Abstract
Phytic acid (PA), though an antinutritional factor that causes mineral deficiency in monogastric animals but also reported to be associated with expression of stress tolerance genes. The present study was conducted to find out association of seed PA with tolerance to yellow mosaic disease (YMD), powdery mildew disease (PMD) and storage pest bruchid in mungbean. Ninety four genotypes were screened for YMD by using infector rows technique in summer 2011 and 2012 and PMD screening was done in rabi 2012 in the field conditions and using excised leaf technique in growth chamber. Screening for bruchid (Callosobruchus chinensis L.) was carried out by following standard procedure. PA and inorganic phosphorous (IP) were estimated from seeds by colorimetric method and Chen’s modified method respectively. Among 94 genotypes, 47 were found susceptible while out of 47 resistant genotypes, 37, 9 and 1 were found resistant to YMD, PMD and bruchid respectively. PA was found to be highest in bruchid resistant genotype followed by PMD and YMD. Along with the resistance genes, high seed PA (>8 mgg-1) was required for resistant reaction to YMD and PMD, while for bruchid resistance very high PA (~18 mgg-1) should be present in seeds. If the concentration of PA is reduced below this level my leads to decrease in tolerance to biotic stresses, even though the resistance gene is present in the plant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mungbean (Vigna radiata L. Wilczek), an important grain legume is popular among consumers as a source of good quality protein and minerals. Among mungbean growing countries, India stands first in area (3.07 million ha.) and production (1.38 million tons). But in India, productivity of mungbean (487 kg/ha) is very less due to various biotic and abiotic stresses (Anonymous 2015). In mungbean, incidence of Yellow Mosaic Disease (YMD) caused by mungbean yellow mosaic virus (MYMV) and powdery mildew disease (PMD) caused by fungus Erysiphe polygoni DC. leads to heavy yield losses from 10 to 100% (Khattak et al. 2000). Further the storage insect pest bruchid (Callosobruchus chinensis L.) damage mungbean seeds and also reduce grain quality. Main objective of mungbean breeding is to develop high yielding, disease and pest resistant cultivars so as to increase the production and productivity. At present, improvement in quality by reducing antinutritional factor like phytic acid (PA) is one of the breeding objectives. PA (myo-inositol hexakisphosphate) is the main storage organic form of phosphorous (P) present in seeds of legumes including mungbean. The mungbean seeds contain 6.17- 9.90 mgg-1 of PA (Dahiya et al. 2013; Dhole & Reddy 2015) and 0.25 to 0.73 mgg-1 of inorganic phosphorous (IP) (Sompong et al. 2010). PA is an effective chelator of positively charged cations of nutritionally important mineral such as calcium, iron and zinc. Phytate is also found responsible for the inhibition of Trypsin (Singh & Krikorian 1982). Humans and non-ruminants lack phytase, hence enable to digest PA which leads to human mineral deficiency (Brown & Solomons 1991).
Earlier breeding efforts have been identified several low phytic acid (lpa) mutants resulting in reduction of seed PA from 50 to >95% in crops, such as wheat, maize, soybean and common bean. While developing lpa varieties, we should not overlook its effect on plant health particularly its response to various biotic and abiotic stresses. During germination, PA is utilized for plant growth and development with the help of phytase (Debnath et al. 2005). Very less information is available on the functional role of PA in the expression of disease resistance gene(s). Some lpa mutants showed yield penalties due to low stress tolerance (Ertl et al. 1998; Bregitzer & Raboy 2006) and increased susceptibility to various viral, bacterial and fungal diseases (Murphy et al. 2008). Association of high PA in seed with reduced infestation by Bruchus pisorum was reported in peas (Marzo et al. 1997). An artificial diet supplemented with 1% PA killed larvae of lepidopteron pest also showed importance of PA in pest resistance (Green et al. 2001). Reduction in PA may decrease tolerance to diseases and pests. Hence, there is need to find out association of PA content in seed with disease reactions. The present study was conducted to find out association of seed PA with YMD, PMD and bruchid tolerance in mungbean germplasm.
