Abstract
In the present study, selected almond genotypes in Keciborlu/Isparta region by Yıldırım (2007) based on late blooming and superior nut chacteristics were used to evaluate seedling growth performances and the reaction to root knot nematodes. Seeds were planted on perlite medium and stored 60 days at +4 °C in order to break seed dormancy. After germinating, seedlings were transferred to plastic pots to evaluate seedling growth characteristics and remaining seedlings were inoculated with Meloidogyne javanica and Meloidogyne incognita in order to asses resistance status of the selected seedlings. The highest germination percentage was obtained from Genotype 54 (96.1 %), the highest plant was observed from Genotype 9 (62.18 cm), the longest primary root was found from Genotype 54 (30.28 cm), the average secondary root lenght was highest in Genotype 9 (31.04 cm) and the widest root collar was observed in Genotype 33 (8.38 mm). Seedling stem diameter changed between 4.76 mm (Genoype 40) to 7.67 mm (Genotype 33). The lowest variation for stem widht was observed in Genotype 55 (0.58 %) while the lowest variation for seedling hight was found in Genotype 33 (0.50 %). Evaluated almond genotypes showed different reactions to studied nematode species, M. javanica and M. incognita. Resistant reactions of almond genotypes to nematode species were classified as susceptible, tolerant and resistant based on gal index values. Genotype 9 and 31 classified as tolerant to M. javanica while Genotype 54 classified as resistant to M. incognita.
Zusammenfassung
In der vorliegenden Studie wurden an den Genotypen der Mandel, die von Yıldırım (2007) aufgrund der späten Blüte und der hervorragenden Fruchtqualitäten in der Region Keciborlu/Isparta selektiert wurden, die Wachstumsentwicklung und die Reaktion auf Wurzelgallennematoden untersucht. Die Samen wurden auf ein Perlite-Medium ausgebracht und 60 Tage bei 4 °C gelagert, um die Keimruhe zu brechen. Nach der Keimung wurden die Sämlinge in Plastiktöpfen ausgepflanzt, um die Wuchseigenschaften der Sämlinge zu untersuchen. Die restlichen Sämlinge wurden mit Meloidogyne javanica und Meloidogyne incognita beimpft, um den Resistenzstatus der ausgewählten Sämlinge zu bewerten. Die höchste Keimrate (96,1 %) wurde bei Genotyp 54, die größte Pflanze (62,18 cm) bei Genotyp 9 und die längste Keimwurzel (30,28 cm) bei Genotyp 54 gefunden. Die mittlere Länge der Seitenwurzeln war bei Genotyp 9 am größten (31,04 cm) und der größte Wurzelhalsdurchmesser (8,38 mm) wurde bei Genotyp 33 erzielt. Der Triebdurchmesser des Sämlings schwankte zwischen 4,76 mm (Genotyp 40) und 7,67 mm (Genotyp 33). Die geringste Schwankung beim Triebdurchmesser zeigte Genotyp 55 (0,58 %), während die geringste Schwankung der Sämlingshöhe bei Genotyp 33 (0,50 %) beobachtet wurde. Die geprüften Mandel-Genotypen zeigten unterschiedliche Reaktionen auf die untersuchten Spezies der Nematoden-Arten M. javanica and M. incognita. Die Resistenzreaktion der Mandel-Genotypen wurde gemäß des Indexwertes für Wurzelgallen in empfindlich, tolerant und resistent eingeteilt. Die Genotypen 9 und 31 zeigten sich tolerant gegenüber M. javanica, während Genotyp 54 als resistent gegenüber M. incognita klassifiziert wurde.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anatolia is among the centers of origin of almond, as it is for many fruit species (Özbek 1978). Almond is a typical plant of the Mediterranean climate and native to the arid and barren areas of the Mediterranean Basin. It is still seen that almond is produced from these seed-grown trees in such countries as Iran, Turkey, Sicily, Spain, Morocco, and Greece (Akçay and Tosun 2005). Its sensitivity to the spring cold and the fact that it does not like much cold quite restrict the growing area of almond in the world (Cangi and Şen 1991). About 2 million tons of almonds are produced worldwide. The USA account for more than half of the almonds produced. Additionally, Spain, Iran and Italy are the other important almond producing countries. Turkey ranks among the top 10 producers of almond with 80 thousand tons production.
