Abstract
Protonic ceramic fuel cells (PCFCs) have been attracting increasing attention because of their advances in high-efficiency power generation in an intermediate-temperature range, as compared to the high-temperature solid oxide fuel cells (SOFCs). The greatest difference between PCFCs and SOFCs is the specific requirement of protonic (H+) conductivity at the PCFC cathode, in addition to the electronic (e−) and oxide-ion (O2−) conductivity. The development of a triple H+/e−/O2− conductor for PCFC cathode is still challenging. Thus, the most-widely used cathode material is based on the mature e−/O2− conductor. However, this leads to insufficient triple phase boundary (TPB), i.e., reaction area. Herein, an efficient strategy that uses a ~ 100 nm-thick proton conductive functional layer (La0.5Sr0.5CoO3−δ, LSC55) in-between the typical La0.8Sr0.2CoO3−δ cathode (a mature e−/O2− conductor, LSC82) and BaZr0.4Ce0.4Y0.1Yb0.1O3−δ electrolyte (11 mm in diameter, 20 μm in thickness) is proposed to significantly enhance the reaction area. Reasonably, the ohmic resistance and polarization resistance are both decreased by 47% and 62%, respectively, compared with that of PCFCs without the functional layer. The power density of the PCFC with such a functional layer can be raised by up to 2.24 times, superior to those described in previous reports. The enhanced PCFC performances are attributed to the well-built TPB and enhanced reaction area via the functional layer engineering strategy.
Graphical abstract

摘要
与高温固体氧化物燃料电池(SOFC)相比,质子陶瓷燃料电池(PCFC)可在中温范围内高效发电,其先进性受到越来越多的关注。PCFC 和 SOFC 之间的主要区别在于,除了电子 (e-) 和氧离子 (O2-) 电导率之外,PCFC 阴极还需要一定的质子 (H+) 电导率。目前开发用于 PCFC 阴极的单相H+/e-/O2- 三型导体仍然具有挑战性。因此,最广泛使用的PCFC阴极材料依旧是成熟的 e-/O2-导体(已经在SOFC中得以充分研究)。然而,这可能导致PCFC阴极三相边界 (triple phase boundary, TPB) 即反应区域的不足。在该工作中,~100 nm质子导电功能层(La0.5Sr0.5CoO3-δ, LSC55)被提出放置在典型的La0.8Sr0.2CoO3-δ阴极(一种成熟的e-/O2- 导体,LSC82)和BaZr0.4Ce0.4Y0.1Yb0.1O3-δ电解质(直径 11 mm,厚度 20 μm)之间,以有效增加反应面积。实验结果表明,与没有功能层的PCFC相比,使用功能层使得欧姆阻抗和极化阻抗分别降低了47%和62%。具有功能层的 PCFC 的功率密度最多可以被提高2.24 倍,明显优于之前报道。PCFC 性能的增强与功能层策略带来的大量TPB 和反应区域密切相关。
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With the optimization of the global energy structure, it is extremely urgent to develop and utilize clean energy, e.g., hydrogen. Solid oxide fuel cells (SOFCs) at high temperature (typically 800−1000 °C) have gained much attention as a high-efficiency technology to convert hydrogen into electrical energy [1, 2]. Although the high working temperature improves the high energy efficiency, the harsh conditions usually lead to short lifetime and high maintaining cost of SOFCs. Alternatively, the protonic ceramic fuel cells (PCFCs) have recently emerged. PCFCs work is based on protonic conducting electrolyte, other than the SOFCs based on oxide-ion (O2−) conducting electrolyte. Because the activation energy for proton conduction is 0.3−0.6 eV, which is much lower than that for O2− conduction (~ 0.8 eV), PCFCs can function below 700 °C. In this regard, PCFCs are a promising device to utilize clean hydrogen energy [3,4,5,6,7].
Developing high-performance PCFCs that operates at a relatively low temperature is particularly desired, e.g., at 600 °C [8,9,10]. However, two obstacles at decreased working temperature must be considered: one is the high ohmic resistance (Ro) of the electrolyte and the other is high polarization resistance (Rp) of the cathode. Great efforts have been devoted to decreasing Ro by using a thinner electrolyte. For example, by decreasing the thickness of the BaZr0.1Ce0.7Y0.2O3–δ electrolyte from 18 to 10 μm, the Ro can be decreased from 0.66 to 0.24 Ω·cm2 at 500 °C [11, 12]. Other works suggest that the Ro can be optimized to 0.1–0.15 Ω·cm2 if the electrolyte thickness can be controlled at ∼2.5 μm [13, 14]. However, controlling the electrolyte to be thin is challenging in terms of both the electrolyte production and PCFC fabrication. Exploring advanced strategies to decrease Ro is thus attractive [5].