Materials and methods
Plant materials
Material consisted of 94 diverse mungbean germplasm lines, which included released varieties, mutants, newly developed genotypes, land races and wild species. These genotypes were grown in a randomized complete block design with two replications at Experimental and Gamma Field Facility Section, Bhabha Atomic Research Centre, Mumbai during summer 2011, 2012 and rabi 2012. Single plants were harvested at maturity and seeds were dried in an oven for 72 h at 50° C. Five seeds from five plants of each replication and each genotype were used to estimate PA and IP contents.
Preparation of sample
Samples of twenty five seeds from each genotype and replication were ground to fine powder and sieved through 40 mesh to remove seed coat fractions. The sieved fine powder was used for estimation of PA and IP.
Estimation of PAP and PA
Fine powder (50 mg) of each genotype was thoroughly mixed with 1mL of 2.4% HCl in 2 mL microcentrifuge tube. These tubes were shaken overnight in a Lab-Line Incubator Shaker (Lab-Line Instruments Inc., Melrose Park, IL, USA). Next day, these tubes were centrifuged at 10,000 rpm in centrifuge (Eppendorf, Hamburg, Germany) at 25°C for 20 min. In 1.5 mL microcentrifuge tubes, crude acid extract was transferred and 100 mg NaCl added in each tube. The salt was dissolved in extract by vertex and incubated at -20°C for 20 min to precipitate remaining matrix components that could interfere with the colorimetric reaction. A clear supernatant was obtained by centrifugation of mixture at 10,000 r min-1 for 20 min at 25°C. The supernatant was diluted 25 times by adding deionized distilled water. Then, 750 μL of the diluted supernatant were mixed with 250 μL of the modified Wade reagent (0.03% FeCl3 and 0.3% sulfosalicylic acid). The absorbance was recorded to determine PAP using the colorimetric method (Latta & Eskin 1980; Gao et al. 2007; Hande et al. 2013). The sodium salt of phytic acid (Sigma, St Louis, MO, USA) was used to prepare a series of calibration standards containing 0, 0.5, 1.0, 1.5, 2.0, 3.0, 4.0, 5.0, 7.5, 10 and 12 mgmL-1 PAP and treated in the same way as described above. The phosphorus content of sodium phytate was 18.38% (Gao et al. 2007). The absorbance of color reaction products for both samples and standards was measured at 500 nm on a UV/V is spectrophotometer (Jasco, Cambridge, UK). The pink color of the Wade reagent is due to the reaction between ferric ion and sulfosalicylic acid with absorbance maxima at 500 nm. PA content calculated by using the formula, PA= 3.552 PAP (Sompong et al. 2010).
Estimation of IP
In 50 mg of the powdered seed sample, 400 μL of 12.5% trichloro-acetic acid with 25 mmolL-1 MgCl2 were added and then vortexed. The suspension was shaken overnight at room temperature (25°C) for proper extraction, and then centrifuged at 10,000 r min-1 for 20 min. The supernatant was diluted with deionized distilled water (1:2). Thereafter, 100 μL of the diluted supernatant were then mixed with 900 μL of Chen’s reagent (6 molL-1 H2SO4, 2.5% ammonium molybdate, 10% ascorbic acid and water, in a 1:1:1:2 proportion) and incubated in a water bath at 50°C for 1 h. A series of standards containing 0.15, 0.31, 0.46, 0.62, 0.77, 0.93, 1.08, 1.24, 1.39 and 1.55 mgmL-1 IP from sodium dihydrogen phosphate were also prepared and processed in the same way as described above (Hande et al. 2013). The absorbance of colour reaction products for both samples and standards was measured at 660 nm. Total IP was estimated by the Chen’s modified method (Chen et al. 1956).