In modern fruit cultivation, it is recommended to use clonal rootstocks due to many advantages they offer such as providing uniform materials, early precocity, cultivar vigor, increased yield and quality, adaptation to different types of soil, and resistance to different diseases. However, seedling rootstocks are used in those species, which are difficult to propagate clonally and have a small number of clonal rootstocks. However, seedling rootstocks are preferred under unsuitable soil conditions especially where clonal rootstocks do not perform well. Moreover, the resistance of seedling rootstocks to various diseases and parasites is higher, and it is generally accepted that they do not contain any virus particles. Particularly in the recent years, the threat of aridity has been increasing due to global warming and environmental pollution which heighten usage of rootstocks.
In almond cultivation, seedling rootstocks are particularly used in the Mediterranean countries with non-irrigated and highly calcarously soils (Akçay and Tosun 2005). It is seen that seedling rootstocks are still considerably used in the production of almond saplings in Turkey (Tekintaş and Dolgun 1996; Kaptan 2012; Soylu 2012; Aktepe 2013). Furthermore, the seeds used to obtain seedling rootstocks in Turkey are taken from the trees with non-specified traits, and the seeds of different trees are mixed. Therefore seedlings obtained from mixed seeds display high heterogeneous development (Yıldırım 2007). On the contrary, the seedlings obtained from the seeds of such cultivars as ‘Texas’, ‘Garrigues’, ‘Atocha’, and ‘Desmayo Rojo’ are used in the production of saplings in the USA and in some European countries (Aktepe 2013).
Soil diseases and plant-parasitic nematodes lead to serious economic losses in cultivated plants. Since the roots of nematode-infected plants fail to perform adequate water and nutrient uptake from soil, they give rise to the retardation of development (Thorne 1961) and reduce the capacity for photosynthesis by leading to chlorosis in leaves. Besides, their indirect effects occur, for they also lead to the entry of other microorganisms due to the injuries they cause in the roots of plants (Stirling 1991). In particular, Pratylenchus penetrans and Pratylenchus vulnus are important pest species in fruit growing and may cause 30 to 80 % damage. This damage in plants may vary according to the pest density and the sensitivity of the plant.
As nematodes are soil-borne organisms and found in the plant roots during their life cycles, they cannot be controlled effectively. Cultural practices between 2 to 70 years may be required to solve the problem of nematodes in perennial plants (e. g. fruit trees) (Dowler and Van Gundy 1984). Especially the chemical compounds used against these pests pose great risks to human and environmental health. Implication of control practices is expensive. The most economical and efficient control method in cultivated plants, especially grown as plantations, is resistant rootstocks. Likewise, it is reported that such clonal rootstocks as GF-677 and GN, used to produce almond saplings, are especially sensitive to root-knot nematode species Meloidogyne incognita and Meloidogyne javanica (Soylu 2012) and GF-677 in particular is sensitive to the root lesion nematodes (Söğüt et al. 2013).
In this study, it was aimed to determine the seedling development characteristics of some almond genotypes selected by Yıldırım (2007) and their host reactions to root-knot nematode species M. incognita and M. javanica.
Materials and Methods
The seeds of 10 genotypes selected from Keçiborlu, Isparta by Yıldırım (2007) were used as the material in the research. The characteristics of the genotypes were showed in Table 1. The seeds were brought to the laboratory and dried for 15 days. The dried seeds had been preserved in a cool and dry place. The seeds of the genotypes used in the research had been subjected to stratification before sowing. To prevent possible infections, the seeds had been disinfected with a fungicide. In this process, the seeds had been treated with 1 ml of fungicide (Maxim XL 035 FS-Syngenta) per liter of water. Stratification had been carried out at +4 oC and in a 90–95 % moisture level until radicles were seen in the cases containing perlite (73 days). Some of the seeds removed from stratification were sown in the polyethylene bags filled with a mix of sand, soil and livestock manure (1:1:1w/v). Additionally, the remaining seeds were planted into the pots with a volume of 5 L with sterile soil mix consisting of 13.3 % clay, 18.4 % silt, and 68.3 % sand to asses reactions of seedlings to the root-knot nematodes.
