Abstract
In this paper, thermoluminescence (TL) properties of rare earth Tb3+-doped α-Sr2P2O7 were examined after β-irradiation and photoluminescence (PL) properties of samples were examined for proper excitation. All the samples were synthesized by high-temperature combustion method. The X-ray diffraction (XRD) and Fourier transform infrared (FTIR) spectroscopy characterization confirms the formation of pure α-phase with crystallized in orthorhombic structure of samples. The PL emission spectra of all samples exhibit characteristic green emission peaks of Tb3+ where the peak at 545 nm has the highest emission intensity for Tb3+ concentration of 5.0 mol%. The TL glow curves of β-irradiated Tb3+-doped α-Sr2P2O7 phosphors were recorded at different heating rates of 2, 4, and 6 K·s−1. TL curves of all sample exhibit combination of two peaks: peak at 420 K shifts toward higher temperature, while peak at 525 K remains unaffected with the increase in Tb3+ concentration as well as fading effect. The activation energy and kinetic parameters of the samples were evaluated using thermoluminescence peak shape method.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Pyrophosphate-based materials are scientifically important because of their luminescent, dielectric, semiconducting, catalytic, magnetic, fluorescent, and ion-exchange properties. Because of remarkable properties, the synthesis of inorganic pyrophosphate materials is a dynamic field of research, especially in luminescence aspects [1–10]. The thermoluminescence (TL) dosimetry properties of Sr2P2O7: Cu, Pr compounds have been studied [1]. In previous work, photoluminescence (PL) and dosimetry properties of Ce-activated α-Sr2P2O7 phosphor were synthesized by a high-temperature combustion method and X-ray-irradiated TL properties of Dy3+-doped α-Sr2P2O7 were studied [2, 3]. Eu2+- and Mn2+-co-doped Sr2P2O7 phosphors were suggested as the potential phosphors for obtaining white-light emission from ultraviolet-light-emitting diode (UV-LED) devices [4].
The TL properties of pure and doped strontium pyrophosphates were widely examined, because the rare earth activated inorganic phosphors are extensively used in a variety of applications in lamp phosphors, color displays, radiation dosimetry, and X-ray imaging [5]. Singly and doubly doped strontium phosphate α-Sr(PO3)2 was compared with equivalently doped diphosphates α-Sr2(P2O7) and SrZn(P2O7), and the crystal structure determination of SrZn(P2O7) based on single-crystal data was evaluated [6]. Eu2+-activated SrCaP2O7 phosphor was suggested as blue-emitting phosphor [7]. Alkaline earth metal pyrophosphates M2P2O7 (M = Ca, Sr, Ba) were proposed for their potential applications as luminescent materials, for example, Sr2P2O7:Eu3+, Tb3+; Ca2P2O7:Eu2+, Y3+; Sr2P2O7:Eu2+, Mn2+; Ca2P2O7:Eu2+, Mn2+ [8–10].
In this paper, PL property of phosphor was examined for various Tb3+ concentrations and the TL property of Tb3+-activated α-Sr2P2O7 was explained on the basis of fading effect. The activation energy, frequency factors, and order of kinetics of the glow curves were calculated by peak shape method with curve fitting information. PL studies suggest the phosphor as excellent green phosphor and TL of phosphor is also quite good important.
2 Experimental
Tb3+-doped Sr2P2O7 phosphors were synthesized by high-temperature combustion method. The samples with various Tb3+ concentrations (0.5 mol%, 1.0 mol%, 1.5 mol%, 2.0 mol%, 2.5 mol% and 5.0 mol%) were synthesized. The starting reactants SrCO3, (NH4)2HPO4 and Tb4O7 (99.99 %) (all in analytical reagent (AR)) were taken as per stoichiometric proportion and grinded in an agate mortar with urea (AR; 15 wt% of the mixture) to make homogeneous mixture. Urea was taken as a flux in this method to raise the combustion process. The grinded mixture was placed in an alumina crucible and heated at 1200 °C for 3 h in a muffle furnace in air and then naturally cooled down to room temperature. The sample in the form of pure white fine powder was obtained after grinding the product [2, 3].