Disease and pest evaluation
Screening for YMD
Infector row method was used to screen 94 genotypes under heavy disease epidemic condition in summer 2011 and 2012 (February-April), at Experimental and Gamma Field Facility of Bhabha Atomic Research Centre, Mumbai, which is a hot spot for YMD caused by MYMV (Dhole & Reddy 2013). All genotypes were grown in two replications using randomized complete block design. Each plot consisted of 3 m rows with inter and intra-row spacing of 30 and 10 cm, respectively. After every five rows, one row of the highly susceptible genotype JL-781 was grown as infector row. All recommended cultural practices were followed, except spraying of insecticide. The disease-rating scale (1-9) was used to score YMD. Disease reactions were scored when 90% of infector rows showed YMD incidence under field conditions. Genotypes showing 1-3 grades were considered as resistant, while those showing 6-9 grades were rated as susceptible.
Field screening for PMD
Randomized complete block design with two replications was used to screen 94 genotypes for PMD in field in rabi 2012 (October-December), when conditions were congenial for disease development. The pathogen was isolated from infected leaves. Conidia from infected leaves were mixed in distilled water with teepol soap to make spore suspension. It was sprayed on leaves at 15, 20 and 25 days after sowing. The disease reactions were recorded by using a 0–5 rating scale (Reddy et al. 1994).
Screening for PMD by excised leaf technique
Those genotypes showed resistance to PMD in the field were further confirmed by using excised leaf technique in growth chamber as standardized earlier (Reddy et al. 1987). Nine resistant genotypes were further tested along with 15 susceptible genotypes in growth chamber in two replications. The pathogen isolated from field was sprayed on leaves and observations were recorded as mentioned above.
Screening for bruchid resistance
Mungbean genotypes were screened for bruchid resistance in two replications by following earlier reported standard procedure (Dongre et al. 1996). Sixty days after insect introduction, numbers of damaged seeds were recorded. Damaged seed, from which an insect had emerged, was considered as the ‘susceptible’ and an undamaged seed, from which no adult insect had emerged, was considered as ‘resistant’. Resistant reaction to bruchid was confirmed by repeated screening.
Data Analysis
Data were subjected to analysis of variance for each year and combined over both years, where replication and years were fitted as random effects and genotypes as fixed effects were tested for significance using PROC GLM of SAS 9.3.1 (SAS Institute Inc., Cary, NC). Regression of phytic acid with disease resistance was estimated with SAS 9.3.1 separately for each season. The mixed model was used to identify significant differences between resistant and susceptible genotypes for PA and IP.
Results
Variation between genotypes and seasons
Differences between genotypes, seasons and the interaction of genotype x season were significant for PA and IP (Table 1). It suggested that genetic variation for these traits exists in the mungbean germplasm which was also affected by environment and its interaction with genotype. Significant differences observed in each season i.e. summer 2011 and rabi 2012 due to genotypes, while differences due to replications were non significant for both traits (Table 2).
PA and IP
In 94 genotypes, PA content was ranged from 5.740 to 18.989 mgg-1 in summer 2011 and 5.854 to 20.024 mgg-1 in rabi 2012. On an average, PA was found to be accumulated more in seeds of rabi 2012 season (8.191 mgg-1) as compared to summer 2011 (7.918 mgg-1). IP content was ranged from 0.699 mgg-1 to 1.390 mgg-1 in summer 2011 and from 0.618 to 1.263 mgg-1 in rabi 2012. Mean IP content was found to be greater in seeds of summer season (0.990 mgg-1) than rabi season (0.917 mgg-1) (Table 3).
Evaluation genotypes for diseases and pest reaction
YMD
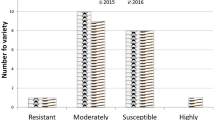
YMD incidence was noticed from 20 days after sowing up to maturity. In both summer 2011 and summer 2012, incidence of YMD in susceptible genotypes and infector rows was found to be high and more than 90% plants were infected in infector rows of JL-781 and Kopergaon (Fig.S1). In 94 genotypes, reactions to YMD were different viz., 1: free from any visible symptoms (grade 1), 22: highly resistant reaction (Grade 2), 14: resistant (grade 3), 43: susceptible (grade 6 and 7) and 14: highly susceptible (grade 8 and 9) in summer 2011. Similar reactions were observed in summer 2012 viz., 1: free from any visible symptoms, 21: highly resistant, 15: resistant, 43: susceptible and 14: highly susceptible. Over two years screening, 37 genotypes found to be resistant while remaining 57 genotypes were susceptible to YMD.
