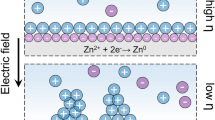
The uncontrolled growth of zinc dendrites on zinc anode hinders the application of zinc ion batteries. To solve this problem, Lu's group proposed a nuclei-rich galvanizing strategy for suppressing the zinc dendrite growth. This allows the nuclei-rich zinc electrode to cycle steadily for 1200 h and have a high Coulombic efficiency of 99.7% at 15 mA·cm−2 with a deposited amount of 10 mAh·cm−2. Moreover, the Zn-MnO2 battery with a low N/P ratio of 1.9 exhibited sustained cycling life.
Graphical abstract

摘要
锌枝晶的不可控生长阻碍了锌离子电池的应用。为了解决这个问题,卢怡君课题组提出了一种富核镀锌策略来抑制锌枝晶的生长。在 15 mA·cm-2电流密度和10 mAh·cm-2 的锌沉积量条件下,该富核锌电极组成的对称电池能够稳定循环 1200 h,并且具有高达99.7%的库仑效率。此外,负极/正极比为1.9的锌-二氧化锰电池展现出优异的循环寿命。
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Zinc ion batteries (ZIBs) are believed to be a promising candidate to replace traditional lithium-ion batteries in some applied fields due to their high safety, large specific capacity and low cost [1,2,3]. However, the limited cycling life and low Coulombic efficiency hinder the use of Zn metal, which is associated with the undesirable growth of Zn dendrites during cycling process [4, 5]. Theoretically, the essence of Zn dendrite growth is attributed to the non-uniform nucleation driven by the uneven distribution of Zn2+ concentration [6,7,8,9]. In order to inhibit Zn dendrite growth and side reactions on Zn anode, various strategies have been presented, e.g., structural construction of Zn electrodes, surface passivation, separator modification, electrolyte optimization, etc. [10,11,12,13]. For ZIBs, the uniform Zn deposition during the galvanizing process is crucial, but remains challenging.
Lu's group recently reported a nuclei-rich galvanizing strategy for constructing a nucleation interface with high ionic conductivity and nucleation catalytic activity by directly contacting hydroxyapatite surface with Zn [14], which could totally change Zn electroplating way through consistent production of numerous crystal nuclei, so that dense Zn crystals with small radii and high uniformity can form. In traditional means (Fig. 1a), the tip of the non-smooth Zn surface would increase the local current density and generate a high-intensity uneven electric field during battery cycling process, so that a large number of free Zn2+ would be adsorbed and concentrated on these tips for preferential deposition. In continuous cycling process, Zn2+ tended to grow on the existing crystal nuclei to form Zn dendrites gradually. In nuclei-rich galvanizing process (Fig. 1b), ZIBs with incubating interface had a high rate of progressive nucleation. Unlike the common progressive nucleation, the amount of nucleation in initial charge/discharge process was five times higher than that of the ordinary battery. Thus, the number of nucleation would increase sharply with the cycling process, while the radius of the nucleus decreases. Therefore, the nuclei incubating interface could exhibit more uniform charge distribution for uniform and dense Zn deposition.

Reproduced with permission from Ref. [14]. Copyright 2023, RSC Publishing
Zn plating: a conventional strategy and b nuclei-rich Zn strategy; c cycling performance of nuclei-rich Zn|Zn cell cycled at 1 mA·cm−2 with a deposit amount of 1 mAh·cm−2; d cycling performance of nuclei-rich Zn|Zn cell cycled at 15 mA·cm−2 with a deposit amount of 10 mAh·cm−2; e compared cycling performance of bare Zn||MnO2 batteries cycled at 10C; f compared cycling performance of pouch cells cycled at 5C.
Compared with traditional Zn|Zn cell in Fig. 1c, Zn|Zn cell was greatly improved after introducing nucleus-rich structure. After cycled at 1 mA·cm−2 after 252 h, the voltage of traditional Zn|Zn cell suddenly dropped due to the battery short circuit. When 15 mA·cm−2 was applied, the cycling performance was obviously enhanced (Fig. 1d). The traditional Zn|Zn cell cycled for below 100 h, while the nuclei-rich Zn|Zn cell exhibited stable cycling over 1200 h. Moreover, the nuclei-rich Zn||MnO2 battery in Fig. 1e kept an average capacity of 1.4 mAh·cm−2 after 3000 cycles with the Coulomb efficiency of 99.97% at 10C, while traditional Zn||MnO2 battery just ran for less than 400 cycles. The pouch cells constructed with nuclei-rich Zn electrode in Fig. 1f could stably cycle for more than 250 times at 5C, indicating potential for commercialization.
In conclusion, Lu's group proposed the nuclei-rich galvanizing strategy which can effectively inhibit Zn dendrite growth. The nuclei-rich Zn anode could significantly inhibit the dendrite growth with a Coulomb efficiency of 99.7%. Such Zn anode achieved the long cycling with low negative/positive ratio of the whole battery and led to pouch cells with good performance. This work not only demonstrates a novel idea to improve the Zn plating/stripping reversibility, but also provides important guidance for other metal anodes (e.g., lithium, sodium, and magnesium).
