Abstract
Astylus atromaculatus Blanchard, 1843 (Coleoptera: Melyridae) is a pollinivorous beetle native to southern South America, which has invaded South Africa more than a century ago. Adults and/or larvae may occasionally damage flowers, seeds, and seedlings of various crops. Severe cattle intoxication has also been reported in Argentina, Uruguay, and South Africa following consumption of alfalfa and forage grasses infested with A. atromaculatus. Despite its economic impact, essential genetic information is lacking for this species. The present paper provides the first DNA barcode reference sequences for A. atromaculatus based on the standard 5’ fragment (658 bp) of the cytochrome c oxidase subunit I gene. The sequences obtained exhibited pairwise distances of ≤ 1.82% among them, and ~ 90% nucleotide identity with the homologous gene fragment in the morphologically similar Astylus variegatus Germar, 1824. The use of this molecular marker to explore the intraspecific variability of A. atromaculatus in central Argentina showed 21 different haplotypes, out of 32 individuals analyzed. A very high haplotype diversity (Hd = 0.962 ± 0.019) and a moderate nucleotide diversity (π = 0.00778 ± 0.00079) were recorded. The haplotype network displayed a diffuse structure due to the abundance of singletons and possible missing haplotypes, with the most common haplotype comprising only 15.6% of the specimens collected. Future research with increased sampling size and geographic coverage will allow for a better understanding of the population genetics of this pest, and consequently, for developing efficient management practices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Astylus atromaculatus (Blanchard, 1843) (Coleoptera: Melyridae), popularly known in Spanish as “siete de oro”, is a common pollen-eating beetle endemic to southern South America, accidentally introduced in South Africa in the early 20th century (Chiesa-Molinari, 1964; Huddleston et al., 1972; Van den Berg et al., 2008). The agricultural pest status of this polyphagous insect across countries and crops is rather ambiguous. In South Africa, A. atromaculatus has been proposed as an efficient pollinator of sunflower (Helianthus annuus L., 1753) (Asteraceae) and cotton (Gossypium hirsutum L., 1763) (Malvaceae) (du Toit, 1990; Pierre & Hofs, 2010). Also, the larvae of this species have shown some predation on noctuid pests (Watmough & Kfir, 1995). On the other hand, larvae and adults of A. atromaculatus were repeatedly reported to damage different plant organs in sorghum (Sorghum Moench., 1794) (Poaceae), corn (Zea mays L., 1753) (Poaceae) and cotton in both continents (Venica, 1969; Huddleston et al., 1972; du Plessis & van den Berg, 2001; Midega et al., 2007). Most importantly, there is strong evidence of the toxicity of A. atromaculatus to livestock that feed on heavily infested grazing areas. A pioneer work in this matter by Kellerman et al. (1972) in South-Africa was further supported by recent studies following outbreaks of intoxication in cattle in Argentina and Uruguay (García et al., 2024; Giannitti et al., 2024). Although no causative toxic compounds could be identified, a direct correlation was found between beetle ingestion and gastroenteric disease in livestock, which led to death in severe cases. Prolonged dry periods experienced in the last years in central Argentina and Uruguay could be at the origin of the intoxication events since the beetles concentrated on blooming alfalfa (Medicago sativa L., 1753) (Fabaceae) due to the shortage of other pollen sources (García et al., 2024). It is envisageable that high A. atromaculatus densities on forage crops in Argentina and neighboring countries will continue to pose sanitary problems in the near future, as drought episodes are becoming more and more frequent owing to climate change.