PMD
Among germplasm, only one genotype Mulmarada was found to be highly resistant i.e. R0 reaction (grade 0), while other 8 genotypes were found to be resistant i.e. R1 reaction (grade 1) to PMD. In remaining 85 susceptible genotypes, 1 genotype was moderately susceptible (grade 3), 54 were susceptible (grade 4) and 30 genotypes were highly susceptible (grade 5). Nine resistant genotypes along with 15 susceptible genotypes showed similar reactions as that of field evaluation in control conditions by using excised leaf technique (Fig.S2). Thus, 9 genotypes were resistant while 85 genotypes were susceptible to PMD.
Bruchid
In the screening for bruchid resistance, only one genotype Thokalwadi wild (Vigna radiata var. sublobata) was found to be resistant to Callosobruchus chinensis L. while rest of 93 genotypes were found to be susceptible. Bruchid resistance of Thokalwadi wild was confirmed by repeated experiments. Bruchid laid eggs on seeds of both resistant and susceptible genotypes, but no adult emergence and seed damage was noticed in Thokalwadi wild. All seeds of remaining 93 genotypes were found to be damaged with holes in each seed. When seeds of Thokalwadi wild were soaked in water and seed coat was removed, the entry spots of grubs were noticed (Fig.S3). The grubs killed at very early stage in seeds of Thokalwadi wild and hence no adult emergence was noticed. Therefore no secondary infestation was observed in Thokalwadi wild.
Association of PA with disease and pest reactions
In mixed analysis, significant differences for PA and IP were found due to disease reactions. PA content of resistant genotypes ranged from 8.094 to 18.989 mgg-1 and 8.030 to 20.024 mgg-1, which was outside the range of susceptible genotypes in both summer 2011 (5.740 to 7.989 mgg-1) and rabi 2012 (5.854 to 7.967 mgg-1) seasons respectively. The mean PA was significantly high in resistant genotypes (8.969 and 9.117 mgg-1) as compared to susceptible genotypes (6.885 and 7.291 mgg-1) in summer 2011 and rabi 2012 seasons respectively (Table 3). Resistant genotypes showed 30.27 and 25.04% higher PA than susceptible genotypes in both summer 2011 and rabi 2012 seasons respectively. PA content was highest in bruchid resistant Thokalwadi wild (18.989 and 20.024 mgg-1), followed by resistant genotypes to PMD (8.877 and 8.966 mgg-1) and YMD (8.772 and 8.859 mgg-1). Resistance genotypes for PMD, YMD and bruchid contain 28.93, 27.41, 173.45% more PA respectively in summer 2011 and 22.97, 21.50, 173.92 % more PA respectively in rabi 2012 than susceptible genotypes. PA in YMD resistant genotypes ranged from 8.129 to 10.422 mgg-1 and 8.081 to 11.478 mgg-1 in summer 2011 and rabi 2012 respectively, which was out side the range of susceptible genotypes. Genotypes resistant to PMD also showed range of PA i.e. 8.094 to 9.951 mgg-1 and 8.030 to 10.397 mgg-1 in summer 2011 and rabi 2012 respectively, which was out side the range of susceptible genotypes. Results indicated that there is some association between PA and biotic stress tolerance. Results of regression analysis further clarified the association of PA with disease reactions (Table 4). Significant regression of YMD and PMD was found with PA in both seasons.
