Abstract
Terbium lithium zinc borosilicate glass systems incorporating copper ions were prepared using a melt quenching technique. The structural and physical properties of the produced glass samples were investigated using X-ray diffraction (XRD), infrared (FTIR), and optical absorption (UV/Vis/NIR) spectroscopy. The non crystalline behavior of the glasses was confirmed by XRD analysis. FTIR clear that changing the ratio of terbium and copper ions did not affect the locations of the borate and silicate groups, but it affected the intensity of absorption and amount of BO4, SiO4 and BO3 groups. The UV–visible–NIR spectra of the glasses showed a strong absorption of Tb3+ ions in the NIR region (2190, 1942, 1867, and 1775 nm for samples free of copper). The highest band gap value was obtained for the sample free of copper glass, which had the lowest amount of non-bridging oxygen. The estimated CIE coordinates for the glasses show their locations in the blue–green region for samples free from copper when excited at 250 nm and green when the sample was excited at 378 nm. And move to green region with the copper content increased. The CIE coordinates deviated towards the yellow region when excited at 378 nm and became green yellowish with increasing Tb concentration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glass prepared using a simple quenching method is easier to prepare than the crystal material, which saves time. Additionally, the ability of soluble rare earth (RE) and transition metal (TM) can be used in transparent media. Glass can drop different amounts of RE and different types that give the chance of high intensity emission in different colors. From literature studied a transparent glass doped with different RE as in Er2O3 gives green emission as studied by Prabhu [1], Kaurvv [2], Deopa [3]. Glass doped with Sm2O3 has a different range of red color as Swapna [4], Marzouk [5] and Eu2O3 in red emission as Hegde [6], Hegde [7], and Tm2O3 in blue emission El-Maaref [8] and Vijaya Babu, Cole [9] and Tb4O7 in green emission by Kaur [10] and Pr6O11 Mahamuda [11], Hegde [12] and studied effect of different RE on mixed together Zhao [13], Sasikala [14], Mungra [15], Zhu [16], He [17], Hegde [18].
The preparation glass using a former has the advantage of low melting point, good solubility of RE and TM, outstanding thermal and chemical stabilities, and broad emission, making it most suitable for high-gain laser applications [19], and is inexpensive and relatively stable [20] like borate. In contrast, borate has a high phonon energy, which provides a good opportunity to overcome this problem through the addition of ZnO. The ability of ZnO as a heavy metal to reduce phonon energy has been reported [21] and decreases the hygroscopic properties with improvement in electrical, mechanical, thermal, and optical properties [10]. In addition, ZnO exhibits a high thermal resistance against crystallization [22]. The addition of silicate as a former in the formation of borosilicate glass offers various advantages, such as preventing crystallization and increasing the emission intensity [23]. Borosilicate glass was chosen because it combines the high stability of silicate with ability to lower the melting temperature of borate [24]. Additionally, the use of borosilicate glass prevents heavy concentration quenching, which improves the efficiency of luminescence materials [25].
Choosing Rare earths such as Tb4O7 and transition metals such as CuO have, the ability to emit green light [26] in addition to their ability to be used in optoelectronic devices [27] and used in green lasers. Tb3+ doped glasses have been used in the development of electromagnetic transmission window of sea water, efficient green emitting phosphors, solid state lasers, white LED's, neutron detection, and medical devices [28] and a little in blue emission that give the chance to characterize the type of material like clustering of normal from the ratio between green intensity to blue intensity. Adding CuO can be used as two ways as the filter-pass or photoluminescence emission depend on the type of the host [29] that copper oxidation formed in prepared glass. This advantage give the chance of glass used as the glass filter when Cu2+ formed inside the network [30] or used in the emission when the Cu+ formed and wide luminescence in the visible region with a d9s → d10 transition in solids[19, 30,31,32].
Incorporating SiO2 into the B2O3 glass matrix improves the heat resistance, chemical durability, and electrical resistivity of borosilicate glasses [33,34,35,36]. These glasses exhibit similar optical properties to fluoride glasses but offer better chemical resistance and superior mechanical properties. The narrow-band emission of Terbium (3+): Tb3+ arises from the transitions between the 4f levels. Trivalent terbium ions, Tb3+, which results in sharp luminescence from 4f to 4f transitions. This sharpness is attributed to the shielding of the 4f electrons by the outer 5s2 and 5p6 orbitals. The green light emission in terbium ions [37,38,39] is attributed to four distinct peaks arising from the 5D4 to 7FJ transitions, where J is 3, 4, or 6. The most prominent green emission peak occurs at 544–550 nm within the visible light spectrum, suggesting its potential for use in electronic devices. Optical rays that emit light and lasers that emit green light [40, 41].
Our interest was focused on determining the effect of the substitution of Tb3+ ions and CuO emitted in green and some blue emission on the photoluminescence properties.
Experimental work
Glass samples with a composition of 35 SiO2 + 35 B2O3 + 10 ZnO + 20 Li2O + (1 − X) Tb4O7 + X CuO (where X= 0, 0.05, 0.1, 0.3, 0.5, and 1 mol% coded as Tb–Cu1, Tb–Cu2, Tb–Cu3, Tb–Cu4, Tb–Cu5, and Tb–Cu6, respectively. The glass samples under investigation were mounted with reagent grade SiO2 (Sigma-Aldrich, 99.9%), H3BO3 (Win LAB U.K., 99%), ZnO (Sigma-Aldrich, 99.9%), CuO (Sigma-Aldrich, 99.9%), and Tb4O7 (Strem chemicals USA, 99.99).
The chemical purity grades were mixed and annealed for 30 min at 450 °C in a porcelain crucible and then melted at 1130 °C. The molten material was cooled and poured between two copper plates in air and annealed at 350 °C for 3 h. All the samples were examined using a Philips Analytical X-ray analysis system (PW3710) with Cu Kα = 1.54°A.