Despite its growing importance, genetic information is lacking on this potentially harmful insect, except for a paper assessing somatic and gametic chromosome numbers (Páez et al., 2020). Even if adult A. atromaculatus specimens (and at a lesser extent, the larvae) are quite conspicuous in appearance, molecular tools have not yet been applied for the accurate identification of this beetle species. Molecular approaches are particularly useful when only damaged specimens, insect parts/debris or DNA traces are found (e. g. after accidental or voluntary ingestion by other animals). In this sense, a 658 bp-long sequence at the 5’ region of the cytochrome c oxidase subunit I mitochondrial gene (COI), the “Folmer region”, is a universally accepted marker for species identification in insects and most animal groups (Folmer et al., 1994; Hebert et al., 2003). Moreover, because of its maternal inheritance and a certain degree of intraspecific variability, COI sequences may help to detect genetic differences at individual or population levels, and to retrace dispersal routes of invasive species. Such knowledge is necessary for establishing control measures. Thus, the present work aimed to provide COI reference sequences for A. atromaculatus and a first insight into the intraspecific genetic diversity of specimens collected in its native range (central Argentina).
Materials and methods
Adult A. atromaculatus specimens were collected on flowers of spontaneous Daucus pusillus Michx., 1803 (Apiaceae) at the Botanical Garden (BG) and grassland areas adjacent to Instituto de Microbiología y Zoología Agrícola (IMYZA), both located in Hurlingham, Argentina. Additional specimens were sampled from two contiguous flowering alfalfa and sunflower plots in Pergamino, Argentina. In all cases, beetles were sampled in April 2023, except for some of the individuals from IMYZA that were captured in February 2024. Sampling points at BG and IMYZA are separated by approximately 0.5–0.7 km, while Pergamino is 190 km (straight-line distance) away from BG/IMYZA. Insects were collected from different plants to minimize sampling siblings and conserved at -20 °C until processing.
Whole DNA was individually extracted from the insect internal organs (excluding bacteriomes) using the CTAB method (Doyle & Doyle, 1990). The 658-bp COI barcoding region was amplified with primers LCO1490/HCO2198 (Folmer et al., 1994) using a Pfu proof-reading polymerase (INBIO, Tandil, Argentina) and a template DNA concentration of 5 ng/µl PCR reaction mixture. PCR products were precipitated with EDTA/Ethanol and bidirectionally sequenced (Sanger technology) in an ABI PRISM 3500 XL genetic analyzer (Applied Biosystems, Foster City, USA) at the Genomic Unit-CICVyA-INTA (Hurlingham, Argentina). For comparison, complete Folmer COI sequences of Astylus spp. were searched in GenBank and BOLD Systems, and intra- and interspecific pairwise distances were computed in MEGA v.11 (Tamura et al., 2021). Based on the newly obtained sequences, haplotype number (Hn), haplotype diversity (Hd) and nucleotide diversity (π) were calculated per sampling site (namely BG, IMYZA and Pergamino) and for the total samples using DNAsp v.6 (Rozas et al., 2017). To infer demographic changes, neutrality tests Tajima’s D (Tajima, 1989), Fu’s Fs (Fu, 1997) and R2 (Ramos-Onsins & Rozas, 2002) were conducted with the same program on the total dataset using coalescent simulations (10,000 replicates). Also, a haplotype network showing the geographical origin of the sequences was reconstructed following a median-joining approach in PopArt (Leigh & Bryant, 2015).
Results
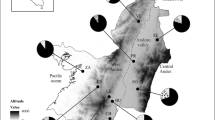
A total of 32 COI sequences (658 bp) were obtained from A. atromaculatus specimens collected in central Argentina, where this species is endemic (GenBank accession numbers PP532884-PP532914 and PP591849). These are the first complete “Folmer region” sequences for A. atromaculatus. The survey showed high genetic polymorphism at each sampling site and overall. In total, 21 haplotypes were found, with a global Hd and π of 0.962 (± 0.019) and 0.00778 (± 0.00079), respectively (Table 1). Pairwise distances among A. atromaculatus COI sequences ranged from 0 to 1.82%. This value rose to 9.27–10.03% when sequences were compared to the only other 658 bp barcode fragment previously reported for the genus (GenBank MH979994), which corresponded to the closely resembling Astylus variegatus Germar, 1824. Neutrality tests yielded negative but non-significant values for Tajima’s D (-1.276, P = 0.0897), large negative significant values for Fu’s Fs (-10.176, P = 0.0006), and small positive significant values for R2 (0.0702, P = 0.0476). The haplotype network revealed the genetic relationships among the haplotypes identified in this study, and their frequency across specimens collected at different sampling points in central Argentina. The network exhibited a scattered pattern, with a high number of private (unique) haplotypes and a maximum of 18 mutational steps between them (Fig. 1). Also, no geographic structure was evident at this geographic scale. The most frequent haplotype (7 C.11) was detected in all sampling sites, but it accounted for just 15.6% of the total individuals analyzed.
