Aqueous Zn-ion batteries (ZIBs) featuring safety, affordability and high energy density may provide a promising solution to the demand for better energy storage devices. ZIBs with high electrochemical performances rely on a stable and reversible Zn metal negative electrode. The side reactions such as the hydrogen evolution reaction (HER) at the anode-electrolyte interface are responsible for the low reversibility, and uneven Zn deposition/dissolution gives rise to the instability of Zn anode. Here, a facile and scalable method of regulating electrolytes has been proposed by Chao and his co-works through adding a low-cost, safe and environmentally-friendly electrolyte additive: glucose. Theoretical calculations and experimental studies reveal that the glucose not only suppresses side reactions by modulating the solvated shell of Zn2+ but also homogenizes Zn deposition/dissolution through forming a special local absorption interface. This bi-functional electrolyte additive endows the Zn anode with both high reversibility and stability, which efficiently improves the performances of ZIBs. This work explores a promising electrolyte strategy for realizing the large-scale application of ZIBs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The frequent spontaneous combustion of electric vehicles and energy storage power stations has intensified the demand for power sources and energy storage devices with high safety. Aqueous batteries are an exceptionally promising technology to store energy from intermittent renewable resources and power electric devices concerning their intrinsic safety of aqueous electrolyte in comparison with non-aqueous batteries such as Li-ion batteries. Zn-ion batteries (ZIBs) based on zinc anode and non-corrosive electrolyte are promising alternatives among diverse aqueous batteries because of not only the safety but also the eco-friendliness and affordability [1]. In particular, Zn metal has merits of high volumetric capacity (5855 mAh·cm−3), suitable redox potential (−0.763 V vs. standard hydrogen electrode), abundance, environmentally benign and safety in comparison with other metals such as lithium [2].
Despite the advantages of Zn anode, its reversibility and stability in mild acidic electrolyte are still far from satisfaction especially at high current density and long cycling, which impedes widespread success of ZIBs [3]. Specifically, the limited reversibility of Zn anode stems from side reactions such as hydrogen evolution reaction (HER) at the anode-electrolyte interface. This parasitic HER is thermodynamically inevitable due to the more negative redox potential of Zn than that of hydrogen according to the Pourbaix diagrams [4]. Therefore, promoting Zn plating/stripping kinetic and keeping solvated water off the anode-electrolyte interface are of importance to suppress HER and improve coulombic efficiency. Secondly, the instability issue of Zn anode refers to its morphologic change and dendrite growth, which would be serious during the repeated Zn deposition/dissolution process. Uneven Zn deposition can be triggered by differential electric field distribution, inhomogeneous Zn-ion distribution or non-uniform charge transfer resistance at different reaction interfaces of Zn electrode. Therefore, averaging the electric field, optimizing the Zn-ion flux and promoting the intrinsic homogeneity of Zn electrodes are vital to achieve their high stability and long cyclic life.
In order to address the issues of reversibility and stability of Zn anode, a variety of strategies have been evaluated including using non-aqueous electrolyte [5], implementing solid-state electrolyte (SSE) [6], employing quasi-solid-state gel electrolyte [7, 8], ex situ coating functional interphase layers [9], constructing in situ solid electrolyte interphase (SEI) layers [10], designing anode with special spatial structure [11], increasing electrolyte concentration [12, 13], and optimizing electrolyte composition [14]. However, most of the approaches cannot be safe, cheap, facile and scalable at the same time. For instance, non-aqueous electrolytes feature high thermodynamic stability and wide electrochemical stability window. Nevertheless, these organic solvents would compromise the original merits of aqueous electrolytes including safety to humans and high ionic conductivity. Secondly, the solid-state electrolyte and gel electrolyte are useful to suppress HER and inhibit Zn dendrite growth. Whereas the ionic conductivity and interface compatibility with Zn anodes are generally unsatisfactory, which would impact the rate performance of ZIBs. In addition, the method of constructing ex situ functional interphase layers is designed to efficiently improve the reversibility and stability of Zn plating/stripping. However, the coating processes are usually not facile and these interphase layers are apt to detach from the Zn anode after repeated deposition/dissolution. Moreover, constructing 3D anodes with high specific surface area could homogenize electric field distribution and regulate the nucleation of Zn, while the fabrication process is complex and rarely applied in mass production. Besides, high electrolyte concentration significantly minimizes the solvated water in Zn2+ solvation sheath, which effectively suppresses HER and promotes the reversibility of Zn anode. Unfortunately, these “water-in-salt” electrolytes encounter the insurmountable obstruction of high cost and high viscosity. Furthermore, forming stable in situ SEI layers, which can address the issues of HER and dendrite growth, is challenging in aqueous electrolytes. Among these strategies, optimizing electrolyte composition through adding additives is most feasible in view of practical applications. Nevertheless, finding electrolyte additives with high human safety, low-cost and environmental friendliness is still not an easy task.