JASCO, V-570 spectrophotometer (with the precision of ± 1 nm), and JASCO FP-8300 spectrofluorometer (comprised of a 150 W Xenon arc lamp) measured the optical properties of glass samples (transmission, absorption, excitation, and emission).
Differential thermal analysis (DTA) of the prepared samples was performed in the temperature range of room temperature to 900 °C at a heating rate of 25 °C/min using Al2O3 powder as a reference material (Shimadzu DTA-50 analyzer).
Attenuated total reflection (ATR) FTIR spectra were obtained using ALPHA II FTIR spectrometer from Bruker Optik GmbH, Germany, equipped with diamond ATR crystal system in the spectral range of 4000–400 cm−1 with the resolution of 4 cm−1.
Results and discussion
When examining the impact of one substance on another's properties, the following methods can be employed: substitution or addition. For oxide glass, a certain percentage of the oxide under study replaces an equivalent percentage of the original elements (substitution). In addition, a percentage of the oxide is added to the original glass composition without altering it, resulting in a total that exceeds 100% (addition). This methodology is documented in numerous scholarly papers [42,43,44].
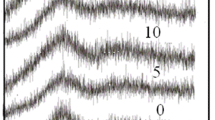
Figure 1 illustrates the amorphous structure of the prepared glass, as determined by XRD measurements. The graph also reveals an increase in the peak amplitude at 25 °C, which is attributed to the increase in the copper concentration. This increase in copper concentration enable the glass to incorporate nanoparticles within its structure.
In our previous studies, we prepared samples of borosilicate glass in porcelain crucibles, at temperatures within the same limits. These samples were analyzed using an EDX device, which verifies the integrity of the samples. Porcelain crucibles react with lead oxide and some heavy elements, and it is recommended to avoid using them [45,46,47].
The infrared spectra of the glass samples are depicted in Fig. 2. The FTIR spectra disclose the structural configuration of the units within the glass samples. Additionally, the FTIR offers data on the stretching and bending vibrations for the silicon and boron groups.
All spectra demonstrate the presence of borate groups. The B–O stretching vibrations of trigonal (BO3)3− units (in meta-, pyro-, and ortho-borate) cause the broad band from 1200 to 1700 cm−1, centered at 1376 cm−1. The anti-symmetrical stretching vibrations with three non-bridging oxygens (NBOs) of B–O–B linkages correspond to the band at 1420–1540 cm−1 [48]. The stretching vibrations of the non-bridging oxygen atoms (O–B–O) in the [BO2O]− units, which are connected with [BO4] or [BO3] units, are found at 1360 cm−1 and 1455 cm−1, respectively [49].
The spectral band between 800 and 1200 cm−1 is attributed to the stretching vibrations of B–O bonds in tetrahedral BO4 units. The band around 1005 cm−1 is due to the non-bridging oxygens (NBOs) within the BO4 group. The silicate and borate groups —comprising BO3 and BO4 units—overlap their spectral contributions and may combine within the 1000–1120 cm−1 range.
The stretching vibration of Si–O–Si bonds in the NBO of SiO4 tetrahedral units causes two bands at 1040 and 1100 cm−1. That is, as the NBO bonds (Si–O–Si links in Q1 units) rose, so did the bridge oxygen bond (Si–O–Si bonds) [48]. The stretching vibrations of Si–O–Si bonds in silicates result in various configurations in the glassy network. These configurations include Q3 (1075 cm−1) with 3 bridging oxygen and one non-bridging, Q2 (1000 cm−1) with 2 bridging oxygen and 2 non-bridging, and Q1 (900 cm−1) with 1 bridging oxygen and 3 non-bridging. The band indicates the presence of B-O-Si bonds at 1020 cm−1. The stretching vibration of Si–O–Si bonds in the non-bridging oxygen of SiO4 tetrahedral units produces two bands at 1040 and 1100 cm−1. Fig. 3 shows the effect of Tb4O7 addition on the SiO4, BO4 and BO3 vibration groups. It is clear the structure variation between samples.
Figure 4a shows the effect of light fall on the sample in the UV, Vis, and near IR ranges. High-intensity peaks were observed in the NIR range for the sample doped with 1 mol% Tb4O7, followed by a decrease in the NIR range with the appearance of a peak at vis, which increased with increasing Cu concentration. Using the deconvolution method shown in Fig. 4b, the positions of the peaks were determined. The appearance of peaks in the NIR range at 2190, 1942, 1867and 1775 nm are represented as 7F6 → 7F3, 7F2, 7F1, and 7F0 respectively [50,51,52]. The UV spectra contribute to the appearance of two low-intensity peaks at 486 and 379 nm, which represent transitions from 7F6 to 5D4 and 5D3, respectively [51]. Generally, copper (Cu) exists in two valence states, Cu+ and Cu2+. The presence of Cu+ is a result of the 3d10 → 3d9 4S1 transition, which appears in the blue UV absorption spectrum and is observed in glass containing 0.5 and 1 mol % CuO.
During the melting process, the presence of Cu2+ results in octahedral coordination. This leads to splitting of the d orbitals into two degenerate T2g levels at a lower energy and a higher energy level of 2Eg. Additionally, tetragonal distortion causes the Jahn–Teller effect, resulting in the splitting of T2g ions into 2B2g (dxy) and 2Eg (dxz, dyz), whereas 2Eg splits into 2B2g (dx2–y2) and 2A2g (dz2).
According to, [53] the appearance of a broad band is due to 2Eg → 2B1g,
2B2g → 2B1g, and 2A1g → 2B1g, which correspond to 709, 864, and 1039 nm, respectively [54]. The intensity and broadness of the peak increased with increasing Cu concentration, and Cu+ appears as previously observed, causing a decrease in the Tb peak intensity [32, 55].