On the other hand, it is essential to decrease Rp is to facilitate the kinetics at cathode side. The design and synthesis of cathode materials have been thus intensively investigated. So far, oxides with double O2−/e− conductivities, such as La0.6Sr0.4Co0.2Fe0.8O3–δ (LSCF), Ba0.5Sr0.5Co0.2Fe0.8O3–δ (BSCF), and La0.8Sr0.2CoO3–δ (LSC82) [15, 16], have been widely used as the cathodes in PCFCs, because these types of oxides are well-understood in the field of SOFCs. However, as previously mentioned, the protonic conductivity is important in a PCFC cathode. Otherwise, protons from the electrolyte cannot transfer across the cathode, leading to fewer cathode−electrolyte−gas triple phase boundaries (TPBs), a low reaction area and low kinetics. Accordingly, the non-existence or insufficiency of proton conductivity in these double oxides demonstrates high Rp (> 0.4 Ω·cm2) at the temperature below 600 °C [3, 15, 16]. An effective solution to address this issue is to develop oxides with simultaneous triple H+/O2−/e− conductivity [17,18,19,20,21,22,23,24]. However, the development of a triple H+/O2−/e− conductor is at its preliminary stage because the proton conductivity in oxides is still limited by the insufficient hydration reaction, e.g., > 1 × 10−5 S·cm−1 is required to active the electrode [5, 8, 25,26,27].
Herein, a strategy based on a cathode functional layer (CFL) with triple conductivity is proposed to decrease both Ro and Rp. Significantly, this is accomplished without employing a thinner electrolyte or triple H+/O2−/e− conducting cathode. Specifically, a thin La0.5Sr0.5CoO3–δ (LSC55) layer is fabricated in-between the conventional BaZr0.4Ce0.4Y0.1Zr0.1O3–δ (BZCYYb) electrolyte and La0.8Sr0.2CoO3–δ (LSC82) cathode. Because of the significantly decreased Ro and Rp, the output power density of a PCFC cell is remarkably improved by 2.24 times compared with that without this functional layer. Moreover, this study investigated the mechanisms for the extended reaction area and enhanced performance. The results demonstrated the potential of this functional layer engineering strategy for developing advanced PCFCs.
2 Experimental
2.1 Synthesis of LSC82 cathode powder
La0.8Sr0.2CoO3–δ (LSC82) powder was synthesized using a previously reported citrate precursor method referring to Refs. [28, 29]. Briefly, La(NO3)3·6H2O, Sr(NO3)2, and Co(NO3)2·6H2O (high purity chemicals, 99.99%, used without pretreatment) following the required stoichiometry were dissolved in H2O (Milli-Q). Citric acid (CA; C6H8O7·H2O) was then added as a chelate agent at a mole ratio of CA:LSC82 = 2:1. The obtained solution was heated to 60 °C and maintained for 3 h under vigorously stirring in order to evaporate H2O and promote polymerization. The obtained gelatinous product was calcinated at 500 °C for 1 h in the air, which yielded a precursor with deep color. Finally, the precursor was annealed at 800 °C for 15 h in pure O2 in a tube furnace, producing the LSC82 cathode powder.
2.2 Synthesis of electrolyte and anode precursors
BaCO3 (high purity chemicals, 99.95%), ZrO2 (high purity chemicals, 98%), CeO2 (high purity chemicals, 99.99%), Y2O3 (high purity chemicals, 99.99%), and Yb2O3 (high purity chemicals, 99.99%), along with 3.56 wt% of Zn(NO3)2·6H2O (Wako chemicals, 99.9%) were ball-milled in ethanol for 48 h and subsequently dried at 80 °C to obtain the precursor powder for BZCYYb electrolyte. The BZCYYb electrolyte precursor and NiO were mixed in ethanol at a weight ratio of 40:60 and ball-milled for 48 h to obtain the anode precursor.
2.3 Fabrication of functional layer and PCFCs
A specific amount of anode precursor was uniaxially pressed under 20 MPa and subsequently pressed isostatically under a hydrostatic pressure of 100 MPa. After that, green anode pellets (~ 12 mm in diameter, ~ 1.2 mm in thickness) were obtained. Then, an electrolyte precursor slurry was prepared by dispersing the electrolyte precursor powder into a solution containing a dispersant (20 wt% polyethylenimine (molecular weight (Mw) = 28,000) dissolved in α-terpineol) and binder (5 wt% surfactant dissolved in α-terpineol). The electrolyte precursor slurry was spin-coated on both sides of the anode pellets, followed by co-sintering at 1450 °C for 12 h in an air atmosphere. Accordingly, the compact ceramic disks in ca. Φ 9 mm × 1 mm were obtained. The backs of the ceramic disks were polished with a mechanical grinder to remove the electrolyte. Thus, the anode | electrolyte configuration was obtained.
A functional layer of La0.5Sr0.5CoO3–δ (LSC55, ~ 100 nm) was coated on the electrolyte side using radio frequency (RF, 40 W) sputtering at 500 °C for 2 h in Ar atmosphere, prior to post annealing at 700 °C for 1 h in O2 atmosphere. The cathode slurry was prepared by dispersing 2 g LSC82 cathode powder in isopropanol (5 ml), α-terpineol (5 ml), molding aids (1 ml) and ethylene (0.3 g). Then the cathode slurry was coated on the LSC55 functional layer by screen printing, forming a configuration of anode | electrolyte | functional layer | cathode. Pt paste was applied to the anode as a current collector, just as in our previous reports [24, 28]. Finally, the cell was annealed at 700 °C for 1 h in air. As a reference, the cell without the functional layer was also prepared.
