Abstract
Nb3Al nanoparticles were directly synthesized by metallothermic reduction process in the molten salts with mNbCl5–nAlCl3 powders as raw materials and sodium as reducing reagent. The effects of different feeding material orders, soaking time, Nb content in raw materials, and 3NbCl5–AlCl3 content in molten salts on the obtained Nb3Al powder were discussed. The as-prepared samples were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), and transmission electron microscopy (TEM). It is found that the only phase Nb3Al nanoparticles are obtained by controlling the variation of the feeding material orders, soaking time, and Nb content in raw materials. And the morphologies of as-prepared nanoparticles change owning to different 3NbCl5–AlCl3 contents in molten salts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nb3Al intermetallics received considerable attention as ultrahigh-temperature structural materials and superconducting materials, due to its high melting points, relatively low density, high strength, excellent oxidation and creep resistance, and excellent superconductivity [1, 2]. In particular, Nb3Al is recognized as excellent source for future superconducting multifilamentary conductors at magnetic fields of much higher than 25 T, under extreme mechanical and irradiation conditions, because of low upper critical field (H C2) values of 21 T at 4.2 K, low critical temperatures (T c), and high critical current density (J c) in low fields of <14 T [3, 4]. Therefore, Nb3Al intermetallics are possibly applied for turbine blades in aircraft engines and stationary gas turbines, perhaps for nuclear magnetic resonance and nuclear magnetic imaging magnets [5–9]. However, it has the lack of deformability and brittle, which leads to fracture and low ductility at room temperature and makes it machine difficultly [10]. In the previous studies, the powder-metallurgical method is considered as an effective way to solve this problem [11], so the preparation of Nb3Al powders is very important.
Recently, in our previous work, a new direct synthesized process for Nb3Al intermetallic nanoparticles was developed. In this process, a pure Nb3Al nanoparticle was directly synthesized from the mixture niobium oxide and aluminum oxide or niobium chloride and aluminum chloride raw material powders by chemical reaction process in the molten salts [12–16]. In this work, the purpose is to control the purity and morphology of the directly synthesized Nb3Al nanoparticles by changing experimental conditions, such as feeding material orders, soaking time, Nb content in raw materials, and 3NbCl5–AlCl3 content in molten salts. And the metallothermic reaction was described as follows:
2 Experimental
CaCl2, NaCl, LiCl, KCl, NbCl5, and AlCl3 were all analytical reagents as solvent and raw materials, respectively. The pure metal sodium as reductant was used. In the LiCl–KCl–NaCl–CaCl2 and LiCl–KCl–NaCl phase diagrams [17], the low eutectic point of these molten salt systems such as the LiCl–KCl–NaCl (54:36:10, mol%, abbreviated as LKN) and LiCl–KCl–NaCl–CaCl2 (53:24:5:18, mol%, abbreviated as LKNC) was chosen.
Because the vapor pressure of NbCl5 and AlCl3 is very large at high temperature, NbCl5 and AlCl3 powder must have pre-treatment in molten salts. In this process, the mixed solvent of reagent in proportion was heated up slowly to 200 °C and dehydrated in vacuum for 6 h in a resistance furnace. After the reagent mixture was melted at 450 °C for 4 h under an argon (Ar) atmosphere, the LKN or LKNC molten salt was cooled to room temperature and milled to powders in the glove box under an argon atmosphere. Secondly, 3NbCl5–AlCl3 mixture powders mixed with blank molten salts were placed inside a sealed quartz reactor in the glove box, after the reactor was quick placed into a 450 °C resistance furnace for 1 h, it was quick took out at room temperature, and the LKN–NbCl5–AlCl3 (96:3:1, mol%, abbreviated as LKNBA) or LKNC–NbCl5–AlCl3 (96:3:1, mol%, abbreviated as LKNCBA) precursor particles were prepared. Also, the small particles were cut from pure metal sodium ingot in the glove box for reduction. Finally, the LKN or LKNC blank molten salts were put into a corundum crucible which was placed inside a resistance furnace under an argon atmosphere and melted at 450 °C for 1 h. And then, the pre-treated precursor particles and the sodium particles (20 % excess) were fed into salt-bath for chemical reaction. After reaction, the products and salts were cooled, washed, and dried in vacuum; the as-prepared products were obtained referred to our previous work [12–14].
