Abstract
Laser-induced breakdown spectroscopy (LIBS) has been used for the identification of inorganic and organic minerals to explore the elemental composition of dried Punica granatum peel samples drying with different techniques, namely hot air oven (T1), microwave (T2), sun-dried (T3), and freeze-dried (T4). The relative concentration of minerals such as potassium, sodium, and magnesium was compared among all the samples using the intensities of the spectral lines of these minerals present in the LIBS spectra of the peel samples. Organic molecules/compound were evaluated using the FTIR spectroscopy technique by identifying the vibrational frequencies of functional groups of the molecules/compounds present in the FTIR spectra of the samples. XRD technique was used to understand the crystallographic structure, and SEM was used for the study of the morphology of different samples. The proposed methods highlighted the potential of the LIBS technique for authenticity certification, providing fast, simple, and clean determinations since no sample pretreatment was required. The main aim of this study is to provide information about the various elements and detection of any adulteration/impurities in the peel samples, which allows to detect their pharmacological properties and serves as a natural medicinal product.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Punica granatum belongs to the family Lythraceace, generally known as pomegranate, is a sweet, tart fruit with a thick leathery skin (rind or peel) enclosing many juicy arils [1]. It is one of the most fascinating fruit in terms of cultural, traditional, and potential therapeutic applications. This fruit first appeared in Iran and subsequently went to northern India's Himalayan region. Iran, India, the East Indies, the Mediterranean region, Southeast Asia, Malaysia, tropical Africa, Russia, Japan, China, and to a lesser extent, the USA are among the places where it is widely grown [2]. Recently, the largest pomegranate acreage in the world is found in India, specifically in the states of Maharashtra, Gujarat, Karnataka, Andhra Pradesh, Tamil Nadu, Madhya Pradesh, and Rajasthan. During 2016–2017, India had 2.16 lakh hectare of pomegranate cultivation, producing 24.42 lakh tons annually at a productivity of 11.70 tons/ha [3]. The peel, arils, and seeds together make up around 50%, 40%, and 10% of the weight of the entire fruit, respectively. Peel is the major byproduct generated from the juice-making industries and a substantial source of antioxidant, polyphenolic, and nutritive compounds [4, 5]. In the modern era, the focus has been switched from the utilization of common agricultural products such as underutilized crops, byproducts, and waste generated from agricultural industries. This would reduce the waste disposal issue while also increasing fresh sources of nourishment. Punica granatum peel is a good source of bioactive compounds as well as minerals like magnesium, potassium, sodium, calcium, phosphorus, and more. These peels can be used as a functional ingredient and in the preparation of natural remedies [1, 6, 7].
As individuals become increasingly aware of the advantages of minerals (macro or micro), which are an essential component of human nutrition for appropriate body functioning and growth, the interest in mineral estimation has risen. Although even in modest amounts, heavy metals including mercury, lead, arsenic, and cadmium can harm human health. Food can get contaminated with metal by the use of raw ingredients, reagents, solvents, and process equipment. In order to assist prevent under or overexposure in the food chain, it is crucial to monitor the beneficial and hazardous components in food items [8]. The methods used to evaluate the elements contained in biological samples often include inductively coupled plasma mass spectroscopy (ICP-MS), inductively coupled plasma atomic emission spectroscopy (ICP-AES), atomic absorption spectroscopy (AAS), etc. However, these processes necessitate preliminary sample preparation, which is time-consuming, labor-intensive, and increases the danger of contamination, thus not eco-friendly. Examples of this preparation include ashing, acid digestion, producing ash solutions, etc. On the other hand, LIBS technique has emerged as a suitable analytical tool for analyzing such types of materials, because LIBS is a fast, non-destructive, and ideal tool for the analysis of in situ multiple elements analysis including the light elements. It is a promising analytical tool for qualitative and quantitative elemental analysis because it is entirely optical, making it suitable as a PAT (process analytical technology) or portable tool. Food technology can use LIBS as a quality control tool to identify nutrients or heavy metals in food samples [6, 8]. Fourier transform infrared (FTIR) spectroscopy, similar to hyperspectral imaging Raman spectroscopy, terahertz spectroscopy, and NIR images, plays an essential role in detecting functional groups of the molecules/compounds present in the food samples. As variations in the permanent dipoles lead to a specific vibration mode, two vibrations related to molecular bonds will stretch and bend. The absorption in the IR range will occur at the feather frequencies, which can be used for identifying the presence of numerous chemical groups [9]. The surface morphology and crystalline structure of the sample were detected by scanning electron microscopy and X-ray diffraction analysis.