2.4 Physical characterization
X-ray powder diffraction (XRD, Rigaku, Ultima IV) was used to evaluate the phase purity using Cu Kα radiation (40 kV, 20 mA) in a 2θ range of 5°–90°. The scan rate and 2θ step were 5 (°)·min−1 and 0.02°, respectively. Scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDX) were performed on a SIGMA500 (ZEISS).
2.5 PCFC performance test
The PCFC cathode was sealed in a glass tube filled with synthetic air (20 vol% O2 and 80 vol% Ar fed bubbled through water at 30 ml·min−1 (H2O pressure of 2330 Pa)), while humidified H2 (10 ml·min−1) with 3% water was fed into the anode side. The same gas flows were used in electrolysis mode tests. The impedance spectra of the PCFCs were obtained with a Solartron 1260A frequency response analyzer implemented with a Solartron 1287 potentiostat in the frequency range of 106–0.1 Hz with an AC amplitude of 30 mV under an open circuit voltage (OCV) condition in a temperature range of 500–700 °C. The current–voltage (I–V) and current–power (I–P) characteristics were recorded on the same apparatus.
3 Results and discussion
3.1 Phase structure and microstructure
Figure 1a shows XRD pattern of the La0.8Sr0.2CoO3–δ (LSC82) cathode powder, which was annealed at 800 °C for 15 h in a pure oxygen atmosphere. The LSC82 powder exhibited a single perovskite structure without the appearance of any second phase. Meanwhile, all the diffraction peaks were identical to those of rhombohedral La0.9Sr0.1CoO3–δ (PDF No. 28-1229). The microstructural features of the LSC82 powder are shown in Fig. 1b, where the grain size was evenly distributed. The calculated average size based on this SEM image was about 82 nm, which was smaller than those of previously reported cathode materials [30,31,32,33]. The small size usually benefits high available surface area. In addition, EDX images shown in Fig. 1c–f suggest that the La, Sr, Co, and O were uniformly distribute in the whole sample. Reasonably, it was desirable to use this LSC82 as the PCFC cathode because it could provide more active sites for the cathodic reaction.
We fabricated PCFCs with and without the CFL in-between the electrolyte and cathode (see the details in Sect. “Experimental”). Figure 2a shows the cross-sectional SEM image of the PCFC with the CFL. It is clearly to observe three distinct layers: the anode BZCYYb + NiO, 20 μm-thick electrolyte BZCYYb (including the LSC55 CFL), and LSC82 cathode from bottom to top. The LSC55 CFL was difficult to be directly observed due to the extremely thin film with the thickness of about 90 nm, which is prepared by the same method with our previous report [34]. SEM image of the electrolyte (Fig. 2b) demonstrates a dense surface while the anode remains good porous structure (Fig. 2c) to provide enough active sites for hydrogen diffusion and reduction. XRD patterns of the anode, electrolyte (BZCYYb) and electrolyte with CFL (BZCYYb + LSC55) are presented in Fig. 2d. The anode exhibits the peaks of NiO and pure perovskite structure. Although the BZCYYb and BZCYYb + LSC55 have the same diffraction features assigned to the pure perovskite structure, the lattice constants are different, as implied from the peak shifts (Fig. 2e and f) for the two cells. The above results suggest that there was no impurity in the anode and electrolyte.
SEM images of a cross section, b surface of electrolyte, and c anode for PCFC consisting of LSC82 cathode, BZCYYb electrolyte, CFL, and NiO-BZCYYb anode; d XRD patterns of anode, electrolyte, and electrolyte with sputtered CFL for PCFC; e–g enlarged XRD peaks of electrolyte and electrolyte with sputtered CFL
3.2 Electrochemical performances of PCFCs
In order to evaluate the role of the CFL, the electrochemical performances of PCFCs with and without the CFL were tested at the temperature range of 500–700 °C. The obtained I–V curves are shown in Fig. 3a, b. The evolution of the OCV versus operating temperature based on these two curves is presented in Fig. 3d. By decreasing the operating temperature from 700 to 500 °C, the OCV increased from 0.99 to 1.14 V for the PCFC without the CFL and from 0.99 to 1.11 V for the PCFC with the CFL. The theoretical OCV could be calculated according to the Nernst equation, which changes from 1.14 to 1.16 V at decreasing temperature from 700 to 500 °C. The gap between the theoretical and practical OCV values was attributed to the hole conduction in the BZCYYb electrolyte [24].
I–V curves and corresponding power densities measured in temperature range of 500–700 °C for PCFCs a without and b with CFL (anode: wet hydrogen with 3% water (10 ml·min−1); cathode: wet air (20 vol% O2 and 80 vol% Ar, H2O pressure of 2330 Pa; 30 ml·min−1); c PPD as function of temperature; d comparison of theoretical and measured OCVs for two PCFCs
On the other hand, the power densities of the PCFCs with the CFL were much higher than those for PCFCs without the CFL at specific temperatures (comparing Fig. 3a with Fig. 3b). The changes of peak power densities (PPDs) vs. temperature for different cells are illustrated in Fig. 3c. The PPDs of PCFCs without the CFL were 414, 233, 162, 137 and 80 mW·cm−2 at 700, 650, 600, 550 and 500 °C, respectively. These values can be significantly improved to 783, 520, 364, 255 and 159 mW·cm−2, respectively, by applying the CFL strategy. What should be emphasized is that the PPD at 600 °C is raised by 2.24 times after introducing CFL. Such an improvement is remarkably better than previous reports [8, 35, 36]. For example, the PPD was increased by 0.37 times by introducing (Pr,Ba,Sr)(Co,Fe)O3 interlayer [8], by 0.8 times when using a BZCYYb1711 layer [35].