The prepared samples were first characterized by X-ray diffractometer (XRD, Bruker D8; Cu Kα radiation, λ = 0.15406 nm, 40 kV and 40 mA) to examine crystal phase formation and structural parameters of phosphors. The XRD measurements were carried out for glancing angle incidence detector at an angle of 2° for 2θ values of 10°–60° in steps of 0.02°. Fourier transform infrared (FTIR) spectra of the samples were recorded by FTIR-4100 type A IR spectrometer in transmittance mode in wavenumber range of 400–4000 cm−1. The PL properties of the phosphor were examined using a Shimadzu spectrofluorometer (1503 R-PC). PL excitation and emission spectra were recorded by a xenon lamp through a monochromator. The TL glow curves of β-irradiated samples were taken using a NUCLEONIX TL analyzer type TL1009 at different heating rates (β) of 2, 4, and 6 K·s−1.
3 Theory
The activation energy (E a), frequency factor (s), and order of kinetics (b) of glow curves were calculated using Chen’s peak shape method [11]. Chen’s method had functional application with a broad energy range between 0.1 and 2.0 eV and the pre-exponential factor (frequency factor) of 1 × 105–1 × 1023 s−1. For analysis of activation energy by Chen’s method, necessary information of the kinetic order is not required. The geometric factor (μ g) was calculated to determine the order of kinetics of glow curve. In this method, three equations for first- and second-order kinetics are derived correlated with the trap depth: a full width of the peak at half maximum (ω = T 2 − T 1), a low-temperature half width (τ = T m − T 1), and a high-temperature half width (δ = T 2 − T m), where T 1 and T 2 are low and high temperatures corresponding to half-maximum intensity, respectively; and T m is the peak temperature at maximum intensity.
The general equation for evaluating activation energy (E a) is given as:
where k is the Boltzmann’s constant, γ = τ, δ, ω, and c γ and b γ are constants which can be calculated using following formula:
Peak shape method is also very useful for estimating the order of kinetics of the TL peaks. As per this method, the order of kinetics (b) was determined by the geometric shape factor (μ g). In general, suggested values of geometric factor are μ g = 0.42 for the first-order kinetics and μ g = 0.52 for the second-order kinetics [11–13].
4 Results and discussion
4.1 XRD analysis
Figure 1 shows XRD patterns of Sr2P2O7 phosphors doped with Tb3+ concentrations of 0.5 mol%, 2.5 mol%, and 5.0 mol%. The XRD patterns illustrate that the structural formation of all samples are very similar to that of the of α-Sr2P2O7 (JCPDS No. 24-1011), in which Sr2P2O7 has an α-phase orthorhombic crystal structure with Pnam space group (JCPDS No. 24–1011). The doping of Tb3+ does not show any measurable influence on the host structure [2, 3, 14, 15]. Tb3+ cannot replace P5+, but it can easily fit into the site of Sr3+ as the ionic radius of Tb3+ (0.0923 nm) is smaller than that of Sr2+ (0.114 nm) and larger than that of P5+ (0.052 nm) [16]. Lattice constants of Sr2P2O7:2.5 mol% Tb calculated are a = 0.8865 nm, b = 1.3142 nm, c = 0.5421 nm, V = 0.631567 nm3 and α = β = γ = 90°. The lattice parameters obtained with different Tb3+ concentrations show very similar nature and the XRD results of Tb3+-doped samples are quite consistent with previous work [2, 3].
4.2 FTIR results
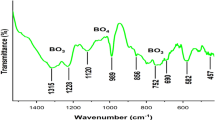
FTIR transmittance spectra of Tb3+-doped Sr2P2O7 were recorded using KBr pellets. KBr pellet of all samples were prepared in a 99:1 weight ratio of KBr to Sr2P2O7 for total weight at a thickness of 1 mm. Figure 2 shows FTIR spectra of Tb3+-doped Sr2P2O7 phosphors in the wavenumber range of 400–2000 cm−1. The FTIR spectra show characteristic absorption bands of pyrophosphate group (P2O7)−4 at 746 and 1190 cm−1, which explain the formation of pyrophosphate groups [2, 3]. Peak observed at 1047 cm−1 is attributed to the antisymmetric stretching mode of (P–O)v 3. Broad peak located at 976 cm−1 is attributed to the symmetric stretching mode of (P–O)v 1. The solder peaks at 620 cm−1 is ascribed the bending mode of (O–P–O)v 4 [2, 3, 17]. The peaks observed in the range of 450–750 cm−1 correspond to the antisymmetric bending modes of PO4, while the peaks between 950 and 1200 cm−1 are attributed to the stretching modes of PO4 [2, 3, 18]. FTIR peaks located at 976, 1014, 1047, 1062, and 1109 cm−1 are attributed to the antisymmetric stretching mode of P–O bond of (PO4)−3 groups in Sr2P2O7 [2, 3]. The bands at 547, 562, 612, and 620 cm−1 are assigned to the antisymmetric bending vibration of (PO4)−3 groups [18–20]. The FTIR spectra show that the positions of all the peaks remain unaffected for various Tb3+ doping concentrations. Thus, the doping of Tb3+ does not affect the bond structure formation of host Sr2P2O7, which is very similar to that of XRD observations.