The seedlings were irrigated regularly, and pesticides were applied when necessary. Furthermore, trace element practices containing Fe, Zn, B, Mn, Cu, and Mo were carried out from the leaf twice (June and July).
The seed germination rate (%), seedling diameter (mm), seedling height (cm), primary root length (mm), root diameter (mm), secondary root length (cm), the number of lateral roots (pieces), the uniformity of seedling heights and seedling diameters were determined in the research in order to discover the seedling development performances.
To determine the resistance of almond seedlings to nematode species M. incognita and M. javanica, the mass production of root-knot nematodes was performed in nematode-sensitive tomato cultivars ‘Rio Grande’ and ‘Tueza F1’. The tomato seedlings were transplanted into the pots containing sterilized sandy soil. The egg packages were removed under a binocular microscope when the tomatoes reached the period with 3 to 4 leaves and put into Eppendorf tubes. The mass production of the nematodes was carried out at 24 ± 1 °C in a climate chamber with 60 ± 5 % moisture. Then, four holes with a distance of 3–4 cm from the plant stem of each plant, a depth of 4–5 cm, and a diameter of 1–1.5 cm were opened into the soil of the almond seedlings transplanted into the pots. The egg packages in the Eppendorf tubes, which had been removed under a binocular microscope, were homogeneously inoculated into each hole to have about 5,000 eggs or second-stage larvae/plant.
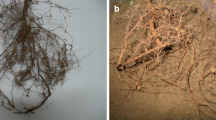
The research was terminated about 12 weeks after the inoculation of nematodes (Fig. 1) into the plants. The experimental plants were removed from their pots, and their roots were cleared of their soil with tap water in such a way that would not cause their roots to disintegrate (Fig. 2). The soil in the pot was further taken into containers and prepared for counting. The root-gall ratio (gall index) was determined in the removed plants; the egg packages and galls were counted by means of a binocular microscope; and the density of second-stage larvae in the soil was determined. Furthermore, such plant growth parameters as seedling diameter, seedling height, primary root length, the longest secondary root length, root diameter, and the number of lateral roots were measured, and the host reactions of the almond rootstocks to the root-knot nematodes were determined.
The gall index values and root-gall ratios in the roots of the seedlings were evaluated according to the egg sac and gall number index of 0–5 stated by Hartman and Sasser (1985) and described below (Table 2). Accordingly, the almond genotypes with a scale value of 0.0–2.0 were expressed as resistant, those with a scale value of 2.1–3.0 as moderately resistant, and those with a scale value of 3.1–5.0 as sensitive.
Statistical Analyses
The research was designed as the randomized block design with 3 replications for determination of the seedling development performances, each replication contained 15 plants. The study was designed as 5 replications and each replication contained 1 plant to asses root-knot nematode resistance. The analysis of variance was performed by using the SPSS package program. The statistical differences in the obtained data were determined with Tukey’s multiple comparison test.
Results and Discussion
To determine the germination rates of the genotypes, the seeds whose radicles were at least 1–2 mm were removed from stratification and used to calculate percentage. The results are presented in Table 3. Accordingly, the highest germination rate was recorded in Genotype 54 (96.1 %), followed by Genotypes 40 (94.8 %) and 42 (90.4 %). On the other hand, the lowest germination rate was found in Genotype 52 (73.6 %). The results of the study showed that the germination rates of the genotypes were quite high. Likewise, in his study, Aktepe (2013) discovered that the germination rates of 15 almond genotypes (Amygdalus orientalis) ranged from 8 % to 62 %. All genotypes displayed a 100 % emergence ratio. In the study, statistically significant differences at the level of p < 0.05 were detected in seedling height and the number of lateral roots among the genotypes, but no difference was seen in the other characteristics (Table 4). In sapling growing, it is desired that seedlings reach a thickness to allow grafting homogeneously until the grafting time (Bolat 1994). In their research, Öylek et al. (2013) reported that the seedlings with diameters between 4 and 7 mm could be grafted and that the bud take ratios of the grafts applied to the seedlings with diameters above 7 mm and their sapling quality were higher than those with small diameters.