To determine the physical transitions of light between the valence and conduction bands as Eg, we used optical absorption values. The value found by Mott and Davis is related to various parameters, including the absorption coefficient, photon energy, and optical band gap. The optical band gap is determined by the following relation [56].
From the Tauc plot as shown in Fig. 5a and 5b, the direct and indirect Eg values were evaluated, where α represents the absorption coefficient, Eg is the optical band gap, and n is the optical transition of the electron. Generally, the addition of a modifier causes changes in the Eg values. This is because the modifier alters the NBOs present in the valence band, thereby forming extrinsic bands between them on the lattice and changing the distance between the valence and conduction bands.
The results obtained drawn in Fig. 6 indicate that glass containing rare earth (RE) has a high value of Eg, which decreases with an increase in transition metal content, consistent with literature sources [10, 55, 57, 58]. Furthermore, as observed in studies [31, 59], adding Cu leads to a decrease in Eg.
This behavior can be attributed to structural changes. A decrease in BO4 units leads to an increase in NBO bonds, which in turn raises the electronic polarizability across the B–O bonds. Notably, an increase in NBOs narrows the band gap between the valence and conduction bands, resulting in a glass with low energy gap and high refractive index. This can also be linked to changes in bonding strength, which are influenced by ionic radius and electronegativity (Tb3+ = 0.106–0.118 and Cu3+–Cu1+ = 0.068–0.091 nm). Furthermore, an increase in CuO relative to Tb4O7 leads to a reduction in BO4 formation and consequently, a decrease in Eg. The optical band gap behavior is generally nonlinear, a trend that can be attributed to the influence of mixed rare-earth and transition elements on the glass network. Additionally, reducing Tb and adding Cu can increase the donor centers, thereby lowering the Eg of the glasses. The smooth absorption edges in the absorption spectra indicate the amorphous nature of the current glass samples, a finding supported by density results [60]. Optical band gap values similar to those for borate glass systems containing dysprosium ions or transition metals [61, 62].
The DTA curve in Fig. 7 illustrates the thermal behavior of the prepared material with increasing temperature. The temperature increase exhibited an endothermic peak, indicating a glass transition temperature (Tg). As the temperature continued to increase, an exothermic peak appeared at the beginning of the crystallization nucleation peak (Tc) at the onset of the crystallization temperature Tx.
The temperature then increased continuously and another endothermic peak appeared, which corresponded to the melting glass temperature (Tm).
Table 1 lists the values of Tg, Tc, Tm, the Kauzmann relation Trg and the glass ability ΔT.
From the Tg values, a slight difference in value appears between the samples, and at the same time, a change in values is expected from the structural change in BOs and NBOs [53]. Glasses with higher BO3 content have a lower Tg [52]. To compare the samples prepared previously with different formers and increasing Cu concentrations [29, 63, 64]. These values agree with the results of the samples under study.
The glass transition temperature (Tg) decreases with the addition of copper, which modifies the glass structure owing to the increase in non-bridging oxygen sites (NBOS). The proximity of the crystallization temperature (Tc) to Tg or the melting temperature (Tm) explains the thermal stability and difficulty of crystallization. The ability of glass to delay crystallization and its good glass-forming ability are due to its stability in performance, with Tc closer to Tm than Tg. The difference between Tc or Tx and Tg, was calculated to determine ΔT [64]. Another important factor is the estimation of the glass-forming ability using the Kauzmann relation Trg = Tg/Tm, which was calculated using the DTA parameter.
By obtaining a value of Trg within the range of 0.5 to 0.66, the system identifies the glass with the highest value that has good forming ability.
In Figs. 8 and 9, the excitation spectra obtained by varying the wavelength of the Cu ions to 460 nm and that of the Tb3+ ions to 543 nm. When glass samples are excited with Tb ions at 543 nm, different excitation peaks can be observed in the ultraviolet (UV), near-ultraviolet (n-UV), and blue regions. The spectra displayed six excitation bands, with peaks at 304, 318, 342, 352, 370, 378, and 486 nm attributed to the transitions from the ground level (7F6) to 5H4, 5H7 + 5D1, 5L6,7, 5G5, 5L10, 5G6 + 5D3, and 5D4 excited states, respectively [65, 66] at different positions.
Figure 8 shows a reduction in the peak intensity as the concentration of Tb4O7 decreased. Moreover, all peaks disappeared in the glass samples containing only CuO. If we choose to excite Cu ions at 460 nm and record the excitation spectrum in the UV range, as shown in Fig. 9, the peak at 250 nm represents the transition from 1Ag to 1T2g of Cu+. As the intensity of the peak changed, the decrease indicated an improvement in Cu2+ [32].
The light produced by Tb3+ ions can be divided into two regions based on the excited level emitted. When the excited level emitted was 5D3 to 7F6-3, emission peaks observed at wavelengths less than 480 nm were observed at 382, 416, 438, and 480 nm, characterized by blue emission. In contrast, the closest level to 5D3 is 5D4, which allows non-radiative energy to increase the emission at 488, 545, 588, and 622. These emissions are formed as a transition to a lower level (5D4 to 7F6-3) as green emissions [67, 68]. According to previous studies, the sum of the emission intensity results from the excited level 5D4 to the emission intensity resulting from the excited state 5D3 indicates the distribution of Tb in a glass sample.
This distribution can be either homogeneous or clustered [28, 67, 69,70,71].The disappearance of the blue emission is a sign of clustering of Tb ions in the glass sample. However, the increase in green emission suggests that glass may be suitable for green laser applications [10]. According to our research, the emission of Cu ions is explained by the presence of Cu+ in both its + 3 and + 2 charge states, which act as luminescence centers (also known as defect centers) that enhance phosphor emission intensity [29]. When present as Cu+ (3d9-4S1), Cu ions act as color centers, which are utilized in the emission properties. In contrast, the presence of Cu2+ has an inverse effect on emissions [20].