We list the published PPD values of typical and recent PCFCs in Table 1 [5, 15, 37,38,39,40,41,42]. Except for those PCFCs with excellent PPDs [5, 41, 42], most PCFCs using a Zr-rich BZCYYb electrolyte usually exhibited limited power densities, and most of the PPDs were lower than 400 mW·cm−2 at 700 °C. The PPD of a PCFC based on a BaZr0.4Ce0.4Y0.2O3 (BZCY442) cathode was 360 mW·cm−2 at 700 °C, [37] while that with the renowned Ba0.5Sr0.5Co0.2Fe0.8O3 (BSCF) cathode presents 381 mW·cm−2 [15]. This comparison indicates that the PPD (783 mW·cm−2) at 700 °C obtained in this study is among the best as compared to those found in the literature.
The electrochemical impedance spectra (EIS) were measured under the OCV condition at 700–500 °C for the PCFCs with and without the CFL. The recorded results and fitting curves are shown in Fig. 4. In general, the x-axis intercept at the high frequency represents the ohmic resistance (Ro) of the cells, which includes the resistance of proton conduction in the electrolyte (main contribution), the contact resistance is associated with the interface, and the electronic resistance of the electrodes. The diameter of the semi arc provides the Rp at the cathode side. It has been widely accepted that the semi arc includes two distinct semi arcs, named as R1 and R2. Accordingly, it is reasonable to fit the EIS with an equivalent circuit model with the configuration of Ro–(R1-CPE1)–(R2-CPE2) as depicted in the inset of Fig. 4a, where R and CPE represent the resistance and constant phase element (i.e., pseudo-capacitance), respectively. S1 and S2 as shown in Fig. 4b are interpreted by the parallel components of (R1-CPE1) and (R2-CPE2), respectively [24, 34]. Therefore, R1 and R2 provide the polarization resistance related to S1 and S2, so that they are renamed as Rp1 and Rp2. The Rp1 from 1 × 105 to 1 × 102 Hz represents the charge transfer resistance (electrochemical proton incorporation, Reaction (3)), and the Rp2 from 1 × 105 to 1 × 10−1 Hz usually reflects the resistance of mass transfer on the cathode (the surface diffusion or association desorption of oxygen species on the cathode side, Reactions (1, 2, 4)) during the cathodic reacting process. Accordingly, EIS curves of the PCFC with and without the CFL at different temperatures are fitted, as shown in Fig. 4a–e; meanwhile, the fitted results of Ro (and calculated proton conductivity), Rp1, Rp2 and Rp are plotted against the temperature, as shown in Fig. 4f–i, respectively.
As shown in Fig. 4f, after applying the LSC55 CFL, the Ro decreased from 0.34 to 0.2 Ω·cm2, 0.47 to 0.25 Ω·cm2, 0.59 to 0.31 Ω·cm2, 0.75 to 0.39 Ω·cm2, and 0.95 to 0.5 Ω·cm2, respectively. The corresponding proton conductivities increase from 0.0059 to 0.01 S·cm−1, 0.004 to 0.008 S·cm−1, 0.0033 to 0.006 S·cm−1, 0.0027 to 0.005 S·cm−1, and 0.002 to 0.004 S·cm−1 at 700, 650, 600, 550 and 500 °C, respectively. Even though the proton conductivities were lower than that recently report (0.028 S·cm−1 of BZCY442 at 700 °C) [43], the significant decreases in terms of Ro and proton conductivity strongly suggests the improved proton conductivity in the PCFCs if using CFL.
On the other hand, the Rp1 (Fig. 4g) and Rp2 (Fig. 4h) of PCFCs with the CFL were smaller than those without the CFL. For example, Rp1 and Rp2 are sharply decreased from 0.55 to 0.11 Ω·cm2 and from 0.58 to 0.32 Ω·cm2 at 600 °C (Rp1 and Rp2 are decreased by 80% and 45% after using the CFL). These results indicate that the CFL can dramatically promote the proton incorporating process, as well as the surface diffusion or association desorption of oxygen species. Specifically, at a low temperature, e.g., 500 °C, the CFL also played an essential role in promoting mass transfer. At this temperature, the Rp1 and Rp2 decreased by 26% and 70%, respectively, indicating that the Reactions (1, 2) may be the rate-determining step for the cell without the CFL. Furthermore, Rp is the sum of Rp1 and Rp2, representing the total resistance of cathodic reaction. Reasonably, the obtained Rp values were 0.34, 1.13, 1.63, and 3.84 Ω·cm2 for PCFCs without the CFL, while those for PCFCs with the CFL significantly decline to 0.25, 0.43, 0.86 and 1.59 Ω·cm2 at 650, 600, 550 and 500 °C, respectively (Fig. 4i).