In the research, the seedling diameters ranged from 4.76 mm (Genotype 40) to 7.67 mm (Genotype 33) and no significant difference was detected in this respect among the genotypes. These results remained lower than the findings obtained from the seedlings of cultivars ‘Texas’ and ‘48-1’ by Güngör et al. (1995) (8.98 and 9.28 mm, respectively). A significant difference in seedling height occurred among the genotypes in the study. The highest seedling height (62.18 cm) was determined in Genotype 9 and the difference between this genotype and Genotype 33 (34.52 cm) was significant. On the other hand, no significant difference was seen among the other genotypes. The obtained results were similar to the findings by Aktepe (2013) (31.2–57.3 cm) but found lower than the findings by Güngör et al. (1995) (101.20–104.50 cm).
Akça and Yıldız (1995) expressed that nut species formed more primary roots than secondary roots which caused considerable damage to the root systems during removal in nurseries. Thus, they emphasized that the bud take ratios of the transplanted saplings decreased and that the growth of saplings therefore slowed down. In the study, the primary root length ranged from 24.63 cm (Genotype 33) to 30.28 cm (Genotype 54) and the root diameter from 5.78 mm (Genotype 40) to 8.38 mm (Genotype 33), and no significant difference in these characteristics was found among the genotypes. Similarly, Güngör et al. (1995) also reported that the root length varied between 27.9 and 31.6 cm. On the other hand, a significant difference in the number of lateral roots was detected among the genotypes. The largest number of lateral roots (18.64 pieces) was determined in Genotype 9. The smallest number of lateral roots (about 14 pieces) was recorded in Genotypes 52, 65, and 32. Güngör et al. (1995) also reported that the amount of secondary roots might vary by cultivar.
The uniformity in the development of the diameters and heights of seedlings is great importance. Hence, those seedlings which are uniform in terms of the characteristics of diameter and height provide advantages in sapling cultivation for such reasons as early grafting, and the carrying out of cultural practices more easily (Güleryüz 1991; Koyuncu and Çelik 2005; Rahemi et al. 2011; Öylek et al. 2013). In the study, the variation in seedling diameter was between 0.38 % (Genotype 55) and 11.26 % (Genotype 42), whereas the variation in seedling height was detected to be between 0.50 % (Genotype 33) and 14.72 % (Genotype 32), and they displayed a high rate of uniformity in this respect.
Different host reactions of the selected almond genotypes to nematode species M. incognita and M. javanica were determined in the study. When the gall index values of the plants infected by nematode species M. incognita and M. javanica were examined (Table 5), significant differences at the level of p < 0.05 were detected among the genotypes.
According to the gall index values obtained from the research, it was not detected any genotype which was resistant to nematode species M. javanica. However, Genotypes 9 and 31 were detected to be moderately resistant (MR). In the research, only Genotype 54 was detected to be resistant to nematode species M. incognita. In the study, Genotypes 55, 65 and 9 were found sensitive, while Genotypes 31, 32, 33, 40, 42 and 52 were found moderately resistant (Table 5). Pinochet et al. (1996a, b) determined that rootstocks GN 15 and GN 9 were resistant to nematode species M. incognita according to the gall index values. The researchers reported that the gall formation in the root in rootstock GF-677 had the highest value (170 pieces) and that it was classified as sensitive. In our research, it was determined that plants with more than 100 gall formations in the root zone were classified as sensitive, a result similar to previously published results (Esmenjaud et al. 1994; Pinochet et al. 1996a, b).
Marull et al. (1991) inoculated nematode species M. incognita into different Prunus rootstocks and determined that of them, rootstocks GN1, GN3 and GN9 were resistant, while rootstock Hansen-5 was moderately resistant. In the previous studies, it was reported that the rootstocks used in production differed in resistance to root-knot nematodes (Esmenjaud et al. 1994; Marull et al. 1991; Fernandez et al. 1994; Lu et al. 1998; Ghelder et al. 2010) and that such factors as ecology, temperature, genetic resistance of the rootstock, and nematode population density were effective on the resistance of rootstocks to the nematode (Esmenjaud et al. 1994). Marull et al. (1991) examined the host reactions of some Prunus rootstocks to nematode species, namely M. incognita, M. javanica, M. arenaria, M. hapla and Pratylenchus vulnus and determined that the nematode species inoculated into the root zone could propagate in very different numbers and that the variation among them was very high, which was similar to the results of our research. In our research, it was concluded that the greatest factor affecting growth of nematode species, and caused variation might be soil temperature (Aydınlı and Mennan 2011) soil temperature influence the reproduction of strain and their levels of influence. Besides, Yüksel (1974) reported that a rootstock might be sensitive to a specific nematode species but resistant to another nematode species or either resistant or sensitive to both nematode species.