Numerous studies have been done to identify the minerals and hazardous substances present in food items that are useful in determining the medicinal effectiveness of a particular food sample. A research done by Kumar et al. [10], that included assessments of dietary supplements and adulteration/toxic components found in different tea leaves. Numerous inorganic elements, including Ca, Na, K, Mg, etc., as well as certain organic elements, including C, N, H, and O, were discovered using the LIBS approach. The findings of this study demonstrated that the nutrients Na, K, and Mg found in tea leaves are beneficial in the prevention of renal disorders and hypertension. In the conclusion, it was determined that food adulteration/contamination is a significant problem in the food sector, but with the aid of the LIBS technology, the quality of food (nutrients) as well as safety (contamination) can be evaluated very quickly, without any sample preparation. In another study, the LIBS approach was used to assess the bioactive potential of the pointed gourd (trichosanthes dioica) leaves. Numerous elemental ratios, such as magnesium/potassium and magnesium/sodium, have been found to have hepatoprotective and hypolipemic effects on hypertriglyceridemia and to manage diabetes mellitus. According to the findings, LIBS is a sophisticated method for identifying the elements that cure diseases and also beneficial in the formulation of ayurvedic remedies [11]. By using LIBS, the mineral composition of milk powder was identified, and the elements were compared to other brands of milk samples. Milk powder has been shown to include both organic and inorganic components in large amounts. Additionally, using FTIR, the functional group of the organic components were confirmed. The results of this study showed that LIBS is a rapid, simple, and affordable method to determine the elemental composition of any food materials [12].
In the present study, LIBS is used to determine the mineral content, FTIR is used for the detection of functional group, SEM is used to detect the surface morphology, and XRD is used to detect the crystallinity and pore structure present in the different dried Punica granatum peel powder. This analysis is significant in view to investigate the viability of LIBS as a simple, rapid, in situ, and synchronized analytical technique for estimation of elements in Punica granatum peel, which have good potential to be used as a functional ingredient in many food preparations and in natural remedies rather than discarded as waste.
Materials and methods
Sample collection
Fruit peels were collected from the local juice vendors of Prayagraj region of Uttar Pradesh. All the fruit peels were randomly selected considering the quality of the fruits and were free from any physical and microbial damage. The peels were cleaned and washed with distilled water to remove dust and unwanted particle and then dried by using four different techniques, i.e., hot air oven drying (T1) (a) (60℃ for 24 h), microwave drying (T2) (b) (for 15 min), sun drying (T3) (c) (for72 hrs), and freeze-drying (T4) (d) (− 20 ℃ for 24 h) depicted in Fig. 1. Dried peels were then ground by using a mixture grinder to find a uniform powder, then stored separately in air-tight zip pouches for further use.
Experimental setup for laser induced breakdown spectroscopy (LIBS)
Atomic emission spectroscopy, known as LIBS, uses a highly intense laser pulse as its excitation source. To record the LIBS spectra, 1 g of each powdered sample was pressed into a pallet by putting it into a 10-mm stainless steel die, which was subjected to press using 10 tons of pressure for 1 min by hydraulic pressure machine (K-Br Press MODEL M-15). These pallets bear the laser pulse thrust without adding any binder. The LIBS experimental setup comprises a laser source, focusing and collecting optics, a spectrometer, a detecting system, and a sample stage with a jack for sample movement [6, 13] Fig. 2 illustrates a detailed schematic diagram of the LIBS setup. The device has a high-power pulse Nd: YAG laser (Continuum Surelite III-10), with a maximum energy of 425 mJ at a rhythmic wavelength of 532 nm. The repetition rate varies from 1 to 10 Hz. The laser beam was focused on the sample's surface by using a plano-convex quartz lens with a focal length of 15 cm to produce intense plasma. The pallets of each sample were placed in a movable platform capable of moving in three directions X, Y, and Z to get the precise and fresh laser short every time, reducing the chances of crater formation on the pallet. Ten spectra were recorded for each sample to reduce the error, in every spectrum laser beam hits the different spots of the pallet and then averaged for analysis by using Origin Software [13, 14].
