Abstract
Among the lead-free solder materials, Sn–Ag–Cu alloys have many advantages, such as good wetting property, superior interfacial properties and high creep resistance. In this article, the organization and welding performance of Sn–Ag–Cu material were investigated. The surface morphology of the two alloys was observed by stereoscopic microscope and scanning electron microscope (SEM). Chemical constitution was examined by X-ray energy-dispersive spectroscopy (EDS). The mechanical properties of Sn–Ag–Cu solder were evaluated systematically compared with those of Sn–Cu solder. The results show that Sn–Ag–Cu solder based on different solder pads has different welding properties. The thickness of intermetallic compound (IMC) at the interface increases with aging time. For the gold-plated pads, there are a large number of IMC graphic, and in the welding interface, it can reduce the reliability of electrical connection. The Sn–Ag–Cu solder joints show a superior mechanical property over the traditional Sn–Cu solder. The number of dimples decreases and that of cavities increases for Sn–Cu0.7 alloy and the fracture surfaces of Sn–Ag3.0–Cu0.5 alloy have many small size dimples which are homogeneously distributed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recently, in microelectronic and optoelectronic industry, it has been widely accepted that the traditional tin–lead solders should be substituted by lead-free solders. One of the reasons for this trend is that both the scientific community and the general public have recognized the harmful influence of lead on environment and health, i.e., lead causes severe health damage [1, 2]. Thus, the materials containing lead are under consuming pressure to reduce or eliminate toxic lead in most products. Consequently, these have pushed forward many recent research and development activities on lead-free solders. After the lead-free solder alloy, the reliability of electronic products is always an important issue that people concerned. Sn–Cu-based solder alloy has good weldability, early on as a high temperature solder used in production and in the wave of lead-free electronic assembly into the field, more attention is focused on the Sn–Cu alloy [3–5]. The Pb-free solders are all potential substitutes for conventional Sn–37Pb solder. Among these solders, the most promising ones are undoubtedly Sn–Ag–Cu ternary alloys, which have advantages of good wetting property, superior interfacial properties and high creep resistance [6, 7].
Studies showed that the formation of intermetallic between lead-free solder and pad compound layer is one of the important issues that affect the reliability of solder joints. Moreover, in the course of electronic products, intermetallic compound (IMC) layer will change [8, 9]. The amount and the thickness of the intermetallic are signs of a good weld, but because the IMC of intrinsic brittleness which is too thick will result in reduced mechanical properties of the solder joint. This will have a direct impact on the reliability of the interconnection [10]. So studying the morphology and distribution of IMC solder interface has important significance.
In this paper, the solder is Sn–Ag–Cu alloy, while the pads are Cu and Au/Ni/Cu, respectively. The welding process and the morphology of the intermetallic phases formed at the interfaces of these solder joints were investigated. The mechanical properties of Sn–Ag–Cu solder were evaluated by a systematical comparison to that of Sn–Cu solder.
2 Experimental
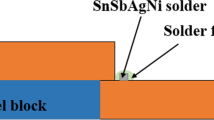
Two solder alloys were selected, Sn–Cu0.7 and Sn–Ag3.0–Cu0.5, while the two pads selected were pure Cu and 0.5Au–1.5Ni–Cu (gold plating of 0.5 μm, nickel plating of 1.5 μm), respectively. After the welding and aging treatment, the samples were prepared for the observation of the cross section of the interface. An etchant consisting of 90 vol% C2H5OH–7 vol% HNO3–3 vol% HCl was used to reveal the cross-sectional microstructures. The tensile test samples with the gauge length of (30.0 ± 0.1) mm, the thickness of (1.00 ± 0.01) mm and the width of (6.00 ± 0.02) mm were cut from the base metal. The tensile tests were carried out at room temperature by AG—I250KN tensile testing machine. The samples for microstructure observation were cut from the gauge length part of the tensile samples deformed. The morphology and composition of the tensile tested samples were examined by scanning electron microscope (SEM, JSM-5800) and energy-dispersive spectroscopy (EDS, Link ISIS 6498). The dislocation configuration of the tensile samples was observed by transmission electron microscope (TEM, JEM-2100F). The mean thickness measurement of the IMCs was taken using SEM and image analysis.
3 Results and discussion
3.1 Microstructure of alloy and interface of solder joint
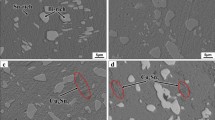
The microstructure of the solder joint was examined by SEM. The features of the microstructure and intermetallics were observed. EDS was used to identify the material elements present in the solder joint. Figure 1 shows the SEM image of Sn–Ag3.0–Cu0.5 solder. It can be seen that there distribute a large number of floc structures. These floc structures are mixtures of Cu6Sn5 and Ag3Sn IMCs. This is because β-Sn initial crystal grains form firstly during solidification, and then the eutectic reaction occurs around them. Figure 2 shows SEM image of Sn–Ag3.0–Cu0.5/Cu joint interface. It is found that at the Sn-containing solder/Cu interface, tin reacts rapidly with Cu to form short rod-like Cu–Sn IMCs. Studies show that it will be formed η-Cu6Sn5 immediately when liquid molten solder contacts with solid phase Cu, and there are holes between Cu6Sn5 phase and Cu substrates.