In conclusion, root-knot nematodes are among the important plant parasites which lead to economic damage in cultivated plants, which cause plant development to regress, and which have spread over an extensive area on world (Akyazı and Felek 2013). Cultural practices between 2 to 70 years may be required to solve the problem of nematodes in perennial plants (Dowler and Van Gundy 1984). Therefore, it is of great importance that the land on which an orchard will be established be free of nematodes. Establishment of orchards which are not infected by nematodes and use of rootstocks with resistance/tolerance to root-knot nematodes in production area has profound importance in terms of nematode control (Pinochet et al. 1996a, b). In our research, the genotypes were found to have different resistance to root-knot nematodes according to their gall index values.
When the results were evaluated collectively, it was seen that the almond genotypes in the experiment had high germination rates and emergence ratios. Nevertheless, they reacted differently to root-knot nematodes M. javanica and M. incognita. Genotype 31, which was moderately resistant to both nematodes, seems to have high potential for being utilized as a seedling rootstock.
References
Akça Y, Yıldız K (1995) A study on nursery tree and seedling with fibrous root in walnut growing II. National Horticultural Congress in Turkey, pp 470–474
Akçay ME, Tosun İ (2005) Determination of some late flowering foreign almond cultivars attitude of growing and yield in Yalova Region. Atatürk Üniversitesi Ziraat Fakültesi Dergisi 36:1–5
Aktepe H (2013) Kahramanmaraş ilinde bulunan bazı yabani badem türlerinin generatif olarak çoğaltılması. Kahramanmaraş Sütçü İmam Üniversitesi Fen Bilimleri Enstitüsü Yüksek Lisans Tezi, Kahramanmaraş, p 78
Akyazı F, Felek AF (2013) Population fluctuations of root-knot nematode species Meloidogyne incognita in kiwifruit orchards in Ordu province. Turkey Akademik Ziraat Dergisi 2:75–82
Aydınlı G, Mennan S (2011) Resistance to nematodes in plants. Türk Entomoloji Bülteni 1:35–47
Bolat İ (1994) A study on the growing of seedlings of some temperate fruits ın Erzincan Hortıcultural Research Instıtute. Atatürk Üniversitesi Ziraat Fakültesi Dergisi 25:67–77
Cangi R, Şen SM (1991) The studies on breeding almond (Prunus amygdalus) by the selection in Vezirköprü. Yüzüncü Yıl University. J Agric Fac 1:131–152
Dowler WM, Van Gundy SD (1984) Importance of agricultural plant nematology. In: Nickle, WR (ed) Plant and insect nematodes. Marcel Dekker, New York, pp 1–12
Esmenjaud D, Minot JC, Voisin R, Pinochet J, Salesses G (1994) Inter and intraspecific resistance variability in myrobalan plum, peach, and peach-almond rootstock using 22 root-knot nematode populations. J Am Soc Hortic Sci 119:94–100
Esmenjaud D, Minot JC, Voisin R, Pinochet J, Simard MH, Salesses G (1997) Differential response to root-knot nematodes in prunus species and correlative genetic implications. J Nematol 29:370–380
Fernandez C, Pinochet J, Esmenjaud D, Salesses G, Felipe A (1994) Resistance among new prunus rootstocks and selections to root-knot nematodes in Spain and France. HortScience 29:1064–1067
Ghelder CV, Lafargue B, Dirlewanger E, Ouassa A, Voisin R, Polidori J, Kleinhentz M, Esmenjaud D (2010) Characterization of the rmja gene for resistance to root-knot nematodes in almond: Spectrum, location and interest for prunus breeding. Tree Genet Genomes 6:503–511
Güleryüz M (1991) Ülkemiz meyve fidancılığında anaç sorunu ve dünyada anaç ıslahı ile ilgili çalışmalar Türkiye I. Fidancılık Sempozyumu, Ankara, pp 273–283