Scanning electron microscopy (SEM)
Structural and morphological characterization of differently dried Punica granatum peel samples were examined using scanning electron microscope (Model: JSM 6490 LV, Make: JEOL, Japan) at 15 kV accelerated voltage. The powdered samples were coated with lead with a 10 nm layer to make them more conductive.
Fourier transform infrared spectroscopy (FTIR) and X-ray diffraction (XRD)
FTIR was used to determine the different functional groups of the molecules in the powdered peel samples. For the characterization, powdered samples were mixed with solid potassium bromide (KBr) homogeneously, which was then compressed with a hydraulic press to form a pallet and used for FTIR analysis. The spectrum was recorded in the range of 500–4000 cm−1 by Fourier transform infrared Spectrometer (FTIR), Model: Nicole 6700, Make: Thermo-Scientific, USA. The degree of crystallinity and porous structure of different dried peel samples were analyzed by using X-ray diffractometer (Model: D8 Advance Eco, Make: Bruker, Germany).
Results and discussion
LIBS analysis
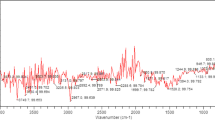
LIBS spectra of different dried pomegranate peel samples were recorded in the spectral range from 200 to 800 nm. Figure 3 illustrates the occurrence of spectral lines of organic elements (H, C, N, and O) and inorganic elements (Na, K, Ca, Mg, and Sr) present in the LIBS spectra of the samples. The spectral profiles of every sample resemble each other. Direct visual analysis of the spectra reveals no discernible differences in spectral signatures of the samples.
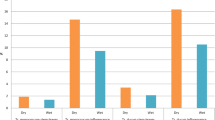
In contrast, the intensities of the spectral lines of the elements vary from sample to sample, which reveals that the concentration of the minerals in each sample are not same. The spectral lines of the various elements present in the LIBS spectra of the samples were identified using the National Institute of Standards and Technology (NIST) atomic spectroscopic database. In humans, sodium (Na) is the main extracellular cation and a vital physiological component. Electrolytes, such as sodium (Na), potassium (K) and magnesium (Mg) manage the proper fluid balance inside and outside of the cells to maintain a healthy acid–base balance in people. Potassium is the primary electrolyte found in cells and stored in muscle fibers with glycogen. It plays an important role in facilitating the transport of glucose into muscle cells. Potassium deficiency causes nerve irritability, cardiac and mental disorders, muscular weakness, and paralysis. Several vital processes rely on calcium, including the growth of bone and dental tissues, the release of hormones, the contraction of muscles, and the metabolism of glycogen. Magnesium (Mg) and calcium (Ca) helps in bone growth, metabolic process stability, blood vessel flexibility, cardiovascular disease prevention, and cerebral cell repair. Besides the spectral lines of the elements, electronic bands of CN are also seen in the LIBS spectra, which provide additional evidence that all four samples contain organic compounds. The error bar of some elements calculated from the area under the peak of the spectral lines in the LIBS spectra for all dried samples is depicted in Fig. 4, which eventually gives an idea about the concentration of elements present in the samples. The highest peaks of Na, K, Ca, and Mg were detected in hot air oven-dried (T1) samples followed by sun-dried (T3), microwave-dried (T2), and freeze-dried (T4). The highest intensity of C was found in T4 and N was in T2 sample, this indicated that the content of all the inorganic elements was high in T1 samples may be due to less time required for drying of sample in hot air oven, in freeze-drying (T4) the model were exposed to very low temperature, beside of this in microwave drying (T2), samples undergo to ultrahigh temperature, which may affect the concentration of elements. In the case of sun-drying (T3), samples were exposed to the sun for a longer time, about 72 h, which may be responsible for the reduction of minerals. This indicated that hot air oven-drying (T1) is the best technique because in this drying, the samples were not directly come in the exposure to heat and were dried with a constant temperature of the air, and supervision necessary to prevent them from burning. Hence, the best results were found in the hot air oven-dried (T1) sample.