From the excitation curve (Fig. 8), it can be observed that the highest intensity occurred at 378 nm. This indicates the most suitable wavelength for exciting Tb3+ions. Figure 10 shows the emission of the glass sample under 378 nm excitation. It shows high-intensity peaks in the green emission region, and low-intensity peaks in the blue emission region.
The glass sample doped with CuO did not show any Cu emission and its peaks disappeared. The figure shows that the emission intensity decreased with decreasing Tb4O7 concentration. Because there was no emission of Cu ions, we determined the distribution of Tb3+ ions inside the network. The intensity ratio of green to blue is a sensitive parameter that can be used to determine the concentration of Tb4O7.
Table 2 shows the calculated ratio between the sum of the intensities of the green emission (5D4-7FJ) and the sum of the peak intensity of the blue emission (5D3-7FJ). The obtained values indicate an increase in the value for the sample containing 0.95 mol% Tb4O7, suggesting that the Tb3+ ions are clustered. As the concentration of Tb4O7 decreased, the ratio began to decrease, explaining the increase in blue emission and indicating a more homogeneous distribution of Tb3+ ions at low concentrations of Tb4O7 [68].
From the perspective of bridging oxygen BO and NBOs, density plays an important role in characterizing the glass structure.
The effect of Tb4O7 concentration on density and molar volume is seen in Table 3.
The density ρ and molar volume Vm {Molar volume = molecular weight/ density (Vm = Mw/ρ)} exhibit a similar tendency in this picture, with the density and Vm increasing as the Tb4O7 concentration increases. Tb3+ enters the interstitial position in the glass structure, breaks bonds to create NBOs, and causes a volume increase. The density increased as the MW (molecular weight) of Tb increased. Vm increases as the oxygen level rises, resulting in the de-polymerization of glass structures. From density and molar volume calculate the number of Tb3+ ions per unit volume according to N = (DρNA)/Mw {D is the concentration of Tb4O7, NA is the Avogadro’s number}, also the distance between Tb3+–Tb3+ ions calculated using the relation R = \({\left(\frac{1}{N}\right)}^{1/3}\). And set in Table 3. From it, can be observed that the Tb3+ ions are too far from each other, disallowing excitation energy to be transmitted between surrounding rare-earth ions, resulting in an increase in emission intensity and increase in radiative energy.
Figure 10 shows a high-intensity peak at 542 nm, which exhibits splitting. This phenomenon is caused by the distortion of the glass network by Tb3+ ions, which leads to a stark splitting of the energy level [28].
Based on the excitation curve of Cu shown in Fig. 9, the excitation wavelength of the Cu ions was chosen as 250 nm to excite the glass samples, as shown in Fig. 11.
Figure 11 shows the ability to excite Tb3+ ions and its effect on the peaks of 488, 542, and 584 nm of green emission. The high-intensity peak of Tb4O7 emission decreases and appears as the emission of a broad emission peak of Cu ions with the increase in Cu concentration. This decrease in the intensity of the peak in the green region reduced the ratio between the green and blue regions. In addition, the very towering peaks inside the broadband at 502 nm represent the boron emission. The towering peak represents the contribution of boron emission, which was previously studied by Lu [72, 73], Collins [74] and Freitas [75]. The broad band signifies the emission mechanism formed by Cu, as when the glasses are excited at 250 nm, the excited electrons populate the higher 1T2g level, and non-radiative relaxation to the 3Eg level occurs. The short life span of this level, from radiative relaxation to the 1Ag ground level appears a broad emission peak of around 470 nm [32, 76].
The color of the glass sample varied from that under day light or UV excitation light. The visible emission color under UV light was evaluated using the CIE chromaticity coordinates. The emitted color when different emission wavelengths are mixed as shown in Figs. 10 and 11 is shown in Fig. 12.
From Fig. 12, shows differences in the emitted colors under different UV lights. The sample excited at 378 nm shows green emission, which is in accordance with a previous report [28, 53, 66], whereas under 250 nm, the green emission is italicized to blue-green. The obtained data for the corresponding (x, y) coordinates are listed in Table 4. The second vital parameter determination is CCT [32, 66] by
where υ = (x-0.332)/(y-0.186). The evaluated values are tabulated in Table 4.
This shows the difference in color brightness with different Tb4O7 concentrations and the ability to use samples doped with Tb4O7 and CuO in daylight fluorescence and samples doped with Cu in neutral-white fluorescence.
Conclusion
Terbium lithium zinc borosilicate glass doped with copper oxide was prepared using a melting technique. The spectroscopic characteristics of the obtained glass systems were examined by recording their excitation and emission spectra. When directly excited, the characteristic luminescence bands arising from the electronic transitions of the Tb3+ ions were observed. Further experiments were conducted to investigate the impact of copper ion concentration on the spectroscopic characteristics of lithium zinc borate glasses. They found that under UV–visible light excitation, our glass system displays different colors between blue, green, and yellow emissions, which is the result of multiple bands of Tb3+ forming simultaneously. The results indicate that lithium zinc borate glasses can be used as potential light emitters.
Data availability
No data was used for the research described in the article.
