarum KC21. Food Sci Biotechnol 19:793–802
López CC, Serio A, Montalvo C, Ramirez C, Alvarez JAP, Paparella A, Mastrocola D, Martuscelli M (2017) Effect of nisin on biogenic amines and shelf life of vacuum packaged rainbow trout (oncorhynchus mykiss) fillets. J Food Sci Technol 54:3268–3277
Luo F, Feng S, Sun Q, Xiang W, Zhao J, Zhang J, Yang Z (2011) Screening for bacteriocin-producing lactic acid bacteria from kurut, a traditional naturally-fermented yak milk from Qinghai-Tibet plateau. Food Control 22:50–53
Lv X, Lin Y, Jie Y, Sun M, Zhang B, Bai F, Zhao H, Li J (2018a) Purification, characterization, and action mechanism of plantaricin DL3, a novel bacteriocin against Pseudomonas aeruginosa produced by Lactobacillus plantarum DL3 from Chinese Suan-Tsai. Eur Food Res Technol 244:323–331
Lv X, Miao L, Ma H, Bai F, Lin Y, Sun M, Li J (2018b) Purification, characterization and action mechanism of plantaricin JY22, a novel bacteriocin against Bacillus cereus produced by Lactobacillus plantarum JY22 from golden carp intestine. Food Sci Biotechnol 27:695–703
Man LL, Meng XC, Zhao RH (2012) Induction of plantaricin MG under co-culture with certain lactic acid bacterial strains and identification of LuxS mediated quorum sensing system in Lactobacillus plantarum KLDS1.0391. Food Control 23:462–469
Man LL, Meng XC, Zhao RH, Xiang DJ (2014) The role of plNC8HK-plnD genes in bacteriocin production in Lactobacillus plantarum KLDS1.0391. Int Dairy J 34:267–274
Merzoug M, Dalache F, Karam HZ, Karam NE (2016) Isolation and preliminary characterisation of bacteriocin produced by Enterococcus faecium GHB21 isolated from Algerian paste of dates “ghars”. Ann Microbiol 66:795–805
Milioni C, Marínez B, Degl'Innocenti S, Turchi B, Fratini F, Cerri D, Fischetti R (2015) A novel bacteriocin produced by Lactobacillus plantarum LpU4 as a valuable candidate for biopreservation in artisanal raw milk cheese. Dairy Sci Technol 95:479–494
Na-Kyoung L, Wook KH, Yeon LJ, UK AD, Hyun-Dong P (2015) Antimicrobial effect of nisin against Bacillus cereus in beef jerky during storage. Korean J Food Sci Anim 35:272–276
Nishie M, Nagao J, Sonomoto K (2012) Antibacterial peptides “bacteriocins”: an overview of their diverse characteristics and applications. Biocontrol Sci 17:1–16
Oladunjoye AO, Singh S, Ijabadeniyi OA (2016) Inactivation of Listeria monocytogenes ATCC 7644 on fresh-cut tomato using nisin in combinations with organic salts. Braz J Microbiol 47:757–763
Pan DD, Zeng XQ, Yan YT (2010) Characterisation of lactobacillus fermentum SM-7 isolated from koumiss, a potential probiotic bacterium with cholesterol-lowering effects. J Sci Food Agric 91:512–518
Peres CM, Peres C, Hernández-Mendoza A, Xavier Malcata F (2012) Review on fermented plant materials as carriers and sources of potentially probiotic lactic acid bacteria with an emphasis on table olives. Trends Food Sci Technol 26:31–42
Perez RH, Zendo T, Sonomoto K (2014) Novel bacteriocins from lactic acid bacteria (LAB): various structures and applications. Microb Cell Factories 13:1–13
Perin LM, Moraes PM, SilvaJr A, Nero LA (2012) Lantibiotics biosynthesis genes and bacteriocinogenic activity of Lactobacillus spp. isolated from raw milk and cheese. Folia Microbiol 57:183–190
Rong JJ, Zheng HF, Liu M, Hu X, Wang T, Zhang XW, Jin F, Wang L (2015) Probiotic and anti-inflammatory attributes of an isolate Lactobacillus helveticus NS8 from Mongolian fermented koumiss. BMC Microbiol 15:196
Rumjuankiat K, Perez RH, Pilasombut K, Keawsompong S, Zendo T, Sonomoto K, Nitisinprasert S (2015) Purification and characterization of a novel plantaricin, KL-1Y, from Lactobacillus plantarum KL-1. World J Microbiol Biotechnol 31:983–994
Rushdy AA, Gomaa EZ (2013) Antimicrobial compounds produced by probiotic Lactobacillus brevis isolated from dairy products. Ann Microbiol 63:81–90
Sedláček I, Nováková D, Svec P (2010) Ribotyping and biotyping of lactobacillus helveticus from the koumiss. Eur Food Res Technol 230:753–758