SEM analysis
In this study, surface structural changes and particle geometry of Punica granatum peel can be seen in a microscopic examination by using scanning electron microscopy, and images are obtained at 5000 × magnification. Figure 5 demonstrates the impact of drying on the morphological structure of Punica granatum peel powder. The obtained images matched to the findings of Pathak et al., [15]. It was observed that the surface of all the samples is uneven, granulated, porous, and fibrous in shape and agglomerated in appearance; in addition to this, particle shape and size vary. The surface of T1 is rough and porous (Fig. 5 a), although the surface of T2 (Fig. 5 b) is less porous and has a smooth surface compared to T1, T3 (Fig. 5 c) demonstrates an irregular surface with insignificant and tiny pores while T4 (Fig. 5 d) shows an asymmetrical surface and rough structure. Every micrograph displayed some atypical morphology with large particle sizes and rough surfaces.
FTIR and XRD analysis
FTIR analysis is mainly used to promptly and positively identify various functional groups of the molecules present in the sample [13]. The FTIR spectrum of all the Punica granatum peel samples (T1, T2, T3, and T4) were recorded in the range of 4000–500 cm−1, and the observed peaks are shown in Fig. 6 a-d. The individual spectra provide information on the presence of bonding for molecules with various functional groups. Broad peaks were observed at 3405.9, 3366.7, 3363.7, and 3379.2 cm−1, which contributed to O–H stretching vibration of hydroxyl, carboxylic, and phenolic groups. Small peaks at 2928.5, 2933.8, 2931.5, and 29,331 cm−1 are due to C-H stretching vibration of the -CH2 alkane group. Strong peaks recorded at 1733.5, 1732.7, 1732.0, and 1731.7 cm−1 denote the C = O stretching vibration of the carbonyl or ketone group [16]. The absorption band at 1616.9, 1617.4, 1616.2, and 1616.5 cm−1 represents the stretching vibration of C-O and C–C bonds [17, 18]. Peaks observed at 1515.7, 1518.6, 1516.6 cm−1 indicated the presence of C = C stretching vibration of the benzene group [16], peaks at 1447.7, 1446.1, 1447.3, and 1447.6 cm−1may be due to the CH2 bending vibration [19] and bands at 1229.5, 1226.9, 1226.7, and 1229.4 cm−1 assigned to the stretching bond of the O–H group of polyphenols [20]. The bands near 1047.1, 1049.2, 1046.5, and 1046.3 cm−1 denote the stretching vibration of the N–H group [17, 21]. The absorption bands at 762.5, 761.0, 761.1, 763.8, 640.9, 633.8, and 630.0 cm−1 indicate the acetylenic C-H bending. As a result, from FTIR spectra, numerous vibrational bands are seen that are caused by various groups, which confirms the presence of C, N, O, and H as well as further confirms the presence of molecular bands of CN as detected in the LIB spectra. Table 1 depicts the different vibrations associated with the different functional groups of the molecules present in P. granatum peel.
The X-ray diffraction patterns of different dried Punica granatum peels are presented in Fig. 7. XRD is an imperative parameter to detect the crystalline and molecular structure of the biopolymers. Punica granatum peel showed a clear broad peak at 21.54°, similar to Cui et al., [22] and Ali et al., [23], indicating the imprecise character of the protein. T1 and T3 exhibit an extremely sharp peak in the range of 14.9°, representing a high degree of crystallinity. However, the intensity of the peaks is different may be due to the drying treatment applied, which changes the surface modification of the Punica granatum peel samples. The highest crystallinity was found in sun-dried (T-3) and hot air oven-dried (T-1) samples compared with the other two along with this vague character, protein was also decreased in T2 and T4 samples, indicating that the degree of crystallinity was increased after hot air oven and sun drying techniques. In other words, all the dried samples of Punica granatum peel showed similar crystallinity; however, hot air oven (T1) and sun-drying (T3) had slightly higher crystallinity followed by microwave (T2) and freeze-dried (T4).