4.3 PL properties
Figure 3a shows excitation spectrum of Tb3+-doped α-Sr2P2O7 phosphor monitored at 545-nm emission wavelength. The excitation spectra exhibits a single band peaked at 232 nm due to the 4f8–4f75d1 (f–d) transition of Tb3+. An excitation band illustrates that the phosphor can be excited through the UV ligth of about 232 nm. Figure 3b illustrates the PL emission spectra, recorded by exciting the samples at their optimized excitation wavelength (i.e., 232 nm) to determine the PL emission from samples. It is important to find out the detailed information of the nature of the Tb3+ luminescence center formed inside host lattice. The emission spectra do not give all information about luminescence center, due to the high degeneracy of the Tb3+ levels involved in several transitions.
Excitation spectra of Tb3+-doped α-Sr2P2O7 recorded at emission wavelength of 545 nm a, PL excitation spectra of Tb3+-doped α-Sr2P2O7 recorded at excitation wavelength of 232 nm b, International Commission on Illumination CIE (Commission internationale de l’eclairage) co-ordinates of Tb3+-doped α-Sr2P2O7 phosphors c, and effect of doping Tb3+ concentration on emission intensity of host α-Sr2P2O7 d
The PL emission spectra of the Tb3+-doped α-Sr2P2O7 show peaks at 415, 436, 469, 491, 545 and 584 nm as a consequence of the characteristics radiative transitions of Tb3+. All defined transitions occurring correspond to the 5D3–7F5, 5D3–7F4, 5D3–7F3, 5D4–7F6, 5D4–7F5, and 5D4–7F4, respectively. The observed high intense peak at 545 nm can be attributed to green emission which is a characteristic emission of Tb3+. Tb3+ replaces the host Sr2+ from lattice site without any structural deformation, which is mainly responsible for contraction of unit cell of host Sr2P2O7. The doping ions occupy the host lattice site and create metastable states inside the energy gap of host, which is the reason for the occurrence of PL emissions. It is inferred that the energy transfer process occurs between host (Sr2P2O7) and activator Tb3+, and a very strong green emission occurs at 545 nm because of 5D4–7F5 electronic transition.
Tb3+ doping ion is effectively observed through the characteristic emission lines, where the emission intensity is lower for small Tb3+ concentrations, indicating that the dopant can be clustered or incorporated onto the lattice sites of host α-Sr2P2O7. The chromaticity coordinates of PL emission spectra recorded at 232-nm excitation are shown in Fig. 3c. The chromaticity coordinates of Tb3+-doped α-Sr2P2O7 phosphor are X = 0.316, Y = 0.673, which are falling into the green region in the chromaticity diagram and remain the same for all Tb3+ concentrations. Figure 3d exhibits the variation of intensity for two dominant transitions 5D4–7F6 and 5D4–7F5 with various Tb3+ doping concentrations. The intensity of emission occurred due to 5D4–7F5 transition is 2–3 times higher that of 5D4–7F6 transition. Concentration quenching effect of doping was not studied, but the green emission could be optimized for 5 mol% Tb3+.
4.4 TL properties
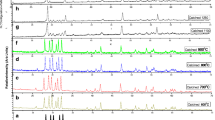
Figure 4 shows the TL glow curves fitted by deconvolution method for α-Sr2P2O7:0.5 mol% Tb, to formulate the trap depth created inside the band structure of α-Sr2P2O7 phosphors by doping Tb3+. All the samples were irradiated for dose of 10 J·kg−1 using Sr90 β-source. The fading effect after β-irradiation on glow curves of all Tb3+-doped α-Sr2P2O7 samples recorded at heating rate of 6 K·s−1 are depicted in Fig. 5. The glow curves for various Tb3+ concentrations demonstrate that the intensity of TL glow curve decreases from Day 1 to Day 20, in which the peak temperature of the first peak at 420 K shifts toward higher temperature, while that of the second peak at 525 K remains unaffected. It is also observed that at highest Tb3+ concentration (5.0 mol%), the second peak at 525 K of glow curve almost vanishes due to energy transfer process; therefore, the intensity of the first peak increases dominantly. TL glow curves analysis were done by Chen’s peak shape method by evaluating order of kinetics (b) and activation energy (E a) recorded at a different heating rates of 2, 4, and 6 K·s−1, and the glow curves were fitted by deconvolution method. From the curve fitting method, it is found that the glow curve consists of two peaks, the first peak is centered at around 420–435 K, and the second peak is centered at around 525 K.