The Arrhenius plots of Ro−1 and Rp−1 for the PCFCs with and without the CFL were performed (Fig. 5). As shown in Fig. 5a, the activation energies (Ea) of Ro−1 were 0.29 and 0.32 eV for PCFCs with and without the CFL. The decreased Ea of Ro−1 for PCFC with CFL suggested that the CFL contributed greatly to the proton conduction of the PCFCs. On the other hand, the Ea of Rp−1 also greatly declined from 1.60 to 1.12 eV when the CFL was sputtered on the electrolyte (Fig. 5b). The dramatically decreased Rp indicated that the CFL can greatly promote hole/proton transfer, water formation and the surface kinetics of the cathode. This Ea value was lower than the widely used cathode materials, such as LSCF6428 (1.40 eV) [15], even comparable to that of the state-of-the-art triple conducting (La0.7Sr0.3)(Mn0.7Ni0.3)O3 (1.13 eV) [44].
In this work, we also preliminarily studied the application of the CFL strategy in protonic ceramic electrolysis cells (PCECs), which works under the reverse mode of fuel cell [45]. The I–V curves for PCECs with the CFL were recorded from 1.8 V to OCV at 500–700 °C, as shown in Fig. 6a. In detail, the current densities at 1.3 V were 1390, 706, 460 and 283 mA·cm−2 at 700, 650, 600 and 550 °C, respectively (Fig. 6b). Interestingly, the current density at 700 °C can reach 1390 mA·cm−2, which was higher than that of the reported Zr-rich BZCY based electrolysis cells in the literatures (Table 2 [46,47,48,49,50]).
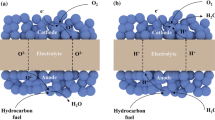
Based on the above results, the functional layer strategy proposed in this work plays an efficient role in promoting the electrochemical performance of PCFCs at intermediate temperatures. Both Ro and Rp were essentially lowered by using the CFL. For PCFCs, particularly those using a double e−/O2− conductor as cathode (like the LSC82 used in this work), the performance is significantly determined by the cathode−electrolyte−gas TPB. As shown in Fig. 7a, the proton transfers from the anode to the cathode side through electrolyte. Since the double e−/O2− conductor is not conductive for protons, the ORR can take place only on the electrolyte/cathode interface. In this regard, some protons cannot directly and immediately participate in the ORR, resulting in the slow kinetics at cathode. By contrast, once a functional LSC55 layer is applied (Fig. 7b), which was reported to be a proton−electron−oxide ion triple conducting phase [34], the O2− and e− can transfer in the whole CFL. Therefore, the protons from the electrolyte can be directly reacted and form water as products. Reasonably, the reaction area is largely expanded to all the interface between the CFL and the electrolyte, thus resulting in the decreased Ro and Rp of PCFCs and improved power density.
4 Conclusion
In summary, BaZr0.4Ce0.4Y0.1Yb0.1O3–δ electrolyte-based PCFCs with a sputtered ultra-thin CFL, i.e., ~ 90 nm La0.5Sr0.5CoO3–δ, was designed and successfully prepared. The PPD can reach 783 mW·cm−2 at 700 °C. At a lower temperature, e.g., 600 °C, the PPD increases by 2.24 times, and the ohmic and polarization resistance declines from 0.59 to 0.31 Ω·cm2 and 1.12 to 0.43 Ω·cm2, respectively, as compared with the PCFC without the CFL. The enhanced electrochemical performances are attributed to the functional layer between the electrolyte and the cathode, which plays the significant role in expanding the cathodic reaction area. We assume that if the CFL with rough surface where the cathode particles can be embedded inside, the electrochemical performance can be further improved, which is still pending in our group. These results indicate that the functional layer engineering strategy holds promise for the development of advanced PCFCs.
References
Madhusudan C, Kasarapu V, Chittimadula M, Reddy YS, Reddy CV. Synthesis and characterization of Y and Dy co-doped ceria solid electrolytes for IT-SOFCs: a microwave sintering. Rare Met. 2021;40(11):3329. https://doi.org/10.1007/s12598-018-1059-1.
Wang ZM, Feng XX, Xie ZX, Li YM. Preparation and characterization of La1–xBaxFeO3–δ cathode materials for intermediate-temperature solid oxide fuel cell. Chin J Rare Met. 2021;45(10):1192. https://doi.org/10.13373/j.cnki.cjrm.xy19080033.
Wang N, Tang CM, Du L, Zhu RJ, Xing LX, Song ZQ, Yuan BY, Zhao L, Aoki Y, Ye SY. Advanced cathode materials for protonic ceramic fuel cells: recent progress and future perspectives. Adv Energy Mater. 2022;12(34):2201882. https://doi.org/10.1002/aenm.202201882.
Wang N, Yuan BY, Tang CM, Du L, Zhu RJ, Aoki Y, Wang W, Xing L, Ye S. Machine learning–accelerated development of efficient mixed protonic–electronic conducting oxides as the air electrodes for protonic ceramic cells. Adv Mate. 2022;34(51):2203446. https://doi.org/10.1002/adma.202203446.
Duan C, Tong J, Shang M, Nikodemski S, Sanders M, Ricote S, Almansoori A, O’Hayre R. Readily processed protonic ceramic fuel cells with high performance at low temperatures. Science. 2015;349(6254):1321. https://doi.org/10.1126/science.aab3987.