References:
N.S. Prabhu, A.N. Meza-Rocha, O. Soriano-Romero, U. Caldino, E.F. Huerta, C. Falcony, M.I. Sayyed, H. Al-Ghamdi, A.H. Almuqrin, S.D. Kamath, Spectroscopic study of Er3+ doped borate glass system for green emission device, NIR laser, and optical amplifier applications. J. Lumin. 238, 118216 (2021). https://doi.org/10.1016/j.jlumin.2021.118216
S. Kaur, O.P. Pandey, C.K. Jayasankar, N. Chopra, Effect of gamma irradiation on physical, optical, spectroscopic and structural properties of Er3+-doped vitreous zinc borotellurite. J. Lumin. 235, 118031 (2021). https://doi.org/10.1016/j.jlumin.2021.118031
N. Deopa, M.K. Sahu, S. Kaur, A. Prasad, K. Swapna, V. Kumar, R. Punia, A.S. Rao, Enhanced visible green and 1.5 μm radiative emission of Er3+ ions in Li2O-PbO-Al2O3-B2O3 glasses for photonic applications. J. Rare Earths 39(5), 520–525 (2021). https://doi.org/10.1016/j.jre.2020.05.002
K. Swapna, Sk. Mahamuda, A.S. Rao, S. Shakya, T. Sasikala, D. Haranath, G.V. Prakash, Optical studies of Sm3+ ions doped Zinc Alumino Bismuth Borate glasses. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 125, 53–60 (2014). https://doi.org/10.1016/j.saa.2014.01.025
M.A. Marzouk, H.A. Elbatal, A.M. Abdelghany, Effect of Gd2O3 and Sm2O3 addition on the properties of CeO2. J. Korean Inst. Electr. Electron. Mater. Eng. 16(11), 979–986 (2003). https://doi.org/10.4313/jkem.2003.16.11.979
V. Hegde, N. Chauhan, V. Kumar, C.S.D. Viswanath, K.K. Mahato, S.D. Kamath, Effects of high dose gamma irradiation on the optical properties of Eu3+ doped zinc sodium bismuth borate glasses for red LEDs. J. Lumin. 207(2019), 288–300 (2019). https://doi.org/10.1016/j.jlumin.2018.11.023
V. Hegde, S.D. Kamath, I. Kebaili, M.I. Sayyed, K.N. Sathish, C.S.D. Viswanath, A.G. Pramod, P. Ramesh, K. Keshavamurthy, G. Devarajulu, G. Jagannath, Photoluminescence, nonlinear optical and gamma radiation shielding properties of high concentration of Eu2O3 doped heavy metal borate glasses. Optik (Stuttg) 251, 168433 (2022). https://doi.org/10.1016/j.ijleo.2021.168433
A.A. El-Maaref, E.A. Abdel Wahab, Kh.S. Shaaban, M. Abdelawwad, M.S.I. Koubisy, J. Börcsök, E.S. Yousef, Visible and mid-infrared spectral emissions and radiative rates calculations of Tm3+ doped BBLC glass. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 242, 118774 (2020). https://doi.org/10.1016/j.saa.2020.118774
K. Vijaya Babu, S. Cole, Luminescence properties of Dy3+-doped alkali lead alumino borosilicate glasses. Ceram. Int. 44(8), 9080–9090 (2018). https://doi.org/10.1016/j.ceramint.2018.02.115
S. Kaur, N. Deopa, A. Prasad, R. Bajaj, A.S. Rao, Intense green emission from Tb3+ ions doped zinc lead alumino borate glasses for laser and w-LEDs applications. Opt. Mater. (Amst.) 4, 318–323 (2018). https://doi.org/10.1016/j.optmat.2018.07.020
S. Mahamuda, K. Swapna, A. Srinivasa Rao, T. Sasikala, L. Rama Moorthy, Reddish-orange emission from Pr3+ doped zinc alumino bismuth borate glasses. Phys. B Condens. Matter 428, 36–42 (2013). https://doi.org/10.1016/j.physb.2013.07.010
V. Hegde, C.S.D. Viswanath, N. Chauhan, K.K. Mahato, S.D. Kamath, Photoluminescence and thermally stimulated luminescence properties of Pr3+-doped zinc sodium bismuth borate glasses. Opt. Mater. (Amst.) 84, 268–277 (2018). https://doi.org/10.1016/j.optmat.2018.06.064
S. Zhao, F. Xin, Sh. Xu, D. Deng, L. Huang, H. Wang, Y. Hua, Luminescence properties and energy transfer of Eu/Tb ions codoped aluminoborosilicate glasses. J. Non Cryst. Solids 357(11–13), 2424–2427 (2011). https://doi.org/10.1016/j.jnoncrysol.2010.11.092
T. Sasikala, L. Rama Moorthy, Photoluminescence properties of singly doped Tm3+ and co-doped Tm3+/Tb3+ions in tellurite glasses. J. Mol. Struct. 1076, 529–534 (2014). https://doi.org/10.1016/j.molstruc.2014.07.051
M. Mungra, F. Steudel, B. Ahrens, S. Schweizer, Tm/Tb/Eu triple-doped lithium aluminoborate glass for white light generation. J. Lumin. 192, 71–76 (2017). https://doi.org/10.1016/j.jlumin.2017.06.028
C. Zhu, D. Wu, J. Liu, M. Zhang, Y. Zhang, Color-tunable luminescence in Ce-, Dy-, and Eu-doped oxyfluoride aluminoborosilicate glasses. J. Lumin. 183, 32–38 (2017). https://doi.org/10.1016/j.jlumin.2016.11.004
D. He, Y. Fang, M. Liao, G. Zhao, Y. Sun, F. Yu, L. Hu, X. Wang, J. Hou, T. Xue, Y. Liu, Luminescence properties and energy transfer behavior of Dy3+/Tm 3+ co-doped phosphate glasses with high moisture-resistance and thermal stability for W-LEDs. J. Lumin. 236, 118087 (2021). https://doi.org/10.1016/j.jlumin.2021.118087
V. Hegde, C.S.D. Viswanath, K.K. Mahato, S.D. Kamath, Warm white light and colour tunable characteristics of Dy3+ co-doped with Eu3+ and Pr3+ zinc sodium bismuth borate glasses for solid state lighting applications. Mater. Chem. Phys. 234, 369–377 (2019). https://doi.org/10.1016/j.matchemphys.2019.05.063