All estimated parameters like energy of trap depths (activation energy) (E a), order of kinetics (b), and frequency factor (s) at different heating rates are mentioned in Table 1. The energy of trap depth for the first fitted curve peaked at 420 K is around 0.8–0.9 eV and that of the second fitted curve peaked at 525 K is around 0.5–0.6 eV. The calculated values of frequency factor (s) are around 1 × 106–1 × 1011 s−1, which is very small compared to that of the expected lattice vibration of 1 × 1012–1 × 1014 s−1 [21]. The observed low-frequency factor is a characteristic of localized transitions in which radiative recombination process can take place without the involvement of a transition of the electrons from conduction band to the valence band. Low activation energy indicates that the probability of localized detrapping process occurs due to the doping of Tb3+, radiative localized transitions, and the extent of wave function overlaps between the trap and the recombination center [22, 23]. TL property of the Tb3+-doped Sr2P2O7 phosphors is mainly due to the defect centers created by substitution of Sr2+ by Tb3+ which does not affect the structure of α-Sr2P2O7. TL sensitivity of α-Sr2P2O7:Tb3+ phosphor predominantly depends on Tb3+ concentration, and optimum concentration is observed to be 5.0 mol% in Fig. 5. The geometric factor estimated from TL analysis is around μ g = 0.50–0.52 for both peaks, which is nearer to that of Chen’s suggested value μ g = 0.52 for the second-order kinetics.
Figure 6 shows the variation in activation energy (E a) with different Tb3+ doping concentrations. The activation energies of TL glow curve calculated for both peaks for the different doping concentrations are significantly uniform. The results indicate that the same TL mechanism occurs in all samples, revealing that the phosphor could be good for thermoluminescence dosimeter (TLD).
5 Conclusion
Tb3+-doped α-Sr2P2O7 phosphors were synthesized by high-temperature combustion method using urea as a flux. XRD and FTIR studies confirm that the host has orthorhombic structure which remains unchanged with various Tb3+ concentrations. The results of PL and TL studies reveal that the luminescence properties increase with doping Tb3+ concentration. PL studies reveal that α-Sr2P2O7 doped with 5.0 mol% Tb3+ shows excellent green emission for a given excitation, and this material has potential to be used as a green luminescent material for display systems in solid state lighting applications. TL analyses of glow curve reveal that the TL glow curve has two peaks analogous to traps created within band structure of host because of Tb3+ doping. The calculated parameters of the glow curve imply that it is of second-order kinetic. The activation energy (E a) and frequency factor (s) are about 0.8–0.9 eV and 1 × 109–1 × 1011 s−1 for the curve with maximum temperature of around 420 K, respectively, and 0.5–0.6 eV and 1 × 106–1 × 107 s−1 for the curve with maximum temperature of 525 K. TL parameters suggest phosphor as a good TL material.
References
Yazici AN, Seyyidoglu S, Toktamis H, Yilmaz A. Thermoluminescent properties of Sr2P2O7 doped with copper and some rare earth elements. J Lumin. 2010;130(10):1744.
Patel NP, Srinivas M, Verma V, Modi D, Murthy KVR. Luminescence study and dosimetry approach of Ce on an α-Sr2P2O7 phosphor synthesized by a high-temperature combustion method. J Biol Chem Lumin. 2015;30(4):472.
Patel NP, Srinivas M, Verma V, Modi D, Murthy KVR. Synthesis and thermoluminescence dosimetry application of Dy3+ activated Sr2P2O7. Int J ChemTech Res. 2014;6(3):1708.
Ma CG, Zheng W, Jin LG, Dong LM. Fluorescence and preparation of Sr2(P2O7):Ce, Tb phosphate by co-precipitation method. Rare Met. 2013;32(4):420.