Pergolesi D, Fabbri E, D’Epifanio A, Di Bartolomeo E, Tebano A, Sanna S, Licoccia S, Balestrino G, Traversa E. High proton conduction in grain-boundary-free yttrium-doped barium zirconate films grown by pulsed laser deposition. Nat Mater. 2010;9(10):846. https://doi.org/10.1038/nmat2837.
Ding XF, Shen JY, Gao XJ, Wang J. Enhanced electrochemical properties of Sm0.2Ce0.8O1.9 film for SOFC electrolyte fabricated by pulsed laser deposition. Rare Metals. 2021;40(5):1294. https://doi.org/10.1007/s12598-014-0396-y.
Choi S, Kucharczyk CJ, Liang Y, Zhang X, Takeuchi I, Ji HI, Haile SM. Exceptional power density and stability at intermediate temperatures in protonic ceramic fuel cells. Nat Energy. 2018;3(3):202. https://doi.org/10.1038/s41560-017-0085-9.
An H, Lee HW, Kim BK, Son JW, Yoon KJ, Kim H, Shin D, Ji HI, Lee JH. A 5 × 5 cm2 protonic ceramic fuel cell with a power density of 13 W cm–2 at 600 °C. Nat Energy. 2018;3(10):870. https://doi.org/10.1038/s41560-018-0230-0.
Bae K, Kim DH, Choi HJ, Son JW, Shim JH. High-performance protonic ceramic fuel cells with 1 µm thick Y:Ba(Ce, Zr)O3 electrolytes. Adv Energy Mater. 2018;8(25):1801315. https://doi.org/10.1002/aenm.201801315.
Nien S, Hsu C, Chang C, Hwang B. Preparation of BaZr0.1Ce0.7Y0.2O3–δ based solid oxide fuel cells with anode functional layers by tape casting. Fuel Cells (Weinh). 2011;11(2):178. https://doi.org/10.1002/fuce.201000147.
Nguyen NTQ, Yoon HH. Preparation and evaluation of BaZr0.1Ce0.7Y0.1Yb0.1O3−δ (BZCYYb) electrolyte and BZCYYb-based solid oxide fuel cells. J Power Sources. 2013;231(213):218. https://doi.org/10.1016/j.jpowsour.2013.01.011.
Choi SM, An H, Yoon KJ, Kim BK, Lee HW, Son JW, Kim H, Shin D, Ji HI, Lee JH. Electrochemical analysis of high-performance protonic ceramic fuel cells based on a columnar-structured thin electrolyte. Appl Energy. 2019;233:29. https://doi.org/10.1016/j.apenergy.2018.10.043.
Bae K, Jang DY, Choi HJ, Kim D, Hong J, Kim BK, Lee JH, Son JW, Shim JH. Demonstrating the potential of yttrium-doped barium zirconate electrolyte for high-performance fuel cells. Nat Commun. 2017;8(1):14553. https://doi.org/10.1038/ncomms14553.
Jeong S, Kobayashi T, Kuroda K, Kwon H, Zhu C, Habazaki H, Aoki Y. Evaluation of thin film fuel cells with Zr-rich BaZrx Ce0.8−xY0.2O3−δ electrolytes (x≥ 0.4) fabricated by a single-step reactive sintering method. RSC Adv. 2018;8(46):26309. https://doi.org/10.1039/C8RA04724C.
Liu Y, Ran R, Tade MO, Shao Z. Structure, sinterability, chemical stability and conductivity of proton-conducting BaZr0.6M0.2Y0.2O3−δ electrolyte membranes: the effect of the M dopant. J Membr Sci. 2014;467:100. https://doi.org/10.1016/j.memsci.2014.05.020.
Yang L, Zuo C, Wang S, Cheng Z, Liu M. A novel composite cathode for low-temperature SOFCs based on oxide proton conductors. Adv Mater. 2008;20(17):3280. https://doi.org/10.1002/adma.200702762.
Yang L, Liu Z, Wang S, Choi Y, Zuo C, Liu M. A mixed proton, oxygen ion, and electron conducting cathode for SOFCs based on oxide proton conductors. J Power Sources. 2010;195(2):471. https://doi.org/10.1016/j.jpowsour.2009.07.057.
Choi J, Kim B, Song SH, Park JS. A composite cathode with undoped LaFeO3 for protonic ceramic fuel cells. Int J Hydrog. 2016;41(22):9619. https://doi.org/10.1016/j.ijhydene.2016.03.115.
Dailly J, Taillades G, Ancelin M, Pers P, Marrony M. High performing BaCe0.8Zr0.1Y0.1O3–δ-Sm0.5Sr0.5CoO3–δ based protonic ceramic fuel cell. J Power Sources. 2017;361:221. https://doi.org/10.1016/j.jpowsour.2017.06.089.
Lee S, Park S, Wee S, Baek HW, Shin D. One-dimensional structured La0.6Sr0.4Co0.2Fe0.8O3–δ-BaCe0.5Zr0.35Y0.15O3–δ composite cathode for protonic ceramic fuel cells. Solid State Ion. 2018;320:347. https://doi.org/10.1016/j.ssi.2018.03.010.