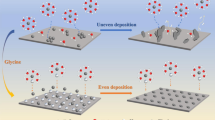
Recently, Chao and his co-works have achieved high-performance ZIBs through adding a low-cost, safe and environmentally-friendly electrolyte additive: glucose (Fig. 1) [15]. With only 0.01 mol·L−1 addition of glucose into the electrolyte consisting of 1 mol·L−1 ZnSO4, the electrolyte endows superior Zn stripping/plating stability of Zn||Zn symmetric cells for 2000 h at 1 mA·cm−2 (Fig. 1c). The glucose additive could be absorbed on the anode-electrolyte interface parallelly, which homogenizes the local electric field and regulates Zn nucleation. In the meantime, the average coulombic efficiency (CE) of Zn||Ti asymmetric cells increases from 85% to 97.3% after adding the glucose additive (Fig. 1d). The glucose functions as a replacement of H2O molecules and SO42− in the primary solvation shell (PSS), which gives rise to the weakened interaction between Zn2+ and water, Zn2+ and SO42−. Therefore, a high CE is achieved, while no by-products such as (Zn(OH)2))3ZnSO4(H2O)3 are detected. Moreover, the Zn-MnO2 full cell exhibits excellent rate performances even at the current density of 3080 mA·g−1 and enhanced capacity retention of 80% after 1000 cycles (Fig. 1e, f). These results indicate that the glucose is a promising electrolyte additive for promoting the stability and reversibility of Zn stripping/plating.

Reproduced with permission from Ref. [15]. Copyright 2021, Wiley–VCH GmbH schematic
Influences of glucose on electrochemical performances of Zn||Zn, Zn||Ti and Zn-MnO2 cells: a, b illustration of different interface reactions with and without glucose; c cycling stability of Zn||Zn symmetric cells; d reversibility and CE measured by Zn||Ti asymmetric cells; e rate and f long-term cycling performances of Zn-MnO2 full cells.
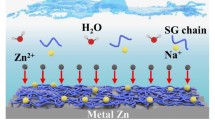
Theoretical calculations including quantum chemistry calculations in atom-lever (sub-nanometer), ab-initio calculations combined with molecular dynamics simulations in molecule-lever (nanometer) and finite element analysis in macro-lever (micrometer) are used to systematically disclose the functional mechanisms of glucose additive. First, quantum chemistry calculation provides the binding energy of Zn2+-H2O, Zn2+-glucose and H2O-glucose (Fig. 2a). Apparently, combining Zn2+ with glucose is more feasible because of the highest binding energy, which indicates that glucose can substitute H2O molecules in the Zn2+ solvation shell from the aspect of binding energy. In addition, the ab-initio calculation shows that glucose has stronger absorption abilities than H2O on different positions of Zn surface (Fig. 2b). Especially, the glucose is preferable to absorb on Zn slab parallelly in view of the largest absorption energy and the absorbed glucose modulates the characteristics of anode-electrolyte interface. Moreover, molecular dynamics simulations further reveal the solvation structure of Zn2+ in ZnSO4-glucose electrolyte from molecule-lever (Fig. 2c–e). The peak at around 0.2 nm away from Zn2+ is related with Zn–O pair from Zn-H2O (Fig. 2c) and Zn-glucose (Fig. 2d), and the average coordination numbers (ACN) are 5.2 and 0.4 for Zn-H2O and Zn-glucose, respectively. Then, the original solvation structure of Zn2+ containing six H2O molecules is changed by replacing one H2O molecule with the glucose (Fig. 2e), accompanying by deceased electrostatic potential in the solvation structure of Zn2+ (Fig. 2f). Additionally, the finite element analysis compares the different Zn stripping/plating processes in electrolyte without and with glucose (Fig. 2g, h). The absorption concentration of glucose can be self-adjusted according to the surface curvature and the concave surface of Zn foil can be filled up, which inhibits the growth of Zn dendrite (Fig. 2h). However, this “super-filling” phenomenon could not exist in the electrolyte without glucose (Fig. 2g). To sum up, based on the above theoretical researches, the glucose replaces the active H2O molecules in the inner shell of the Zn coordination sphere and parallelly absorbs on the Zn surface with the ability to self-adjust its absorption concentration. These results prove that the glucose is beneficial for achieving the dendrite-free Zn deposition/dissolution, and the adverse side reactions are suppressed since active H2O molecules and SO42− are substituted by glucose at the anode-electrolyte reaction interface.
Theoretical calculations in different scales: a binding energy of Zn2+-H2O, Zn2+-glucose and H2O-glucose based on quantum chemistry calculations; b absorption energy of glucose and H2O molecules on different positions of Zn (002) crystal plane based on ab-initio calculations; radial distribution functions of c Zn2+-O (H2O), d Zn2+-O (glucose) and e 3D snapshot of ZnSO4-glucose electrolyte based on molecular dynamics simulations; f electrostatic potential mapping of solvation structures of Zn2+ with and without glucose; interface changes during Zn tripping/plating at 0 s (left), 300 s (middle) and 600 s (right) in ZnSO4 electrolytes g without and h with glucose based on finite element analysis, where down side is anode and upside is cathode. Reproduced with permission from Ref. [15]. Copyright 2021, Wiley–VCH GmbH schematic
In conclusion, Chao and his co-works have proved that the glucose can be an efficient electrolyte additive for achieving the Zn anode with high stability and reversibility in ZIBs through theoretical calculations and experimental analyses. The glucose has merits of low-cost, safe and environmentally-friendly, which is promising to be used in mass production. In future, more researches should be focused on developing practical, feasible and safe methods to realize the goal of large-scale application of ZIBs, and the advantages of aqueous electrolytes should not be compromised. Strategies of exploiting novel electrolytes and optimizing electrolyte composition are promising from the aspects of practical application. Meanwhile, the influences of electrolyte additives on the whole battery system including the cathode should not be ignored.