P.R. Rani, M. Venkateswarlu, Sk. Mahamuda, K. Swapna, N. Deopa, A.S. Rao, G.V. Prakash, Structural, absorption and photoluminescence studies of Sm3+ ions doped barium lead alumino fluoro borate glasses for optoelectronic device applications. Mater. Res. Bull. 110, 159–168 (2019). https://doi.org/10.1016/j.materresbull.2018.10.033
Y.S.M. Alajerami, S. Hashim, W.M.S. Wan Hassan, A.T. Ramli, M.A. Saleh, The effect of MgO on the optical properties of lithium sodium borate doped with Cu+ ions. Opt. Spectrosc. (Engl. Transl. Opt. i Spektrosk.) 114(4), 537–543 (2013). https://doi.org/10.1134/S0030400X1304022X
M. Kumar, A.S. Rao, Influence of Tb3+ ions concentration and temperature on lithium bismuth alumino borosilicate glasses for green photonic device applications. Opt. Mater. (Amst.) 120, 111439 (2021). https://doi.org/10.1016/j.optmat.2021.111439
A. Thulasiramudu, S. Buddhudu, Optical characterization of Cu2+ ion-doped zinc lead borate glasses. J. Quant. Spectrosc. Radiat. Transf. 97(2), 181–194 (2006). https://doi.org/10.1016/j.jqsrt.2005.04.006
D. Aboutaleb, B. Safi, Structure and properties of the soda-borate glasses: effect of adding Fe2O3 concentration. J. Chem. Eng. Process Technol. 7(1), 2–5 (2015). https://doi.org/10.4172/2157-7048.1000268
S. El-jadal, The influence of iron oxide on the structural and optical properties of borosilicate. Sci. J. Appl. Sci. Sabratha Univ. 9, 33–46 (2022)
H. Wen, P.A. Tanner, Optical properties of 3d transition metal ion-doped sodium borosilicate glass. J. Alloys Compd. 625, 328–335 (2015)
K. Tonooka, O. Nishimura, Effect of calcination temperature on the luminescent properties of Tb-doped borosilicate glasses. J. Mater. Sci. 34(20), 5039–5044 (1999). https://doi.org/10.1023/A:1004792529119
I.R. Mohammed, A study on optical, spectroscopic and structural properties of copper-doped calcium lithium borate glasses. J. Opt. 49(4), 556–563 (2020). https://doi.org/10.1007/s12596-020-00641-3
K. Swapna, S. Mahamuda, A.S. Rao, M. Jayasimhadri, S. Shakya, G.V. Prakash, Tb3+ doped Zinc Alumino Bismuth Borate glasses for green emitting luminescent devices. J. Lumin. 156, 180–187 (2014). https://doi.org/10.1016/j.jlumin.2014.08.019
H.K. Obayes, H. Wagiran, R. Hussin, M.A. Saeed, Structural and optical properties of strontium/copper co-doped lithium borate glass system. Mater. Des. 94, 121–131 (2016). https://doi.org/10.1016/j.matdes.2016.01.018
A. Okasha, S.Y. Marzouk, A.M. Abdelghany, Design a tunable glasses optical filters using CuO doped fluoroborate glasses. Opt. Laser Technol. 137, 106829 (2021). https://doi.org/10.1016/j.optlastec.2020.106829
Y. Shi, R. Wei, J. Guo, X. Tian, F. Hu, H. Guo, Influence of optical basicity on Cu+ luminescence in aluminosilicate oxyfluoride glasses. Front. Mater. 6, 1–6 (2019). https://doi.org/10.3389/fmats.2019.00246
O. Soriano-Romero, R. Lozada-Morales, A.N. Meza-Rocha, S. Carmona-Tellez, U. Caldino, B. Flores-Desirena, R. Palomino-Merino, Cold bluish white and blue emissions in Cu+-doped zinc phosphate glasses. J. Lumin. 217, 1–7 (2020). https://doi.org/10.1016/j.jlumin.2019.116791
W. Rittisut, P. Manyum, N. Wantana, Y. Ruangtaweep, S. Rujirawat, K. Kamonsuangkasem, R. Yimnirun, A. Prasatkhetragarn, S. Kothan, H.J. Kim, J. Kaewkhao, Synthesis and characterization of eco-friendly, stable green-emitting Tb3+-doped borosilicate glasses using waste silica gel. J. Non Cryst. Solids 638, 123045 (2024)
N. Singkiburin, N. Srisittipokakun, R. Rajaramakrishna, S. Kothan, N. Intachai, J. Kaewkhao, Investigation of europium oxide (Eu2O3) doped in cobalt boro-silicate glasses from waste glass for photonics material application. Optik (Stuttg) 291, 171146 (2023)
M. Zhao, Q. Zhang, Z. Xia, Structural engineering of Eu2+-doped silicates phosphors for LED applications. Acc. Mater. Res. 1(2), 137–145 (2020)
N. Intachai, S. Kothan, N. Wantana, S. Kaewjaeng, P. Pakawanit, N. Vittayakorn, P. Kanjanaboos, N. Phuphathanaphong, H.J. Kim, J. Kaewkhao, Eu2O3 doped silicoborate glasses for scintillation material application: luminescence ability and X-ray imaging. Optik (Stuttg) 294, 171439 (2023)
Y.-C. Li, Y.-H. Chang, Y.-S. Chang, Y.-J. Lin, C.-H. Laing, Luminescence and energy transfer properties of Gd3+ and Tb3+ in LaAlGe2O7. J. Phys. Chem. C 111(28), 10682–10688 (2007)
C.R. Kesavulu, H.J. Kim, S.W. Lee, J. Kaewkhao, E. Kaewnuam, N. Wantana, Luminescence properties and energy transfer from Gd3+ to Tb3+ ions in gadolinium calcium silicoborate glasses for green laser application. J. Alloys Compd. 704, 557–564 (2017)
D. Valiev, E. Polisadov, S. Stepanov, K. Belikov, N. Yegorov, H. Othman, V. Vaganov, Luminescence spectroscopy of scintillating glasses doped with Tb3+/Ce3+ with different concentrations of cerium under photo-and electron excitation. J. Lumin. 162, 128–133 (2015)