Natarajan V, Bhide MK, Dhobale AR, Godbole SV, Seshagiri TK, Page AG, Lu CH. Photoluminescence, thermally stimulated luminescence and electron paramagnetic resonance of europium-ion doped strontium pyrophosphate. Mater Res Bull. 2004;39(13):2065.
Henning AH, Michael D, Brohmer MC. Coactivation of α-Sr(PO3)2 and SrM(P2O7) (M = Zn, Sr) with Eu2+ and Mn2+. Chem Mater. 2007;19(25):6358.
Kohale RL, Dhoble SJ. Eu2+ luminescence in SrCaP2O7 pyrophosphate phosphor. J Biol Chem Lumin. 2013;28(5):656.
Xu M, Wang L, Jia D, Zhao H. Tuning the color emission of Sr2P2O7:Tb3+, Eu3+ phosphors based on energy transfer. J Am Ceram Soc. 2015;98(5):1536.
Hao Z, Zhang J, Zhang X, Lu S, Luo Y, Ren X, Wang X. Phase dependent photoluminescence and energy transfer in Ca2P2O7: Eu2+, Mn2+ phosphors for white LEDs. J Lumin. 2008;128(5–6):941.
Ye S, Liu ZS, Wang JG, Jing XP. Luminescent properties of Sr2P2O7:Eu, Mn phosphor under near UV excitation. Mater Res Bull. 2008;43(5):1057.
Chen R. Glow curves with general order kinetics. J Electrochem Soc. 1969;116(9):1254.
McKeever SWS. Thermoluminescence of Solids. In: Cahn RW, Davis EA, Ward IM, editors. Cambridge: Cambridge University Press; 1985. 87.
Tiwari B, Rawat NS, Desai DG, Singh SG, Tyagi M, Ratna P, Gadkari SC, Kulkarni MS. Thermoluminescence studies on Cu-doped Li2B4O7 single crystals. J Lumin. 2010;130(11):2076.
Xua M, Wang L, Liu L, Jia D, Sheng R. Influence of Gd3+ doping on the luminescent of Sr2P2O7:Eu3+ orange–red phosphors. J Lumin. 2014;146:475.
Pang R, Li C, Shi L, Su Q. A novel blue-emitting long-lasting proyphosphate phosphor Sr2P2O7:Eu2+, Y3+. J Phys Chem Solids. 2009;70(2):303.
Shannon RD. Crystal physics, diffraction, theoretical and general crystallography. Acta Crystallogr A. 1976;A32(5):751.
Bow JS, Liou SC, Chen SY. Structural characterization of room-temperature synthesized nano-sizedb-tricalcium phosphate. Biomaterials. 2004;25(16):3155.
Ledent MTP. Vibrational spectra and structure of LiB2+PO4 compounds with B = Sr, Ba, Pb. J Solid State Chem. 1978;23(1–2):147.
Khay N, Ennaciri A, Harcharras M. Vibrational spectra of double diphosphates RbLnP2O7 (Ln = Dy, Ho, Y, Er, Tm, Yb). Vib Spectrosc. 2001;27(2):119.
Velchuri R, Vijaya Kumar B, Rama Devi V, Jaya Prakash D, Vithal M. Solid-state syntheses of rare-earth-doped Sr1−x Ln2x/3MgP2O7 (Ln = Gd, Eu, Dy, Sm, Pr, and Nd; x = 0.05) by metathesis reactions and their spectroscopic characterization. Spectrosc Lett. 2011;44(4):258.
McKeever SWS, Moscovitch M, Townsend PD. Thermoluminescent Dosimetry Materials: Properties and Uses. Ashford: Nuclear Technology Publishing; 1995. 63.
Lephoto MA, Ntwaeaborwa OM, Pitalea SS, Swart HC, Botha JR, Mothudi BM. Synthesis and characterization of BaAl2O4:Eu2+ co-doped with different rare earth ions. Phys B Condens Matter. 2012;407(10):1603.
Tanoria OA, Melendreza R, Monteroa MP, Casta-nedab B, Chernova V, Yenc WM, Barboza-Floresa M. Persistent luminescence dosimetric properties of UV-irradiated SrAl2O4:Eu2+, Dy3+ phosphor. J Lumin. 2008;128(1):173.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patel, N.P., Srinivas, M., Modi, D. et al. Optimization of luminescence properties of Tb3+-doped α-Sr2P2O7 phosphor synthesized by combustion method. Rare Met. 37, 587–593 (2018). https://doi.org/10.1007/s12598-015-0688-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-015-0688-x