3.2 Effect of aging time on IMC
The cross-sectional SEM images of Sn–Ag3.0–Cu0.5/Cu joints are shown in Fig. 3. It can be seen that from the side view at the interface, the interfaces of Sn–Ag3.0–Cu0.5/Cu solder joints are mainly composed of rod-like IMC after aging for 300 h. β-Sn phases around them form in the solder matrix. These rod-like IMCs are mixtures of Cu6Sn5 and Cu3Sn IMCs. After aging for longer duration, Cu6Sn5 will grow into solder joints in solid-state aging. During the aging process, the dissolved elements from the metal pads usually have fast diffusion in the molten solder, such as Cu, and can quickly diffuse across the molten solder and potentially influence the interfacial reaction on the other side [11]. With isothermal aging conducted, IMCs begin to grow. The thickness and morphology of this Cu6Sn5 intermetallic layer significantly affect the mechanical performances of the solder. The intermetallic often acts as a crack initiation site, leading to contact failure when IMCs grow to certain number and size.
The Cu pad is made up of a thin layer of Au for oxidation protection on top of a Ni layer. The Ni layer is a diffusion barrier preventing a diffusion couple between the Sn-rich solder and the Cu pad. Figure 4 shows SEM images of joint interface of Sn–Ag3.0–Cu0.5/0.5Au–1.5Ni–Cu after aging treatment at 180 °C for 300 h. The growth of the IMCs in solid-state aging for these solder alloys is a complex progress. Au layer is very thin and will be very easy to completely dissolve the solder when touching the liquid solder. Au in the solder first forms metal compound between AuSn4 (AuSn4 phase is not the most stable structure) [12, 13]. Subsequently, Ni will be diffused to solder to form Ni–Sn IMC. As the reaction proceeds, Cu layer will join the reaction after Ni layer is depleted [14, 15]. The gold layer diffuses readily into the solder, IMCs form between Sn–Ag–Cu solder, and Ni–Au surface could be ternary (Cu,Ni)6Sn5 and/or complex quad (Cu,Ni,Au)6Sn5 IMCs [16]. During thermal aging, the amount of free Cu atoms that can diffuse to the solder increases and those Cu atoms are taken to form IMCs. After aging for longer duration, IMCs transform into lump type in solid-state aging, and the number of IMCs increases significantly. The average thickness of IMC layer is plotted as a function of aging time as shown in Fig. 5. It can be seen that with aging time increasing from 0 to 300 h, the thickness of IMC layers increases and has dependence on time, implying that the growth of IMC layers is controlled by diffusion mechanism. IMC formation between solder and Ni–Au surface finishes on copper pad and its subsequent growth can have a serious consequence on the reliability performance of solder joint, even causing the fault between solder and pad, which seriously reduces the mechanical and electrical properties and reliability of solder joints.
3.3 Mechanical properties and fracture analysis
The mechanical properties of Sn–Ag3.0–Cu0.5 and Sn–Cu0.7 alloy were measured by the tensile tests. The tensile strength of Sn–Ag3.0–Cu0.5 and Sn–Cu0.7 alloys can reach about 19.3 and 14.7 MPa, respectively. Figure 6 shows SEM image of Sn–Ag3.0–Cu0.5 alloy ruptured. It can be seen that the microstructures change under stress, and the microcracks appear in the fracture area. SEM secondary electron images of fracture surface of Sn–Cu0.7 and Sn–Ag3.0–Cu0.5 alloys are presented in Fig. 7. Fracture surfaces of Sn–Ag3.0–Cu0.5 alloys have many small size dimples which are homogeneously distributed, as shown in Fig. 7a. The necking at fracture surface occurs and the reduction in area reaches 32 %. Figure 7b shows that the number of dimples decreases and that of cavities increases for Sn–Cu0.7 alloy. The bearing area at place where necking occurs decreases quickly, resulting in fracture finally. Study shows that Sn–Ag–Cu with a small amount of Ag adding in Sn–Cu alloy is ternary alloy, which can improve the mechanical properties of the solder. When Ag content is less than 3.2 wt%, Ag3Sn is not easy to form, Sn–Ag eutectic composition is Sn–3.5Ag, and the eutectic alloy is formed by β-Sn phase and fine Ag3Sn [17, 18].
Figure 8 shows TEM images of Sn–Cu0.7 and Sn–Ag3.0–Cu0.5 alloys after test. It can be seen from Fig. 8a that the dislocations occur at the grain boundary. When the stress concentration reaches a certain level, movement dislocations cut into the grains and result in the decreases in mechanical properties. At the same time, as seen in Fig. 8b, a large number of IMC particles are observed in the grains. EDS results (not shown here) show that these particles are Ag3Sn phases. The dislocation moves in the grains and the motion of dislocations are prevented by the Ag3Sn particles. The formation of fine and continuous IMC phase provides an ideal structure for resistance because the mobile dislocations will be hindered from passing these IMC phase.