The composition and microstructure of as-prepared products were determined by X-ray diffractometer (XRD, MAC Science Co. Ltd., M21X) with Cu Kα radiation, field emission scanning electron microscopy (FESEM, Z1ISS, ULTRA55, Germany) with an energy dispersive spectrometer (EDS, XMAX50, England), and transmission electron microscopy (TEM, JEM-2010, JEOL Co., Japan), respectively.
3 Results and discussion
3.1 Controlling homogeneous reaction system
3.1.1 Feeding material order effects
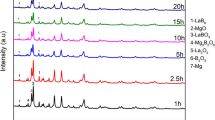
The XRD patterns of the as-prepared powders by chemical reduction of 3NbCl5–1AlCl3 with 20 % excessive sodium at 450 °C for 4 h in LKN molten salts are shown in Fig. 1. For Na → precursor → Na order, Nb particles with a large amount of NbAl3 and Nb2Al are obtained, as shown in Fig. 1a. For precursor → Na → precursor order, Nb3Al powders are obtained, as shown in Fig. 1b. In addition, the morphology and EDS analysis of the obtained Nb3Al products are shown in Fig. 2. The particle size distribution of Nb3Al nanoparticles is 30–150 nm as shown in Fig. 2a. The majority elements of product nanoparticles are niobium and aluminum with a trace amount of oxygen by EDS element analysis, as shown in Fig. 2b. Nb/Al/O content in as-prepared Nb3Al nanoparticles is evaluated to be 77.16 mol%/20.91 mol%/1.93 mol%. The molar ratio of Nb/Al in the products is consistent with data in Nb–Al phase diagram [18].
According to the experimental results in LKNBA reaction systems, the feeding precursor particle and sodium particle (20 % excess) orders are important to prepare pure Nb3Al nanoparticles via chemical reduction process from NbCl5 and AlCl3 powders by sodium in molten salts.
3.1.2 Soaking time effects
Figure 3 shows XRD patterns of as-prepared powders by reducing 3NbCl5–AlCl3 with sodium for different soaking time at 450 °C in LKNC molten salts. After soaking for 0.5 h, Nb particles without Nb3Al are obtained, as shown in Fig. 3a. After 1.0 h, the as-prepared Nb nanoparticles with a large amount of NbAl3 and Nb2Al are obtained as shown in Fig. 3b. With the increase in soaking time from 1.0 to 3.0 h, Nb, NbAl3, and Nb2Al disappear and Nb3Al nanoparticles without any impurity are obtained, as shown in Fig. 3c, d. In addition, FESEM and TEM images, EDS spectrum, and diffraction pattern of as-prepared products are shown in Fig. 4 (referred to Ref. [16]). The FESEM and TEM images of as-prepared powders are shown in Fig. 4a, c, respectively. Figure 4a, c shows that the particle sizes of pure Nb3Al nanoparticles are about 50–200 and 90–180 nm, respectively. And the content of Nb/Al/O element in as-prepared particles is 91.58 wt%/6.43 wt%/1.99 wt%, as shown in Fig. 4b. Moreover, as presented in Fig. 4d, TEM image of Nb3Al nanoparticles illustrates that the as-prepared particles are polycrystalline powders. Therefore, the optimum soaking time on LKNCBA reaction system plays an important role in the formation of pure Nb3Al powders in molten salts.
The above results demonstrate that only Nb3Al nanoparticles without any impurity are obtained when LKNBA and LKNCBA molten salts form a homogeneous reaction system, which may be ascribed to the fact that all Nb and Al ions diffuse into reaction molten salt system and form a liquid phase. Thus, the optimum feeding raw material orders and soaking time play important roles in the formation of pure Nb3Al particles in molten salts.