C. Zu, Y. Wang, J. Chen, B. Han, H. Tao, Luminescent properties and applications of Tb3+ doped silicate glasses with industrial scales. J. Non Cryst. Solids 357(11–13), 2435–2439 (2011)
Z. Chenggang, Z. Zhihua, Z. Ligang, X. Anguo, C. Yuandao, Z. Xiangyang, Z. Yongbing, L. Xiaoyang, G. Qizhi, Luminescence properties of Tb3+-doped borosilicate scintillating glass under UV excitation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 147, 324–327 (2015). https://doi.org/10.1016/j.saa.2015.03.097
I. Kashif, A. Ratep, Impact of europium ions Eu3+ on thermal, optical, and luminescence properties of lithium zinc borate glasses. Optik (Stuttg) 300, 171621 (2024)
I. Kashif, A. Ratep, Various color light emission from single, double, and triple Eu 3+/Tb 3+/Tm 3+ doped borate glass excited by UV light. J. Fluoresc. (2023). https://doi.org/10.1007/s10895-023-03502-x
S. Dayioglugil, N. Solak, Effect of ZnO on luminescence performance of terbium-activated zinc borosilicate glasses. Materials (Basel) 17(9), 2154 (2024)
I. Kashif, M.L. Montes, M.A. Taylor, A. Ratep, Influence of Fe substitution on the Eu-doped lithium borosilicate glass system’s physical, thermal, magnetic, and luminescent properties. J. Mater. Sci. Mater. Electron. 35(4), 1–15 (2024)
I. Kashif, A. Ratep, Effect of addition of dysprosium oxide on spectroscopic properties and Judd-Ofelt analysis of lithium borosilicate glass system. SILICON 15(7), 3365–3378 (2023). https://doi.org/10.1007/s12633-022-02249-7
I. Kashif, A. Ratep, Judd–Ofelt and luminescence study of Dysprosium-doped lithium borosilicate glasses for lasers and w-LEDs. Boletín la Soc. Española Cerámica y Vidr. 61(6), 622–633 (2022)
R. Kaur, S. Singh, O.P. Pandey, Influence of CdO and gamma irradiation on the infrared absorption spectra of borosilicate glass. J. Mol. Struct. 1049, 409–413 (2013). https://doi.org/10.1016/j.molstruc.2013.06.072
Y. Lai, Y. Zeng, X. Tang, H. Zhang, J. Han, H. Su, Structural investigation of calcium borosilicate glasses with varying Si/Ca ratios by infrared and Raman spectroscopy. RSC Adv. 6(96), 93722–93728 (2016). https://doi.org/10.1039/c6ra20969f
T. Sambasiva Rao, D.V.K. Reddy, S.K. Taherunnisa, A.S.S. Reddy, P.V. Rao, N. Veeraiah, M.R. Reddy, Studies on structural characterization and near white light emission through energy transfer between Ce3+ and Tb3+ in barium gallium borosilicate glasses. J. Mol. Struct. 1190, 184–195 (2019). https://doi.org/10.1016/j.molstruc.2019.04.065
V.X. Quang, P. Van Do, N.X. Ca, L.D. Thanh, V.P. Tuyen, P.M. Tan, V.X. Hoa, N.T. Hien, Role of modifier ion radius in luminescence enhancement from 5D4 level of Tb3+ ion doped alkali-alumino-telluroborate glasses. J. Lumin. 221, 117039 (2020). https://doi.org/10.1016/j.jlumin.2020.117039
S.N.S. Yaacob, M.R. Sahar, E.S. Sazali, Z.A. Mahraz, K. Sulhadi, Comprehensive study on compositional modification of Tb3+ doped zinc phosphate glass. Solid State Sci. 81, 51–57 (2018). https://doi.org/10.1016/j.solidstatesciences.2018.05.006
E.O. Taha, A. Saeed, The effect of cobalt/copper ions on the structural, thermal, optical, and emission properties of erbium zinc lead borate glasses. Sci. Rep. 13(1), 1–13 (2023). https://doi.org/10.1038/s41598-023-39256-6
I. Kashif, A. Ratep, Blue, red, and green emission from chromium and copper metal doped lithium borate glass. IOP Conf. Ser. Mater. Sci. Eng. (2020). https://doi.org/10.1088/1757-899X/956/1/012013
G. Sangeetha, K.C. Sekhar, A. Hameed, G. Ramadevudu, M.N. Chary, M. Shareefuddin, Influence of CaO on the structure of zinc sodium tetra borate glasses containing Cu2+ ions. J. Non Cryst. Solids 563, 120784 (2021). https://doi.org/10.1016/j.jnoncrysol.2021.120784
I. Kashif, H. Farouk, A. Ratep, M. Al Mahalawy, White light emission in Dy3+ doped SiO2B2O3Bi2O3TeO2 glass system. J. Non Cryst. Solids 522, 119581 (2019). https://doi.org/10.1016/j.jnoncrysol.2019.119581
Y.S. Mustafa Alajerami, S. Hashim, W.M. Saridan Wan Hassan, A.T. Ramli, The effect of CuO and MgO impurities on the optical properties of lithium potassium borate glass. Phys. B Condens. Matter 407(13), 2390–2397 (2012). https://doi.org/10.1016/j.physb.2012.03.029
O.A. Zamyatin, V.G. Plotnichenko, M.F. Churbanov, E.V. Zamyatina, V.V. Karzanov, Optical properties of zinc tellurite glasses doped with Cu2 + ions. J. Non Cryst. Solids 480, 81–89 (2018). https://doi.org/10.1016/j.jnoncrysol.2017.08.025
H. Aboud, H. Wagiran, I. Hossain, R. Hussin, S. Saber, M. Aziz, Effect of co-doped SnO2 nanoparticles on the optical properties of Cu-doped lithium potassium borate glass. Mater. Lett. 85, 21–24 (2012). https://doi.org/10.1016/j.matlet.2012.06.033