Conclusion
LIBS illustrate the capability of quick identification of elements found in different dried P. granatum peel samples. The spectral lines of various elements in the LIBS spectra of the samples demonstrate that all the dried samples are good sources of minerals like sodium, potassium, calcium, magnesium, nitrogen, and carbon. LIBS is a fast, precise, and low-cost technology for determining nutritional components in food samples at the ppm level. Although all of the samples have comparable nutrient elements, demonstrating its potential usefulness for quickly discerning huge data sets of different dried peel samples. In addition, the results of SEM, FTIR, and XRD examination showed the characteristic shape, functional group, and crystalline structure of the P. granatum peel, respectively. The influence of drying temperature and duration is also investigated, revealing small variations in the shape and functional group in the powdered peel samples. The findings also revealed that P granatum peels contain a high concentration of several minerals needed for the proper functioning of the human body system. Thus, rather than being dumped as garbage, these peels could be used as food or as a culinary ingredient.
Availability of data and material
Not applicable.
Code availability
Not applicable
References:
H. Jalal, M.A. Pal, S.R. Ahmad, M. Rather, M. Andrabi, S. Hamdani, Physico-chemical and functional properties of pomegranate peel and seed powder. J. Pharm. Innov. 7(4), 1127–1131 (2018)
U. Noor, S. Soni, E. Gupta, Punica granatum peel waste-an underutilized substrate of therapeutic potential: an overview. Recent Pat. Food Nutr. Agric. 13(2), 103–119 (2022). https://doi.org/10.2174/2772574X14666221124163344
R. Kumar, R. Meena, B.D. Sharma, P.L. Saroj, Production Technology of Pomegranate in Arid Region, (ICAR-Central Institute for Arid Horticulture, 2018), pp.1–34
R.R. Mphahlele, O.A. Fawole, N.P. Makunga, U.L. Opara, Effect of drying on the bioactive compounds, antioxidant, antibacterial and antityrosinase activities of pomegranate peel. BMC Complement. Altern. Med. 16(1), 1–12 (2016). https://doi.org/10.1186/s12906-016-1132-y
S.C. Kushwaha, M.B. Bera, P. Kumar, Antioxidants in Fruits: Properties and Health Benefits, (Springer, 2020), 295–316, https://doi.org/10.1007/978-981-15-7285-2_15
J. Singh, R. Kumar, S. Awasthi, V. Singh, A.K. Rai, Laser Induced breakdown spectroscopy: A rapid tool for the identification and quantification of minerals in cucurbit seeds. Food Chem. 221, 1778–1783 (2017). https://doi.org/10.1016/j.foodchem.2016.10.104
K. Sharma, C.E. Akansha, Comparative studies of proximate, mineral and phytochemical compositions of pomegranate (Punica granatum) in peel, seed and whole fruit powder. Methods 3, 192–196 (2018)
M. Markiewicz-Keszycka, X. Cama-Moncunill, M.P. Casado-Gavalda, Y. Dixit, R. Cama-Moncunill, P.J. Cullen, C. Sullivan, Laser-induced breakdown spectroscopy (LIBS) for food analysis. Rev Food Sci Technol. 65, 80–93 (2017)
C.H. Feng, Optimizing Procedures of Ultrasound-Assisted Extraction of Waste Orange Peels by Response Surface Methodology. Molecules, 27(7), 2268 (2022). https://doi.org/10.3390/molecules27072268
T. Kumar, A.K. Rai, A. Dwivedi, R. Kumar, A.K. Rai, Investigation and comparison of nutritional supplements (elements and compounds) in various tea leaves using spectroscopic techniques. Proc. Natl. Acad. Sci., India, Sect. A Phys. Sci. 93(2), 413–422 (2023). https://doi.org/10.1007/s40010-023-00815-1
T. Kumar, P.K. Rai, A.K. Rai, N.K. Rai, A.K. Rai, C.G. Parigger, G. Watal, S. Yadav, Elemental laser-plasma analysis of pointed gourd leaves for diabetes management. Foundations 2(4), 981–998 (2022). https://doi.org/10.3390/foundations2040066