Discussion
So far there are no reports available in mungbean and other crops regarding the association of PA with disease resistance. During germination, phytase degraded PA to produce free organic P, which is utilized for plant growth and development (Debnath et al. 2005). Yield penalties in some lpa mutants reported due to low stress tolerance (Bregitzer & Raboy 2006; Ertl et al. 1998). Decreased levels of PA in transgenic potato plants constitutively expressing an antisense gene sequence for myo-inositol 3-phosphate synthase and lpa mutant plants of Arabidopsis thaliana showed increased susceptibility to various viral, bacterial and fungal diseases (Murphy et al. 2008). In the present study, high seed PA found to be associated with resistant reaction to YMD and PMD as revealed in regression coefficients. Low PA genotypes like YBSM, JL-781, and VC-6379(58-97) were found highly susceptible to YMD, PMD and bruchid. The bruchid resistant genotype Thokalwadi wild contain 169.04 and 175.5 % greater PA than susceptible genotypes. It must be resistant to all other biotic stresses if PA alone imparts resistance. But it was resistant to bruchid only and susceptible to YMD and PMD. The PA inhibits trypsin by binding to trypsin calcium (Singh & Krikorian 1982). Thus PA acts as a trypsin inhibitor which results in the starvation and death of bruchid grubs in the cotyledons (Srinivasan & Durairaj 2007). Hence, no adult emergence was noticed in Thokalwadi wild which contain almost double seed PA as compared to bruchid susceptible genotypes. It is a constitutive type of defense against bruchid. Association of high PA in seed with reduced infestation by Bruchus pisorum was reported in peas (Marzo et al. 1997). The earlier report showed that an artificial diet supplemented with 1% PA was enough to kill larvae of lepidopteron pest (Green et al. 2001). The Thokalwadi wild contained almost 2% PA in seeds which was sufficient to kill bruchid grubs.
The YMD resistant genotypes were susceptible to PMD and bruchid and vice versa indicating that resistant gene is required along with high PA. It confirmed earlier results and suggested that PA itself is not imparting resistance to various biotic stresses but it may enhance the expression of resistance genes already present in the plant. The data obtained in the present study well supported this hypothesis that along with high PA, genotype should possess resistant genes. In crop improvement programme for stress tolerance, breeders often found it difficult to maintain the intensity of resistance present in donor parent after transfer of resistance genes in the recipient parent or their recombinants. This might be due to shuffling of resistance genes and genes for biochemical like PA having role in stress tolerance. As a result of this intensity of resistance in recombinants comes down. It can be conform if the lpa mutant identified in the resistant genotype.
Conclusion
High PA in seed is associated with resistance reaction to diseases like YMD and PMD and insect pest bruchid. To maintain resistance against YMD and, seed PA should be >8 mgg-1 while for bruchid resistance seed PA should be very high (~18 mgg-1). Results of present study also revealed that PA itself is not imparting resistance to various biotic stresses but it may enhance the expression of resistance genes already present in the plant. PA content as high as 2% in seeds has antibiosis mechanism against bruchid. If the concentration of PA is reduced below this level my leads to disease susceptibility even though the resistance gene is present in the plant.
References
Anonymous. (2015). Project coordinator’s report (mungbean and urdbean) 2014-15. All India coordinated research project on MULLaRP. Kanpur, India: Indian Institute of Pulses Research.
Bregitzer, P., & Raboy, V. (2006). Effects of four independent low phytate mutations on barley agronomic performance. Crop Science, 46, 1318–1322.
Brown, K. H., & Solomons, N. W. (1991). Nutritional problems of developing countries. Infectious Disease Clinics of North America, 5, 297–317.
Chen, P. S., Toribara, T. Y., & Warner, H. (1956). Micro determination of phosphorus. Analytical Chemistry, 28, 1756–1758.
Dahiya, P. K., Linnemann, A. R., Nout, M. J. R., Boekel, M. A. J. S., & Grewal, R. B. (2013). Nutrient composition of selected newly bred and established mungbean varieties. LWT - Food Science and Technology, 54, 249–256.
Debnath, D., Sahu, N. P., Pal, A. K., Baruah, K., Yengkokpam, S., & Mukherjee, S. C. (2005). Present scenario and future prospects of phytase in aquafeed -review. Asian-Australasian Journal of Animal Sciences, 18, 1800–1812.
Dhole, V. J., & Reddy, K. S. (2013). Development of a SCAR marker linked with a MYMV resistance gene in mungbean (Vigna radiata L. Wilczek). Plant Breeding, 132, 127–132.
Dhole, V. J., & Reddy, K. S. (2015). Genetic variation for phytic acid content in mungbean (Vigna radiata L. Wilczek). The crop Journal, 3, 157–162.