Median-joining haplotype network based on 32 COI Folmer sequences of Astylus atromaculatus. Short transverse bars between haplotypes indicate number of mutations. Colors illustrate haplotype distributions by sampling sites: Botanical Garden –BG- and IMYZA (Hurligham), and Pergamino. Circle sizes are proportional to the number of specimens of each haplotype
Discussion
This paper reports the first molecular characterization and genetic diversity assessment of A. atromaculatus. The newly obtained COI reference sequences provide a complementary tool to classical morphology-based identification. Given the interspecific pairwise distances observed (~ 10%), this molecular marker could be useful for distinguishing A. atromaculatus from A. variegatus, a common agricultural pest in NE Argentina and Brazil very similar in aspect, except for the color of the pronotum (Souza & Carvalho, 1994). However, to corroborate consistent “barcoding gaps” (i.e. interspecific variations higher than intraspecific variations) (Meier et al., 2008), additional COI sequence data are needed for A. variegatus and other Astylus spp. The results presented here advance essential information in this direction. Also, sequencing of further mitochondrial and nuclear genes will allow molecular phylogenetic analysis on this poorly known genus.
To the author’s knowledge, there are no prior intraspecific diversity studies on Melyridae to compare with. Within Coleoptera, literature abounds, especially on weevil pests. Similar values of COI haplotype and nucleotide diversity were found, for instance, in populations of Anthonomus eugenii Cano, 1894 (Coleoptera: Curculionidae) collected in México, where that species originated (Fernández et al., 2022). It is assumed that populations at the centre of origin of a species generally exhibit higher genetic variability with respect to invasive populations (Javal et al., 2019; Anooj et al., 2020). Here, the large amount of private and possible “missing” haplotypes suggests that only a portion of the actual diversity was sampled in this initial survey covering part of the beetle’s original geographic range. Due to the limited sampling area, the lack of geographic structure in the haplotype network is not surprising; further analyses considering a wider spatial coverage will return more precise information on this issue. Neutrality tests indicated an excess of rare mutations, which may reflect recent population expansion. However, statistically significant values were obtained for Fu’s Fs and R2 but not for Tajima’s D (the former two being more powerful for detecting past demographic events) (Ramos-Onsins & Rozas, 2002). The extent of diversity detected in the COI sequences highlights the need to increase the number of individuals analyzed. It also validates the suitability of this marker for further population genetics studies on A. atromaculatus.
A broader sampling and sequencing effort at a regional, continental and intercontinental scale should shed more light on the haplotype diversity, possible population structure and demographic history of this important pest across South America and South Africa. These data will help to determine, for instance, whether A. atromaculatus populations should be treated as single or separate “management units”, with possibly different susceptibility to insecticides and/or natural enemies, and implement appropriate control measures.
Data availability
Sequences are openly available at GenBank under accession numbers PP532884-PP532914 and PP591849. Additional information available on request from the authors.
References
Anooj, S. S., Raghavendra, K. V., Shashank, P. R., Nythia, C., Sardana, H. R., & Vaibhav, V. (2020). An emerging pest of radish, striped flea beetle Phyllotreta striolata (Fabricius), from Northern India: Incidence, diagnosis and molecular analysis. Phytoparasitica, 48, 743–753. https://doi.org/10.1007/s12600-020-00825-4
Chiesa-Molinari, O. (1964). Investigaciones sobre el control de Astylus atromaculatus Blanchard (Dasytidae = Melyridae). EEA-INTA Manfredi, Divulgación Técnica n° 3, pp. 7.