References
Chao D, Zhou W, Xie F, Ye C, Li H, Jaroniec M, Qiao SZ. Roadmap for advanced aqueous batteries: from design of materials to applications. Sci Adv. 2020;6(21):eaba4098.
Song M, Tan H, Chao D, Fan HJ. Recent advances in Zn-ion batteries. Adv Funct Mater. 2018;28(41):1802564.
Zhu QN, Wang ZY, Wang JW, Liu XY, Yang D, Cheng LW, Tang MY, Qin Y, Wang H. Challenges and strategies for ultrafast aqueous zinc-ion batteries. Rare Met. 2021;40(2):309.
Chao D, Ye C, Xie F, Zhou W, Zhang Q, Gu Q, Davey K, Gu L, Qiao SZ. Atomic engineering catalyzed MnO2 electrolysis kinetics for a hybrid aqueous battery with high power and energy density. Adv Mater. 2020;32(25):2001894.
Wang N, Dong X, Wang B, Guo Z, Wang Z, Wang R, Qiu X, Wang Y. Zinc-organic battery with a wide operation-temperature window from −70 to 150 °C. Angew Chem Int Ed. 2020;59(34):14577.
Wang Z, Hu J, Han L, Wang Z, Wang H, Zhao Q, Liu J, Pan F. A MOF-based single-ion Zn2+ solid electrolyte leading to dendrite-free rechargeable Zn batteries. Nano Energy. 2019;56:92.
Chen Z, Li X, Wang D, Yang Q, Ma L, Huang Z, Liang G, Chen A, Guo Y, Dong B, Huang X, Yang C, Zhi C. Grafted MXene/polymer electrolyte for high performance solid zinc batteries with enhanced shelf life at low/high temperatures. Energy Environ Sci. 2021;14(6):3492.
Jin Y, Han KS, Shao Y, Sushko ML, Xiao J, Pan H, Liu J. Stabilizing zinc anode reactions by polyethylene oxide polymer in mild aqueous electrolytes. Adv Funct Mater. 2020;30(43):2003932.
Yang H, Chang Z, Qiao Y, Deng H, Mu X, He P, Zhou H. Constructing a super-saturated electrolyte front surface for stable rechargeable aqueous zinc batteries. Angew Chem Int Ed. 2020;59(24):9377.
Zeng X, Mao J, Hao J, Liu J, Liu S, Wang Z, Wang Y, Zhang S, Zheng T, Liu J, Rao P, Guo Z. Electrolyte design for in situ construction of highly Zn2+-conductive solid electrolyte interphase to enable high-performance aqueous Zn-ion batteries under practical conditions. Adv Mater. 2021;33(11):2007416.
Chao D, Zhu C, Song M, Liang P, Zhang X, Tiep NH, Zhao H, Wang J, Wang R, Zhang H. A high-rate and stable quasi-solid-state zinc-ion battery with novel 2D layered zinc orthovanadate array. Adv Mater. 2018;30(32):1803181.
Chao D, Qiao S-Z. Toward high-voltage aqueous batteries: super- or low-concentrated electrolyte? Joule. 2020;4(9):1846.
Wang F, Borodin O, Gao T, Fan X, Sun W, Han F, Faraone A, Dura JA, Xu K, Wang C. Highly reversible zinc metal anode for aqueous batteries. Nat Mater. 2018;17(6):543.
Hao J, Yuan L, Ye C, Chao D, Davey K, Guo Z, Qiao SZ. Boosting zinc electrode reversibility in aqueous electrolytes by using low-cost antisolvents. Angew Chem Int Ed. 2021;60(13):7366.
Sun P, Ma L, Zhou W, Qiu M, Wang Z, Chao D, Mai W. Simultaneous regulation on solvation shell and electrode interface for dendrite-free Zn ion batteries: achieved by a low-cost glucose additive. Angew Chem Int Ed. 2021;60(33):18247.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (No. 22075115), the Natural Science Foundation of Jiangsu Higher Education Institutions of China (No. 19KJA430020) and Jiangsu Qing Lan Project (2020)
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Song, M., Zhong, CL. Achieving both high reversible and stable Zn anode by a practical glucose electrolyte additive toward high-performance Zn-ion batteries. Rare Met. 41, 356–360 (2022). https://doi.org/10.1007/s12598-021-01858-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-021-01858-2