Dongre, T. K., Pawar, S. E., Thakare, R. G., & Harwalkar, M. R. (1996). Identification of resistant source to cowpea weevil [Callosobruchus maculatus (F.)] in Vigna sp. and inheritance of their resistance in black gram (Vigna mungo var. mungo). Journal of Stored Products Research, 32, 201–204.
Ertl, D. S., Young, K. A., & Raboy, V. (1998). Plant genetic approaches to phosphorus management in agricultural production. Journal of Environmental Quality, 27, 299–304.
Gao, Y., Shang, C., Saghai Maroof, M. A., Biyashev, R. M., Grabau, E. A., Kwanyuen, P., Burton, J. W., & Buss, G. R. (2007). A modified colorimetric method for phytic acid analysis in soybean. Crop Science, 47, 1797–1803.
Green, E. S., Zangerl, A. R., & Berenbaum, M. R. (2001). Effect of phytic acid and xanthotoxin on growth and detoxification in caterpillar. Journal of Chemical Ecology, 27, 1763–1773.
Hande, P., Mondal, S., Badigannavar, A. M., & D’Souza, S. F. (2013). Genetic variability of phytic acid- phosphorus and inorganic phosphorus in cultivated groundnut (Arachis hypogaea L.). Plant Genetic Resources: Characterization and utilization, 1-6.
Khattak, G. S. S., Haq, M. A., Rana, S. A., Abass, G., & Irfag, M. (2000). Effect of mungbean yellow mosaic virus (MYMV) on yields and yield components of mungbean (Vigna radiata L. Wilczek). Kasetsart Journal (Natural Sciences), 34, 12–16.
Latta, M., & Eskin, M. A. (1980). Simple and rapid colorimetric method for phytate determination. Journal of Agricultural and Food Chemistry, 28, 1315–1317.
Marzo, F., Andres, A., Maria, V. C., & Ruben, A. (1997). Fertilization effects of phosphorus and sulfur on chemical composition of seeds of Pisum sativum L. and relative infestation by Bruchus pisorum L. Journal of Agricultural and Food Chemistry, 45, 1829–1833.
Murphy, A. M., Otto, B., Brearley, C. A., Carr, J. P., & Hanke, D. E. (2008). A role for inositol hexakisphosphate in the maintenance of basal resistance to plant pathogens. The Plant Journal, 56, 638–652.
Reddy, K. S., Pawar, S. E., & Bhatia, C. R. (1987). Screening for powdery mildew (Erysiphe polygoni DC) resistance in mungbean (Vigna radiata L. Wilczek) using excised leaves. Proceedings of Indian Academy of Science (Plant Science), 97, 365–369.
Reddy, K. S., Pawar, S. E., & Bhatia, C. R. (1994). Inheritance of powdery mildew (Erysiphe polygoni DC) resistance in mungbean (Vigna radiata L. Wilczek). Theoretical and Applied Genetics, 88, 945–948.
Singh, M., & Krikorian, A. D. (1982). Inhibition of Trypsin Activity in Vitro by Phytate. Journal of Agricultural and Food Chemistry, 30, 799–800.
Sompong, U., Kaewprasit, C., Nakasathien, S., & Srinives, P. (2010). Inheritance of seed phytate in mungbean (Vigna radiata L. Wilczek). Euphytica, 171, 389–396.
Srinivasan, T., & Durairaj, C. (2007). Biochemical basis of resistance in rice bean, Vigna umbellata Thunb. (Ohwi and Ohashi) against Callosobruchus maculatus F. Journal of Entomology, 4, 371–378.
Acknowledgments
The authors are grateful to Dr. S. P. Kale, Head, NABTD, BARC, Mumbai, for his guidance and support throughout this research work. We are especially thankful to Mr. S. M. Bhatkar and P. N. Thokal for the help in conducting the field experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dhole, V.J., Reddy, K.S. Association of phytic acid content with biotic stress tolerance in mungbean (Vigna radiata L. Wilczek). Phytoparasitica 44, 261–267 (2016). https://doi.org/10.1007/s12600-016-0514-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-016-0514-5