T. Kumar, A.K. Rai, A.K. Rai, Rapid authentication and evaluation of minerals in milk powder using laser-induced breakdown spectroscopic (LIBS) technique. J. Opt. 1–13. (2023). https://doi.org/10.1007/s12596-023-01265-z
T. Kumar, A. Dwivedi, Z. Gazali, A. Rai, A.K. Rai, Compositional Analysis of Green Tea Leaves Using Laser Induced Breakdown Spectroscopy (libs). International Symposium on Molecular. Spectroscopy (2021). https://doi.org/10.15278/isms.2021.RF04
T. Kumar, A.K. Rai, A. Dwivedi, R. Kumar, M. Azam, V. Singh, A.K. Rai, Chemical characterization for the detection of impurities in tainted and natural curcuma longa from india using LIBS coupled with PCA. Atoms 10(3), 91 (2022). https://doi.org/10.3390/atoms10030091
P.D. Pathak, S.A. Mandavgane, B.D. Kulkarni, Fruit peel waste: characterization and its potential uses. Curr. Sci. 113(3), 444–454 (2017)
M.T.H. Siddiqui, S. Nizamuddin, N.M. Mubarak, K. Shirin, M. Aijaz, M. Hussain, H.A. Baloch, Characterization and process optimization of biochar produced using novel biomass, waste pomegranate peel: a response surface methodology approach. Waste Biomass Valor. 10, 521–532 (2019). https://doi.org/10.1007/s12649-017-0091-y
S. Rafiaee, M.R. Samani, D. Toghraie, Removal of hexavalent chromium from aqueous media using pomegranate peels modified by polymeric coatings: effects of various composite synthesis parameters. Synth. Met. 265, 116416 (2020). https://doi.org/10.1016/j.synthmet.2020.116416
D. Kanaujia, V. Kumar, S.K. Dwivedi, G. Prasad, Photobiosynthesis of silver nanoparticle using extract of Aspergillus flavus CR500: its characterization, antifungal activity and mechanism against Sclerotium rolfsii and Rhizoctonia solani. J. Clust. Sci. 31, 1041–1050 (2020). https://doi.org/10.1007/s10876-019-01709-2
C. Trilokesh, K.B. Uppuluri, Isolation and characterization of cellulose nanocrystals from jackfruit peel. Sci. Rep. 9(1), 1–8 (2019). https://doi.org/10.1038/s41598-019-53412-x
S. Ben-Ali, A. Akermi, M. Mabrouk, A. Ouederni, Optimization of extraction process and chemical characterization of pomegranate peel extract. Chem. Pap. 72(8), 2087–2100 (2018). https://doi.org/10.1007/s11696-018-0427-5
X. Liu, IR spectrum and characteristic absorption bands (Organic Chemistry I; Kwantlen Polytechnic University, Surrey, BC, Canada, 2021)
H. Cui, D. Surendhiran, C. Li, L. Lin, Biodegradable zein active film containing chitosan nanoparticle encapsulated with pomegranate peel extract for food packaging. Food Packag. Shelf Life 24, 100511 (2020). https://doi.org/10.1016/j.fpsl.2020.100511
A. Ali, Y. Chen, H. Liu, L. Yu, Z. Baloch, S. Khalid, L. Chen, Starch-based antimicrobial films functionalized by pomegranate peel. Int. J. Biol. Macromol. 129, 1120–1126 (2019). https://doi.org/10.1016/j.ijbiomac.2018.09.068
Acknowledgements
None declared.
Funding
There was no specific grant for this research from any funding source in the public, private, or non-profit sectors.
Author information
Authors and Affiliations
Contributions
Material preparation, data collection, and analysis of the LIBS experiment were performed by U.N, S.S, and T.K, and first draft of the manuscript was written by U.N, S.S, and T.K. All authors edited and commented on previous version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest:
No author has disclosed any conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Noor, U., Soni, S., Kumar, T. et al. An approach to evaluate and characterize Punica granatum peel through multiple spectroscopic techniques. J Opt (2023). https://doi.org/10.1007/s12596-023-01479-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12596-023-01479-1