Song YF, Chen YB, Wang W, Zhou C, Zhong YJ, Yang GM, Zhou W, Liu ML, Shao ZP. Self-assembled triple-conducting nanocomposite as a superior protonic ceramic fuel cell cathode. Joule. 2019;3(11):2842. https://doi.org/10.1016/j.joule.2019.07.004.
Zhao Z, Cui J, Zou M, Mu S, Huang H, Meng Y, He K, Brinkman KS, Tong J. Novel twin-perovskite nanocomposite of Ba–Ce–Fe–Co–O as a promising triple conducting cathode material for protonic ceramic fuel cells. J Power Sources. 2020;450:227609. https://doi.org/10.1016/j.jpowsour.2019.227609.
Wang N, Toriumi H, Sato Y, Tang C, Nakamura T, Amezawa K, Kitano S, Habazaki H, Aoki Y. La0.8Sr0.2Co1–xNixO3–δ as the efficient triple conductor air electrode for protonic ceramic cells. ACS Appl Energy Mater. 2020;4(1):554. https://doi.org/10.1021/acsaem.0c02447.
Zohourian R, Merkle R, Raimondi G, Maier J. Mixed-conducting perovskites as cathode materials for protonic ceramic fuel cells: understanding the trends in proton uptake. Adv Funct Mater. 2018;28(35):1801241. https://doi.org/10.1002/adfm.201801241.
Ren R, Wang Z, Xu C, Sun W, Qiao J, Rooney DW, Sun K. Tuning the defects of the triple conducting oxide BaCo0.4Fe0.4Zr0.1Y0.1O3–δ perovskite toward enhanced cathode activity of protonic ceramic fuel cells. J Mater Chem A. 2019;7(31):18365. https://doi.org/10.1039/C9TA04335G.
Zohourian R, Merkle R, Maier J. Proton uptake into the protonic cathode material BaCo0.4Fe0.4Zr0.2O3–δ and comparison to protonic electrolyte materials. Solide State Ion. 2017;299:64. https://doi.org/10.1016/j.ssi.2016.09.012.
Wang N, Hinokuma S, Ina T, Toriumi H, Katayama M, Inada Y, Zhu C, Habazaki H, Aoki Y. Incorporation of bulk proton carriers in cubic perovskite manganite driven by interplays of oxygen and manganese redox. Chem Mater. 2019;31(20):8383. https://doi.org/10.1021/acs.chemmater.9b02131.
Aoki Y, Tsuji E, Motohashi T, Kowalski D, Habazaki H. La0.7Sr0.3Mn1–xNixO3–δ electrocatalysts for the four-electron oxygen reduction reaction in concentrated alkaline media. J Phys Chem C. 2018;122(39):22301. https://doi.org/10.1021/acs.jpcc.8b06741.
Zhou Y, Zhang W, Kane N, Luo Z, Pei K, Sasaki K, Choi Y, Chen Y, Ding D, Liu M. An efficient bifunctional air electrode for reversible protonic Ceramic electrochemical cells. Adv Funct Mater. 2021;31(40):2105386. https://doi.org/10.1002/adfm.202105386.
Zhou Y, Liu E, Chen Y, Liu Y, Zhang L, Zhang W, Luo Z, Kane N, Zhao B, Soule L, Niu Y, Ding Y, Ding H, Ding D, Liu M. An active and robust air electrode for reversible protonic ceramic electrochemical cells. ACS Energy Lett. 2021;6(4):1511. https://doi.org/10.1021/acsenergylett.1c00432.
Liu B, Jia L, Chi B, Pu J, Li J. A novel PrBaCo2O5+σ-BaZr0.1Ce0.7Y0.1Yb0.1O3 composite cathode for proton-conducting solid oxide fuel cells. Compos B Eng. 2020;191:107936. https://doi.org/10.1016/j.compositesb.2020.107936.
Li W, Guan B, Ma L, Hu S, Zhang N, Liu X. High performing triple-conductive Pr2NiO4+δ anode for proton-conducting steam solid oxide electrolysis cell. J Mater Chem A. 2018;6(37):18057. https://doi.org/10.1039/c8ta04018d.
Tang C, Akimoto K, Wang N, Fadillah L, Kitano S, Habazaki H, Aoki Y. The effect of an anode functional layer on the steam electrolysis performances of protonic solid oxide cells. J Mater Chem A. 2021;9(24):14032. https://doi.org/10.1039/d1ta02848k.
Shimada H, Yamaguchi Y, Ryuma MM, Sumi H, Nomura K, Shin W, Mikami Y, Yamauchi K, Nakata Y, Kuroha T, Mori M, Mizutani Y. Protonic ceramic fuel cell with Bi-layered structure of BaZr0.1Ce0.7Y0.1Yb0.1O3–δ functional interlayer and BaZr0.8Yb0.2O3–δ electrolyte. J Electrochem Soc. 2021;168(12):124504. https://doi.org/10.1149/1945-7111/ac3d04.
Shimada H, Yamaguchi T, Sumi H, Nomura K, Yamaguchi Y, Fujishiro Y. Improved transport property of proton-conducting solid oxide fuel cell with multi-layered electrolyte structure. J Power Sources. 2017;364:458. https://doi.org/10.1016/j.jpowsour.2017.08.038.