Doyle, J. J., & Doyle, J. L. (1990). Isolation of plant DNA from fresh tissue. Focus, 12, 13–15.
du Plessis, H., & van den Berg, J. (2001). Laboratory assay to determine the efficacy of insecticides for control of the spotted maize beetle Astylus atromaculatus Blanchard (Coleoptera: Melyridae), a pest of sorghum. South African Journal of Plant and Soil, 18, 136–138. https://doi.org/10.1080/02571862.2001.10634418
du Toit, A. P. (1990). The importance of certain insect as pollinators of sunflower (Helianthus annuus L). South African Journal of Plant and Soil, 7, 159–162. https://doi.org/10.1080/02571862.1990.10634559
Fernández, D. C., Van Laerhoven, S. L., Rodríguez-Leyva, E., Zhang, Y. M., & Labbé, R. (2022). Population structure and genetic diversity of the pepper weevil (Coleoptera: Curculionidae) using the COI barcoding region. Journal of Insect Science, 22, 25. https://doi.org/10.1093/jisesa/ieac012
Folmer, O., Black, M., Hoeh, W., Lutz, R., & Vrijenhoek, R. (1994). DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology, 3, 294–299.
Fu, Y. X. (1997). Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics, 147, 915–925. https://doi.org/10.1093/genetics/147.2.915
García, J. A., Livio, J. M., Matto, C., Dutra, F., Scioli, V., Giannitti, F., Langston, J., Poppenga, R. H., Cantón, G. J., & Uzal, F. A. (2024). Pollen beetle (Astylus atromaculatus)-associated gastroenteric disease in cattle: Reports of 6 natural outbreaks. Journal of Veterinary Diagnostic Investigation, 36, 95–102.
Giannitti, F., Machado, M., Silva-Silveira, C., da, Cibils-Stewart, X., Baráibar, N., Queiroz-Machado, C. R. R., Poppenga, R. H., Menchaca, A., Uzal, F. A., García, J. A., Matto, C., Dutra, F., Ruprechter, G., Caffarena, D., & Saravia, A. (2024). Experimental oral administration of pollen beetle (Astylus atromaculatus) to cattle results in an acute lethal gastrointestinal disease. Veterinary Pathology, 61, 590–603. https://doi.org/10.1177/03009858241231557
Hebert, P. D. N., Cywinska, A., Ball, S. L., & de Waard, J. R. (2003). Biological identifications through DNA barcodes. Proceedings of the Royal Society London B, 270, 313–321. https://doi.org/10.1098/rspb.2002.2218
Huddleston, E. W., Ward, C. R., & Parodi, R. A. (1972). Chemical control of Astylus atromaculatus attacking grain sorghum in Argentina. Journal of Economic Entomology, 65, 892–894. https://doi.org/10.1093/jee/65.3.892
Javal, M., Roques, A., Haran, J., Hérard, F., Keena, M., & Roux, G. (2019). Complex invasion history of the Asian long-horned beetle: Fifteen years after first detection in Europe. Journal of Pest Science, 92, 173–187. https://doi.org/10.1007/s10340-017-0917-1
Kellerman, T. S., Adelaar, T. F., & Minne, J. A. (1972). The toxicity of the pollen beetle Astylus atromaculatus Blanch. Journal of the South African Veterinary Association, 43, 377–381.