P.P. Pawar, S.R. Munishwar, D.D. Ramteke, R.S. Gedam, Physical, structural, thermal and spectroscopic investigation of Sm2O3 doped LAB glasses for orange LED. J. Lumin. 208, 443–452 (2019)
A. Ichoja, S. Hashim, S.K. Ghoshal, I.H. Hashim, R.S. Omar, Physical, structural and optical studies on magnesium borate glasses doped with dysprosium ion. J. Rare Earths 36(12), 1264–1271 (2018)
M. Abdel-Baki, F.A. Abdel-Wahab, F. El-Diasty, One-photon band gap engineering of borate glass doped with ZnO for photonics applications. J. Appl. Phys. 111(7), 073506 (2012)
Y. Shang, M. Wang, E. Li, C. Zhong, T. Qin, Enhancing thermal and dielectric properties of MgO–Al2O3–SiO2–B2O3 glass-ceramics through CuO doping. Ceram. Int. 49(19), 32078–32085 (2023). https://doi.org/10.1016/j.ceramint.2023.07.175
U.G. Issever, G. Kilic, E. Ilik, The Impact of CuO on physical, structural, optical and thermal properties of dark VPB semiconducting glasses. Opt. Mater. (Amst.) 116, 111084 (2021). https://doi.org/10.1016/j.optmat.2021.111084
S. Qian, L. Huang, S. Zhao, S. Xu, Luminescent properties of Tb3+ doped high density borogermanate scintillating glasses. J. Rare Earths 35(8), 787–790 (2017). https://doi.org/10.1016/S1002-0721(17)60977-3
M. Kumar, A.S. Rao, Influence of Tb3+ ions concentration and temperature on lithium bismuth alumino borosilicate glasses for green photonic device applications. Opt. Mater. (Amst.) (2021). https://doi.org/10.1016/j.optmat.2021.111439
W. Chewpraditkul, Y. Shen, D. Chen, A. Beitlerova, M. Nikl, Luminescence of Tb3+-doped high silica glass under UV and X-ray excitation. Opt. Mater. (Amst.) 35(3), 426–430 (2013). https://doi.org/10.1016/j.optmat.2012.09.012
D. Umamaheswari, B.C. Jamalaiah, T. Sasikala, T. Chengaiah, I.G. Kim, L. Rama Moorthy, Photoluminescence and decay behavior of Tb3+ ions in sodium fluoro-borate glasses for display devices. J. Lumin. 132(5), 1166–1170 (2012). https://doi.org/10.1016/j.jlumin.2011.12.080
A.J. Silversmith, D.M. Boye, K.S. Brewer, C.E. Gillespie, Y. Lu, D.L. Campbell, 5D3→7FJ emission in terbium-doped sol-gel glasses. J. Lumin. 121(1), 14–20 (2006). https://doi.org/10.1016/j.jlumin.2005.09.009
P. Nachimuthu, R. Jagannathan, Tb3+ fluorescence as a probe of cluster formation in lead oxyfluoride glasses. J. Non Cryst. Solids 183(1–2), 208–211 (1995). https://doi.org/10.1016/0022-3093(94)00586-9
C.H. Kam, S. Buddhudu, Luminescence and decay behaviour of Tb3+:ZrF 4-BaF2-LaF3-YF3-AlF3-NaF optical glasses. Phys. B Condens. Matter 337(1–4), 237–244 (2003). https://doi.org/10.1016/S0921-4526(03)00409-5
H.C. Lu, J.I. Lo, Y.C. Peng, S.L. Chou, B.M. Cheng, W.H. Hung, Far-UV photoluminescence of boron-doped diamond: cross interaction between boron and diamond. Carbon N. Y. 134, 448–451 (2018). https://doi.org/10.1016/j.carbon.2018.04.017
H.C. Lu, Y.C. Peng, M.Y. Lin, S.L. Chou, J.I. Lo, B.M. Cheng, Analysis of boron in diamond with UV photoluminescence. Carbon N. Y. 111, 835–838 (2017). https://doi.org/10.1016/j.carbon.2016.10.082
A.T. Collins, The characterisation of point defects in diamond by luminescence spectroscopy. Diam. Relat. Mater. 1(5–6), 457–469 (1992). https://doi.org/10.1016/0925-9635(92)90146-F
J.A. Freitas, K. Doverspike, P.B. Klein, Y.L. Khong, A.T. Collins, Luminescence studies of nitrogen- and boron-doped diamond films. Diam. Relat. Mater. 3(4–6), 821–824 (1994). https://doi.org/10.1016/0925-9635(94)90277-1
S. Hemalatha, M. Nagaraja, A. Madhu, K. Suresh, N. Srinatha, Spectroscopic studies of transition metal ion-doped borate glasses for optical applications. Mater. Today Proc. 49, 1875–1879 (2021). https://doi.org/10.1016/j.matpr.2021.08.075
Acknowledgements
The authors thank Material Science and Glass research Lab., Physics Department, Faculty of science, AL-AZHAR University for providing the measurement facilities.
Funding
No funding for research.
Author information
Authors and Affiliations
Contributions
AR: investigation, writing—original draft, methodology, formal analysis. IK: writing—review and editing, administration, formal analysis, investigation. SSA: preparation samples, measurements, writing draft. NMK: preparation samples, measurements, writing draft. MAE: preparation samples, measurements, writing draft.
Corresponding author
Ethics declarations
Ethical approval
This paper meets the ethical standards of this journal.
Consent to participate
All authors agree with the review of this paper in this journal.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kashif, I., Ali, S.S., Khodeir, N.M. et al. Effect of copper addition on lithium zinc borosilicate glass containing terbium oxide. J Opt (2024). https://doi.org/10.1007/s12596-024-02025-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12596-024-02025-3