4 Conclusion
Sn–Ag–Cu solder has different welding properties on different pads. The thickness of IMC at the interface increases with aging time because the chemical force causes the growth of IMC. After aging, the number of IMC compounds increases significantly. For the gold-plated pads, there are a large number of IMC graphic, and in the welding interface, it can reduce the reliability of electrical connection. The number of dimples decreases and that of cavities increases for Sn–Cu0.7 alloy and the fracture surfaces of Sn–Ag3.0–Cu0.5 alloy have many small size and homogeneously distributed dimples. The necking at fracture surface occurs and the reduction in area reaches 32 %. The results show that Ag can improve the mechanical properties of the solder.
References
Gourlay CM, Read J, Nogita K. The maximum fluidity length of solidifying Sn–Cu–Ag–Ni solder alloys. J Electron Mater. 2012;37(1):51.
Ventura T, Nogita K. The influence of 0–0.1 wt% Ni on the microstructure and fluidity length of Sn–0.7Cu–xNi. J Electron Mater. 2008;37(1):32.
Pang HL, Xiong BS. Low cycle fatigue study of lead free 99.3Sn–0.7Cu solder alloy. Int J Fatigue. 2004;26(5):865.
Zribi A, Clark A, Zavalij L. The growth of intermetallic compounds at Sn–Ag–Cu solder/Cu and Sn–Ag–Cu solder/Ni interfaces and the associated evolution of the solder microstructure. J Electron Mater. 2001;30(9):1157.
Xu BS, Zang LK, Yuan ZF, Wu Y, Zhou Z. Dissolutive wetting process and interfacial characteristic of molten Sn–17Bi–0.5Cu alloy on copper substrate. Rare Met. 2013;32(6):537.
Amalu EH, Lau WK, Ekere NN. A study of SnAgCu solder paste transfer efficiency and effects of optimal reflow profile on solder deposits. Microelectron Eng. 2011;88(7):1610.
Naoyuki H, Tokuteru U, Yorinobu T. Effects of Zn addition and aging treatment on tensile properties of Sn–Ag–Cu alloys. J Alloy Compd. 2012;527(25):226.
Nogita K, Read J, Nishimura T. Microstructure control in Sn–0.7 mass% Cu alloys. Mater Trans. 2005;46(11):2419.
Fakpan K, Otsuka Y, Mutoh Y. Creep-fatigue crack growth behavior of Pb-contained and Pb-free solders at room and elevated temperatures. Procedia Eng. 2011;10(5):1238.
Jiang L, Chawla N. Mechanical properties of Cu6Sn5 intermetallic by micropillar compression testing. Scripta Mater. 2010;63(4):480.
Jeong WY, Won CM, Seung JB. Interfacial reaction of ENIG/Sn–Ag–Cu/ENIG sandwich solder joint during isothermal aging. Microelectron Eng. 2006;83(6):2329.
Ochoa F, Deng X, Chawla N. Effects of cooling rate on creep behavior of a Sn–3.5 Ag alloy. J Electron Mater. 2004;33(12):1592.
Wang JX, Xue SB, Fang DS, Zhang ZS. Effect of diode-laser parameters on shear strength of micro-joints soldered with Sn–Ag–Cu lead-free solder on Au/Ni/Cu pad. Trans Nonferrous Metals Soc China. 2006;16(6):1374.
Nianduan L, Donghua Y, Liang L. Interfacial reaction between Sn–Ag–Cu solder and Co–P films with various microstructures. Acta Mater. 2013;61(2):4581.
Kim KS, Huh SH, Suganuma K. Effects of intermetallic compounds on properties of Sn–Ag–Cu lead-free soldered joints. J Alloy Compd. 2009;352(2):226.
Shiau LC, Ho CE, Kao CR. Reactions between Sn–Ag–Cu lead-free solders and the Au/Ni surface finish in advanced electronic packages. Solder Surf Mount Technol. 2002;14(3):25.
Nishikawa H, Piao JY, Takemoto T. Interfacial reaction between Sn–0.7Cu solder and Cu substrate. J Electron Mater. 2006;35(5):1127.
Ma D, Wang WD, Lahiri SK. Scallop formation and dissolution of Cu–Sn intermetallic compound during solder reflow. J Appl Phys. 2012;91(5):3312.
Acknowledgments
This study was financially supported by the Chinese Universities Scientific Fund (No. 2013RC0402), the Science & Technology Planning Project of Jinan City, China (No. 201401056), the Natural Science Foundation of Shanxi Province, China (No. 2013011023-5), and the Taiyuan University of Science & Technology Doctoral Fund (No. 20122029).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kong, YG., Kong, ZG. & Shi, FM. Microstructure and mechanical property of Sn–Ag–Cu solder material. Rare Met. 36, 193–197 (2017). https://doi.org/10.1007/s12598-015-0579-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-015-0579-1