References
Zhu ZX, Lin ZW, Sun ZW, Zhang PX, Li C-P, Dong R, Mi HW. Deciphering H+/Zn2+ co-intercalation mechanism of MOF-derived 2D MnO/C cathode for long cycle life aqueous zinc-ion batteries. Rare Met. 2022;41(11):3729. https://doi.org/10.1007/s12598-022-02088-w.
Zhang SJ, Chen H, Xu YX, An CS, Xiang KX. Facile preparation of Nb2O5 microspheres and their excellent electrochemical performance in aqueous zinc-ion hybrid supercapacitors. Rare Met. 2022;41(9):3129. https://doi.org/10.1007/s12598-022-02016-y.
Su ZH, Wang RH, Huang JH, Sun R, Qin ZX, Zhang YF, Fan H-S. Silver vanadate (Ag0.33V2O5) nanorods from Ag intercalated vanadium pentoxide for superior cathode of aqueous zinc-ion batteries. Rare Met. 2022;41(8):2844. https://doi.org/10.1007/s12598-022-02026-w.
Song M, Zhong CL. Achieving both high reversible and stable Zn anode by a practical glucose electrolyte additive toward high-performance Zn-ion batteries. Rare Met. 2022;41(2):356. https://doi.org/10.1007/s12598-021-01858-2.
Yufit V, Tariq F, Eastwood DS, Biton M, Wu B, Lee PD, Brandon NP. Operando visualization and multi-scale tomography studies of dendrite formation and dissolution in zinc batteries. Joule. 2018;3:485. https://doi.org/10.1016/j.joule.2018.11.002.
Yu HM, Li QY, Liu W, Wang H, Ni XY, Zhao QW, Wei WF, Ji XB, Chen YJ, Chen LB. Fast ion diffusion alloy layer facilitating 3D mesh substrate for dendrite-free zinc-ion hybrid capacitors. J Energy Chem. 2022;73:565. https://doi.org/10.1016/j.jechem.2022.06.028.
Liu XY, Lu QQ, Yang AK, Qian YT. High ionic conductive protection layer on Zn metal anode for enhanced aqueous zinc-ion batteries. Chin Chem Lett. 2023;34: 107703. https://doi.org/10.1016/j.cclet.2022.07.046.
Zhang T, Tang Y, Guo S, Cao XX, Pan AQ, Fang GZ, Zhou J, Liang SQ. Fundamentals and perspectives in developing zinc-ion battery electrolytes: a comprehensive review. Energy Environ Sci. 2020;13:4625. https://doi.org/10.1039/D0EE02620D.
Tao F, Liu Y, Ren XY, Wang J, Zhou YZ, Miao YJ, Ren FZ, Wei SZ, Ma JM. Different surface modification methods and coating materials of zinc metal anode. J Energy Chem. 2022;66:397. https://doi.org/10.1016/j.jechem.2021.08.022.
Wang D, Li Q, Zhao Y, Hong H, Li HF, Huang ZD, Liang GJ, Yang Q, Zhi CY. Insight on organic molecules in aqueous Zn-ion batteries with an emphasis on the Zn anode regulation. Adv Energy Mater. 2022;12:2102707. https://doi.org/10.1002/aenm.202102707.
Wang TT, Li CP, Xie XS, Lu BA, He ZX, Liang SQ, Zhou J. Anode materials for aqueous zinc ion batteries: mechanisms, properties, and perspectives. ACS Nano. 2020;14:16321. https://doi.org/10.1021/acsnano.0c07041.
Zhao Z, Zhao J, Hu Z, Li JD, Li JJ, Zhang YJ, Wang C, Cui GL. Long-life and deeply rechargeable aqueous Zn anodes enabled by a multifunctional brightener-inspired interphase. Energy Environ Sci. 2019;12:1938. https://doi.org/10.1039/C9EE00596J.
Yuan Z, Yin Y, Xie C, Zhang HM, Yao Y, Li XF. Advanced materials for zinc-based flow battery: development and challenge. Adv Mater. 2019;31:1902025. https://doi.org/10.1002/adma.201902025.
Zou Q, Liang ZJ, Wang WW, Dong DJ, Lu YC. A nuclei-rich strategy for highly reversible dendrite-free zinc metal anodes. Energy Environ Sci. 2023;16:6026. https://doi.org/10.1039/D3EE03246A.
Acknowledgements
This study was financially supported by the Natural Science Foundation of China (No. 22202098) and the Key Scientific and Technological Project of Henan Province, China (No. 212102210638).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, HX., Guo, KL. Nuclei-rich galvanizing strategy for suppressing zinc dendrite growth. Rare Met. 43, 4016–4018 (2024). https://doi.org/10.1007/s12598-024-02745-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-024-02745-2