Sun ZH, Liu WJ, Zhang JC, Yu J, Zhang WY, Cai C, Menghe B, Sun TS, Zhang HP (2010) Identification and characterization of the dominant lactobacilli isolated from koumiss in China. J Gen Appl Microbiol 56:257–265
Tiwari SK, Srivastava S (2008) Purification and characterization of plantaricin LR14: a novel bacteriocin produced by Lactobacillus plantarum LR/14. Appl Microbiol Biot 79: 759–767
Todorov SD, Dicks LMT (2004) Screening of lactic-acid bacteria from South African barley beer for the production of bacteriocin-like compounds. Folia Microbiol 49:406–410
Todorov SD, Dicks LMT (2006) Effect of medium components on bacteriocin production by Lactobacillus plantarum strains ST23LD and ST341LD, isolated from spoiled olive brine. Microbiol Res 161:102–108
Todorov SD, Dicks LMT (2009) Bacteriocin production by Pediococcus pentosaceus isolated from marula (Scerocarya birrea). Int J Food Microbiol 132:117–126
Todorov S, Gotcheva B, Dousset X, Onno B, Ivanova I (2014) Influence of growth medium on bacteriocin production in Lactobacillus plantarum ST31. Biotechnol Biotechnol Equip 14:50–55
Wang HK, Yan H, Shin J, Huang L, Zhang HP, Qi W (2011) Activity against plant pathogenic fungi of Lactobacillus plantarum IMAU10014 isolated from Xinjiang koumiss in China. Ann Microbiol 61:879–885
Wayah SB, Philip K (2018) Characterization, yield optimization, scale up and biopreservative potential of fermencin SA715, a novel bacteriocin from Lactobacillus fermentum GA715 of goat milk origin. Microb Cell Factories 17:125s
Wu R, Wang LP, Wang JC, Li HP, Menghe B, Wu JR, Guo MG, Zhang HP (2009) Isolation and preliminary probiotic selection of lactobacilli from koumiss in Inner Mongolia. J Basic Microb 49:318–326
Xi Q, Wang J, Du R, Zhao F, Han Y, Zhou Z (2018) Purification and characterization of Bacteriocin produced by a strain of Enterococcus faecalis TG2. Appl Biochem Biotechnol 184:1106–1119
Xie Y, An HR, Hao YL, Qin QQ, Huang Y, Luo YB, Zhang LB (2011) Characterization of an anti-Listeria bacteriocin produced by Lactobacillus plantarum LB-B1 isolated from koumiss, a traditionally fermented dairy product from China. Food Control 22:1027–1031
Yang E, Fan L, Jiang Y, Doucette C, Fillmore SAE (2012) Antimicrobial activity of bacteriocin-producing lactic acid bacteria isolated from cheeses and yogurts. AMB Exp 2:48
Yu HJ, Chen YF, Yang HJ, Yang J, Xue JG, Li CK, Kwok LY, Zhang HP, Sun TS (2015) Screening for Lactobacillus plantarum with potential inhibitory activityagainst enteric pathogens. Ann Microbiol 65:1257–1265
Zeng X, Xia W, Wang H, Wang J, Jiang Q, Xu Y, Qiu Y, Wang H (2014) Technological properties of Lactobacillus plantarum strains isolated from Chinese traditional low salt fermented whole fish. Food Control 40:351–358
Zhu X, Zhao Y, Sun Y, Gu Q (2014) Purification and characterization of plantaricin ZJ008, a novel bacteriocin against Staphylococcus spp. from Lactobacillus plantarum ZJ008. Food Chem 165:216–223
Funding
This study was supported by the Doctoral Research Start-up Fund of Inner Mongolia University for Nationalities (No. BS403), Natural Sciences Foundation of Inner Mongolia Autonomous Region of China (No. 2018MS03060).
Author information
Authors and Affiliations
Contributions
Li-Li Man and Dian-Jun Xiang contributed equally to this article. Li-Li Man and Dian-Jun Xiang performed the experiments and contributed significantly to the data analysis, results discussion and manuscript preparation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics statement
No animals or humans were used in this study.
Open access
This article is distributed under the terms of the Creative Commons At tribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Man, LL., Xiang, DJ. Characterization of a broad spectrum bacteriocin produced by Lactobacillus plantarum MXG-68 from Inner Mongolia traditional fermented koumiss. Folia Microbiol 64, 821–834 (2019). https://doi.org/10.1007/s12223-019-00697-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-019-00697-0