3.2 Controlling Nb content of products
Figure 5 shows Nb–Al phase diagram [18], and only Nb3Al phase exists in Nb content of 77.5 mol%–82.0 mol% area. Therefore, controlling Nb content in raw materials is important to synthesize and obtain pure Nb3Al nanoparticles. Figure 6 shows XRD patterns of as-prepared powders with Nb content in raw materials changing from 72 mol% to 85 mol% by stoichiometric ratio at 450 °C in LKNCBA homogeneous reaction system. In the case of 85 mol% Nb, the Nb3Al powders with a small amount of Nb phase were synthesized, as shown in Fig. 6a. With Nb content decreasing, the content of Nb phase decreases and Nb2Al phase appears, as shown in Fig. 6. Pure Nb3Al nanoparticles without any impurity are obtained, as shown in Fig. 6b, c. As-prepared Nb3Al nanoparticles with a small amount of Nb2Al are obtained, as shown in Fig. 6d. In addition, the formation of Nb3Al nanoparticles in the reduction process is related to Nb content in raw materials (78 mol%–82 mol%). Therefore, it is necessary to conduct at the optimum amount of Nb ion in raw materials in the homogeneous reaction system.
3.3 Controlling morphologic of products
FESEM images of as-prepared Nb3Al powders by sodiothermic reduction with different 3NbCl5–AlCl3 contents and 20 % excessive sodium at 450 °C in LKNC molten salts are shown in Fig. 7. For 3NbCl5–AlCl3 content of 15 wt%, as-prepared globular-like Nb3Al nanoparticles are obtained, and the particle size distribution of pure Nb3Al nanoparticles is 450–1200 nm as shown in Fig. 7a. With the content of 3NbCl5–AlCl3 decreasing from 15 wt% to 2 wt%, the particle size of Nb3Al nanoparticles sharply decreases and the morphology of as-prepared Nb3Al nanoparticles changes from globular-like to fine filamentary, as shown in Fig. 7b–d. The fine filamentary Nb3Al nanoparticles of as-prepared Nb3Al powders, as shown in Fig. 7d, have diameters of 100–200 nm and length of 500–1600 nm. In addition, the EDS analysis of the obtained fine filamentary Nb3Al nanoparticles is shown in Fig. 8. The content of Nb/Al/O element in as-prepared particles is 92.21 wt%/6.44 wt%/1.35 wt%, and the weight ratio of Nb/Al in the products is consistent with the data in Nb–Al phase diagram [18]. The above results demonstrate that various morphologies of as-prepared Nb3Al powders without any impurity are obtained when the mixture raw material powder content in LKNCBA reaction system changes. Thus, the optimum content of raw materials in the LKNCBA reaction system plays an important role in the formation of Nb3Al powders with different morphologies in molten salts.
4 Conclusion
The pure Nb3Al nanoparticles could be successfully produced via chemical reaction in molten salts. It is found that controlling homogeneous reaction system and Nb content in raw materials is a very important operation for the formation of pure Nb3Al product, that is, if the LKNBA and LKNCBA molten salts are not homogeneous reaction system, the pure Nb3Al nanoparticles cannot be prepared. The morphology of as-prepared powders would change when the raw mixture material content in the homogeneous reaction system is different. Based on the experimental results, it is observed that via the precursor → Na → precursor order and soaking time of >3 h by chemical reaction in the molten salts with 78 mol%–82 mol% Nb in raw materials, single pure Nb3Al nanoparticles can be prepared well.
References
Hanada S. Niobium aluminides. Curr Opin Solid State Mater Sci. 1997;2(3):279.
Peng LM. Synthesis and mechanical properties of niobium aluminide-based composites. Mater Sci Eng A. 2008;480(15):232.
Takeuchi T, Kuroda T, Itoh K, Kosuge M, Iijima Y, Kiyoshi T, Matsumoto F, Inoue K. Development of Nb tube processed Nb3Al multifilamentary superconductor. J Fusion Energy. 1992;11(01):7.