Leigh, J. W., & Bryant, D. (2015). PopART: Full-feature software for haplotype network construction. Methods in Ecology and Evolution, 6, 1110–1116. https://doi.org/10.1111/2041-210X.12410
Meier, R., Zhang, G., & Ali, F. (2008). The use of mean instead of smallest interspecific distances exaggerates the size of the barcoding gap and leads to Misidentification. Systematic Biology, 57, 809–813. https://doi.org/10.1080/10635150802406343
Midega, A., van den Berg, O., & Khan, Z. R. (2007). Habitat management in control of Astylus atromaculatus (Coleoptera: Melyridae) in maize under subsistence farming conditions in South Africa. South African Journal of Plant and Soil, 24, 188–191. https://doi.org/10.1080/02571862.2007.10634807
Páez, V. A., Andrada, A. R., Moreno-Ruiz-Holgado, M. M., Silenzi-Usandivaras, G. M., Oviedo, A., & de Ruiz, G. E. (2020). Chromosome numbers in insects of Argentina. I. Cytogenetic characterization of 15 species with economic importance. Revista Colombiana de Entomología, 46, e8536. https://doi.org/10.25100/socolen.v46i1.8536
Pierre, J., & Hofs, J. L. (2010). Astylus atromaculatus (Coleoptera: Melyridae): Abundance and role in pollen dispersal in Bt and non-Bt cotton in South Africa. Environmental Entomology, 39, 1523–1531. https://doi.org/10.1603/EN09142
Ramos-Onsins, S. E., & Rozas, J. (2002). Statistical properties of new neutrality tests against population growth. Molecular Biology and Evolution, 19, 2092–2100. https://doi.org/10.1093/oxfordjournals.molbev.a004034
Rozas, J., Ferrer-Mata, A., Sanchez-Del Barrio, J. C., Guirao-Rico, S., Librado, P., Ramos-Onsins, S. E., & Sánchez-Gracia, A. (2017). DnaSP 6: DNA sequence polymorphism analysis of large data sets. Molecular Biology and Evolution, 34, 3299–3302. https://doi.org/10.1093/molbev/msx248
Souza, B., & Carvalho, C. F. (1994). Aspectos morfológicos do adulto de Astylus variegatus (Germar, 1824) (Coleoptera, Melyridae). Pesquisa Agropecuaria Brasileira, 29, 689–694.
Tajima, F. (1989). Statistical methods for testing the neutral mutation hypothesis by DNA polymorphism. Genetics, 123, 585–595. https://doi.org/10.1093/genetics/123.3.585
Tamura, K., Stecher, G., & Kumar, S. (2021). MEGA11: Molecular evolutionary genetics analysis version 11. Molecular Biology and Evolution, 38, 3022–3027. https://doi.org/10.1093/molbev/msab120
van den Berg, J., Torto, B., Pickett, J. A., Smart, L. E., Wadhams, L. J., & Woodcock, C. M. (2008). Influence of visual and olfactory cues on field trapping of the pollen beetle, Astylus atromaculatus (Col.: Melyridae). Journal of Applied Entomology, 132, 490–496. https://doi.org/10.1111/j.1439-0418.2007.01259.x
Venica, N. (1969). Biología de Astylus atromaculatus Blanch., insecto perjudicial del sorgo. Acta Zoológica Lilloana, 24, 161–163.
Watmough, R. H., & Kfir, R. (1995). Predation on pupae of Helicoverpa armigera Hbn. (Lep., Noctuidae) and its relation to stem borer numbers in summer grain crops. Journal of Applied Entomology, 119, 679–688. https://doi.org/10.1111/j.1439-0418.1995.tb01358.x
Acknowledgements
To Bárbara Pidal and Marcela Sánchez (Instituto de Recursos Biológicos – INTA) for identifying the host plant Daucus pusillus. Joel D. Arneodo is member of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina) research staff.
Funding
This work was funded by Instituto Nacional de Tecnología Agropecuaria (INTA, Argentina).
Author information
Authors and Affiliations
Contributions
J.D.A.: Conceptualization; funding acquisition; investigation; writing – original draft; writing – review and editing. C.D.F: Investigation; writing – review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arneodo, J.D., Decker-Franco, C. Molecular identification and preliminary diversity analysis of Astylus atromaculatus Blanchard, 1843 (Coleoptera: Melyridae) based on mitochondrial COI sequences. Phytoparasitica 52, 76 (2024). https://doi.org/10.1007/s12600-024-01195-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12600-024-01195-x