Güngör MK, Kaşka N, Çağlar S, Küden A (1995) Badem yetiştiriciliğinde saçak köklü çöğür ve fidan eldesi üzerinde araştırmalar Türkiye II. Ulusal Bahçe Bitkileri Kongresi, pp 384–388
Hartman KM, Sasser JN (1985) Identification of meloidogyne species on the basis of different host test and perineal pattern morphology. In: Barker KR, Carter CC, Sasser JN (eds) North Carolina State University Graphics, pp 69–77
Kaptan D (2012) Genetic diversity of almonds (Prunus dulcis) of Datça. Bogazici University, Molecular and Genetic Department, master thesis, İstanbul, p 96 (Unpublished)
Koyuncu F, Çelik M (2005) Katlama uygulaması ve tohum kabuğunun “nemaguard” şeftalisinde tohum çimlenmesi ve çöğür gelişimi üzerine etkileri. Süleyman Demirel Üniversitesi Fen Bilimleri Enstitüsü Dergisi 9:47–50
Lu Z‑X, Sosinski B, Reighard GL, Baird WV, Abbott AG (1998) Construction of a genetic linkage map and identification of AFLP markers for resistance to root-knot nematodes in peach root-stocks. Genome 41:199–208
Marull J, Pinochet J, Verdejo S, Soler A (1991) Reaction of prunus rootstocks to Meloidogyne incognita and M. arenaria in Spain. Suppl J Nematol 23:64–569
Öylek HŞ, Aslan A, Demirtaş MN, Avcı S (2013) Farklı çaplara sahip zerdali çöğürlerinin aşı başarısı ve fidan gelişimine etkisi. Tarım Bilimleri Araştırma Dergisi 6:103–107
Özbek S (1978) Özel Meyvecilik. Çukurova Üniversitesi Ziraat Fakültesi Yayınları, p 128
Pinochet J, Angles M, Dalmau E, Fernandez C, Felipe A (1996a) Prunus rootstock evaluation to root-knot and lesion nematodes in Spain. J Nematol 28:616–623
Pinochet J, Esmenjaud D, Salesses G, Felipe A (1996b) Resistance among new prunus rootstocks and selections to root-knot nematodes in Spain and France. HortScience 29:1064–1067
Rahemi A, Taghavi T, Fatahi R, Ebadi A, Hassani D, Chaparro J (2011) Seed germination and seedling establishment of some wild almond species. African J Biotechnol 10:7780–7786
Söğüt MA, Devran Z, Arici SE, San B, Yildirim AN (2013) Host reactions of root lesion nematodes (Pratylenchus spp.) on the rootstocks of pome and stone fruits. Turkısh J Entomol 37:239–248
Soylu A (2012) Meyve yetiştiriciliğinin temel ilkeleri. Hasad Yayıncılık, İstanbul
Stirling GR (1991) Biological control of plant-parasitic nematodes. CAB International, Wallingford, UK
Tekintaş FE, Dolgun O (1996) Badem çöğürlerine aşılı bazı şeftali ve nektarin çeşitlerinin uyuşma durumlarının incelenmesi üzerine bir araştırma. Yüzüncü Yıl Üniversitesi Ziraat Fakültesi Dergisi 6:51–54
Thorne G (1961) Principles of nematology. Mc Graw-Hill Book Company, New York
Yıldırım AN (2007) The selection of almond (Prunus amygdalus L.) in Isparta province. Adnan Menderes University, Graduate School of Natural and Applied Sciences, Department of Horticulture, Aydın, Ph.D. Thesis, p 168 (Unpublished)
Yüksel H (1974) Kök-ur nematodlarının (MeloidogyneSpp.) Türkiye’deki durumu ve bunların populasyon problemleri üzerine düşünceler. Atatürk Üniversitesi Ziraat Fakültesi Dergisi 5:83–105
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kızıltan, M.F., Yıldırım, A.N. & Söğüt, M.A. The Determination of Nematode Tolerance and Seedling Performance of The Selected Almond (Prunus amygdalus L.) Genotypes in Isparta Province. Erwerbs-Obstbau 58, 233–239 (2016). https://doi.org/10.1007/s10341-016-0282-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10341-016-0282-x
Keywords
- Almond
- Prunus amygdalus L
- Genotypes
- Seedling growth
- Root knot nematodes
- Meloidogyne javanica
- Meloidogyne incognita
- Resistant reaction