Liu Y, Guo Y, Ran R, Shao Z. A novel approach for substantially improving the sinterability of BaZr0.4Ce0.4Y0.2O3−δ electrolyte for fuel cells by impregnating the green membrane with zinc nitrate as a sintering aid. J Membr Sci. 2013;437:189. https://doi.org/10.1016/j.memsci.2013.03.002.
Nasani N, Ramasamy D, Mikhalev S, Kovalevsky AV, Fagg DP. Fabrication and electrochemical performance of a stable, anode supported thin BaCe0.4Zr0.4Y0.2O3–δ electrolyte protonic ceramic fuel cell. J Power Sources. 2015;278:582. https://doi.org/10.1016/j.jpowsour.2014.12.124.
Bi L, Fabbri E, Traversa E. Effect of anode functional layer on the performance of proton-conducting solid oxide fuel cells (SOFCs). Electrochem commun. 2012;16(1):37. https://doi.org/10.1016/j.elecom.2011.12.023.
Guo Y, Lin Y, Ran R, Shao Z. Zirconium doping effect on the performance of proton-conducting BaZryCe0.8−yY0.2O3−δ (0.0≤ y≤ 0.8) for fuel cell applications. J Power Sources. 2009;193(2):400. https://doi.org/10.1016/j.jpowsour.2009.03.044.
Kim J, Sengodan S, Kwon G, Ding D, Shin J, Liu M, Kim G. Triple-conducting layered perovskites as cathode materials for proton-conducting solid oxide fuel cells. ChemSusChem. 2014;7(10):2811. https://doi.org/10.1002/cssc.201402351.
Choi S, Davenport T, Haile S. Protonic ceramic electrochemical cells for hydrogen production and electricity generation: exceptional reversibility, stability, and demonstrated faradaic efficiency. Energy Environ Sci. 2019;12:206. https://doi.org/10.1039/c8ee02865f.
Han D, Liu X, Bjoheim T, Uda T. Yttrium-doped barium zirconate-cerate solid solution as proton conducting electrolyte: why higher cerium concentration leads to better performance for fuel cells and electrolysis cells. Adv Energy Mater. 2021;11:2003149. https://doi.org/10.1002/aenm.202003149.
Wang N, Hinokuma S, Ina T, Zhu C, Habazaki H, Aoki Y. Mixed proton–electron–oxide ion triple conducting manganite as an efficient cobalt-free cathode for protonic ceramic fuel cells. J Mater Chem A. 2020;8(21):11041. https://doi.org/10.1039/d0ta03899g.
Duan C, Kee R, Zhu H, Sullivan N, Zhu L, Bian L, Jennings D, O’Hayre R. Highly efficient reversible protonic ceramic electrochemical cells for power generation and fuel production. Nat Energy. 2019;4(3):230. https://doi.org/10.1038/s41560-019-0333-2.
Kobayashi T, Kuroda K, Jeong S, Kwon H, Zhu C, Habazaki H, Aoki Y. Analysis of the anode reaction of solid oxide electrolyzer cells with BaZr0.4Ce0.4Y0.2O3–δ electrolytes and Sm0.5Sr0.5CoO3–δ anodes. J Electrochem Soc. 2018;165(5):F342. https://doi.org/10.1149/2.0891805jes.
Huan D, Shi N, Zhang L, Tan W, Xie Y, Wang W, Xia C, Peng R, Lu Y. New, efficient, reliable air electrode material for proton-conducting reversible solid oxide cells. ACS Appl Mater Inter. 2018;10(2):1761. https://doi.org/10.1021/acsami.7b16703.
Gan Y, Zhang J, Li Y, Li S, Xie K, Irvine JTS. Composite oxygen electrode based on LSCM for steam electrolysis in a proton conducting solid oxide electrolyzer. J Electrochem Soc. 2012;159(11):F763. https://doi.org/10.1149/2.018212jes.
Gan L, Ye L, Liu M, Tao S, Xie K. A scandium-doped manganate anode for a proton-conducting solid oxide steam electrolyzer. RSC Adv. 2016;6(1):641. https://doi.org/10.1039/C5RA19844E.
Bi L, Shafi SP, Traversa E. Y-doped BaZrO3 as a chemically stable electrolyte for proton-conducting solid oxide electrolysis cells (SOECs). J Mater Chem A. 2015;3(11):5813. https://doi.org/10.1039/C4TA07202B.
Acknowledgements
This work was financially supported by China Post-doctoral Science Foundation (No. 2022M710856), Guangzhou Postdoctoral Research Project (No. 62104380), the Outstanding Youth Project of Natural Science Foundation of Guangdong Province (No. 2022B1515020020), the Funding by Science and Technology Projects in Guangzhou (Nos. 202206050003 and 202201010603) and Guangdong Engineering Technology Research Center for Hydrogen Energy and Fuel Cells.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, N., Huang, ZY., Tang, CM. et al. Functional layer engineering to improve performance of protonic ceramic fuel cells. Rare Met. 42, 2250–2260 (2023). https://doi.org/10.1007/s12598-022-02257-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-022-02257-x