Inoue K, Iijima Y, Takeuchi T, Kiyoshi T, Fukuda K, Nakagawa K, Iwaki G, Moriai H. New Nb3Al multifilamentary conductor and its application to high field superconducting magnet. Phys B. 1998;246–247(29):364.
Gauthier V, Josse C, Bernard F, Gaffet E, Larpin JP. Synthesis of niobium aluminides using mechanically activated self-propagating high temperature synthesis and mechanically activated annealing process. Mater Sci Eng A. 1999;265(15):117.
Mitsui H, Habazaki H, Asami K, Hashimoto K, Mrowec S. High temperature corrosion behavior of sputter-deposited Al–Nb alloys. Trans Mater Res Jpn A. 1994;309(14):243.
Lee KM, Lee JS, Lee DJ, Kim SS, Ahn IS, Ahn IS, Park MW. Effect of thermal treatment on the atomic ordering of mechanically alloyed Al3Nb. J Alloys Compd. 2000;313(1–2):214.
Mostaan H, Karimzadeh F, Abbasi MH. Investigation of in situ synthesis of NbAl3/Al2O3 nanocomposite by mechanical alloying and its formation mechanism. J Alloys Compd. 2010;503(02):294.
Inoue K, Kikuchi A, Yoshida Y, Yoshida Y. A new practical superconductor: rapidly heated and quenched Nb3Ga wire. Phys C. 2003;384(03):267.
Togano K, Kumakura H, Yoshida Y, Tachikawa K. Fabrication of superconducting composite tapes by a newly developed liquid quenching technique. J IEEE Trans Magn. 1989;21(01):463.
Akihama R, Murphy RJ, Foner S. Fabrication of multifilamentary Nb–Al by a powder metallurgy process. J IEEE Trans Magn. 1981;17(02):274.
Wang N, Du C, Jiao SQ, Huang K, Zhu HM. Direct synthesis of niobium aluminides powders by sodiothermic reduction in molten salts. In: TMS Annual Meeting 2011, EPD Congress 2011. San Diego; 2011. 629.
Wang N, Huang K, Jiao SQ, Zhu HM. Synthesis of NbAl3 powder by sodiothermic reduction in molten salt. J Rare Earths. 2011;29:48.
Wang N, Du C, Huo JG, Zhang Y, Huang K, Jiao SQ, Zhu HM. Direct synthesis of Nb–Al intermetallic nanoparticles by sodiothermic homogeneous reduction in molten salts. Intermetallics. 2013;43:45.
Du C, Wang N, Hou JG, Jiao SQ, Zhu HM. Facile synthesis of Nb–Al alloy powders via sodiothermic reduction in molten salts. J Alloys Compd. 2013;555:405.
Du C, Wang N, Zhang Y, Jiao SQ, Zhu HM. Production of NbAl3 powders through sodium reduction of oxides in molten salts. In: TMS Annual Meeting 2012, 3rd International Symposium on High-Temperature Metallurgical Processing. Orlando; 2012. 251.
Bale CW, Chartrand P, Decterov SA, Eriksson G, Hack K, Mahfound RB, Melancon J, Pelton AD, Petersen S. FactSage thermochemical software and databases-recent developments. Calphad. 2002;26(2):189.
Bale CW, Bélisle E, Chartrand P, Eriksson G, Hack K, Mahfound RB, Pelton AD, Petersen S. FactSage thermochemical software and databases-recent developments. Calphad. 2008;32(01):1.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos. 50934001, 21071014, 51102015, and 51401004), the Fundamental Research Funds for the Central Universities (Nos. FRF-AS-11-002A, FRF-TP-12-023A, and FRF-MP-09-006B), the National High Technology Research and Development Program of China (No. 2012AA062302), the Program of the Co-Construction with Beijing Municipal Commission of Education of China (Nos. 00012047 and 00012085), and the Program for New Century Excellent Talents in University (No. NCET-11-0577).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, N., Huang, CY., Zhang, Y. et al. Preparation of Nb3Al powder by chemical reaction in molten salts. Rare Met. 41, 1671–1676 (2022). https://doi.org/10.1007/s12598-015-0523-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-015-0523-4