Abstract
A number of reports during the last few decades have shown that human fertility has been declining with rising environmental pollutants. Epidemiological and experimental studies performed during the last three decades have established that exposure to excess hexavalent chromium [Cr(VI)] as one of the major threats to male reproductive health. Rapid industrialization and inappropriate discharge of Cr-contaminated effluents from a variety of industries contribute to environmental Cr(VI) contamination. Consumption of excess Cr(VI) either through drinking water or air or food adversely affects spermatogenesis and steroidogenesis in the testis and post-testicular sperm maturation in the epididymis, leading to subfertility/ infertility. The multiprong attacks of Cr(VI) lead to sperm anomalies such as low sperm count, reduced sperm motility and viability, compromise in the integrity of acrosome, damage to blood-testis barrier in Sertoli cells leading to disruption of spermatogenesis at round spermatid stage. Leydig cells, present in the interstitial compartment of the testis, are extremely vulnerable to Cr(VI) toxicity resulting in subdued activity /function of the key components of steroidogenic machinery, culminating in hypoandrogenism, which in turn affects the regulation of spermatogenesis and post-testicular sperm maturation. The present review attempts to enlighten the readers about the points mentioned above. We propose the hypothesis “exposure to excess Cr(VI) during critical periods of differentiation and maturation of the testis disrupts the hypothalamo-hypophyseal-testicular axis by inducing oxidative stress and thus, affecting male fertility.”
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human fertility has been dwindling, and the male factor is thought to be responsible for roughly 15–20% of infertility cases (Choy and Eisenberg 2018; Calogero et al. 2021). Men's sperm count and sperm quality have been consistently declining worldwide (Geoffroy-Siraudin et al. 2012). The causes of male infertility are complex and the reason for around half of infertility is unclear. Although genetic factors can account for a small portion of male infertility, rising environmental pollution might also contribute to the steady rise in male infertility (Nordkap et al. 2012; Gao et al. 2015). Males are exposed to environmental pollutants throughout their life. Fetal and postnatal exposure to environmental pollutants inhibit spermatogenesis leading to male subfertility/infertility (Skakkebaek et al. 2001). Fetal exposure to environmental chemicals leads to reproductive tract anomalies such as cryptorchidism and hypospadias, testicular cancer, and subfertility/infertility in males, referred to as Testicular Dysgenesis Syndrome (TDS) (Skakkebaek et al. 2001). A rise of cryptorchidism and testicular cancer might significantly contribute to male subfertility/infertility (Jorgensen et al. 2011; Loebenstein et al. 2019).
Many molecules present in the environment such as xenobiotics, chemicals, and heavy metal pollutants, classified as ‘environmental endocrine disruptors or endocrine-disrupting chemicals (EDCs)’ interfere with the synthesis, secretion, and action of endogenous hormones in humans and wildlife (Kavlock et al. 1996; Diamanti-Kandarakis et al. 2009; Gore et al. 2015). These EDCs are linked to genetic mutations, stunted growth, repeated miscarriages, fetal growth restriction, preeclampsia, preterm birth, and declining fertility (Diamanti-Kandarakis et al. 2009; Krieg et al. 2016; Schjenken et al. 2021). The reproductive system is highly sensitive to EDCs and their adverse effect on reproductive health include reduced fertility, poor semen quality, and growth rate among humans and other animals, which may be a stress response too (Rehman et al. 2018; Sharma et al. 2020). Among various heavy metals, chromium (Cr) is an established endocrine disruptor and reproductive toxicant. It is extremely deleterious to the growing fetus, as these can cross the feto-placental barrier (Saxena et al. 1990a, b; Kumar et al. 2017; Banu et al. 2018). A few epidemiological studies and many experimental studies on animals clearly indicate Cr(VI) as a reproductive toxicant (World Health Organization 2003; Campbell 2009; Banu et al. 2008, 2011, 2015, 2016, 2018; Sivakumar et al. 2014; Kumar et al. 2017; Shobana et al. 2020; Navin et al. 2017; 2021). Increased incidence of birth and developmental defects among children born in families living around various industries using Cr(VI) further bolster this metal as a potent threat to human reproductive health (Blacksmith Report 2007). Extensive studies in monkeys and rats have established that Cr(VI) directly targets the testis and epididymis (Aruldhas et al. 20042005, 2006; Subramanian et al. 2006). The purpose of this review is to define Cr(VI)-induced reproductive toxicity, which includes substantial research on the metal's negative effects on spermatogenesis and steroidogenesis.
Environmental Endocrine Disruptors
The Wingspread work session in 1991 on ‘‘Chemically Induced Alterations in Sexual Development: The Wildlife /Human Connection’’ (Colborn and Clement 1992) kickstarted the research on environmental endocrine disruptors. A consensus from that meeting, reached by a panel of expert scientists was that ‘‘many compounds introduced into the environment by human activity are capable of disrupting the endocrine system of animals, including fish, wildlife, and humans (Hotchkiss et al. 2008). As per United States Environmental Protection Agency (US EPA), “EDC is an exogenous agent that interferes with the production, release, transport, metabolism, binding, action, or elimination of natural hormones in the body and is responsible for homeostasis, reproduction, and developmental processes (Kavlock et al. 1996). EDCs exert their effects on endocrine and reproductive systems through nuclear receptors, non-nuclear steroid hormone receptors (membrane estrogen receptors), non-steroid receptors (neurotransmitters such as serotonin, dopamine, norepinephrine receptors), orphan receptors (aryl hydrocarbon receptor), and enzymatic pathways controlling steroid biosynthesis and/or metabolism (Erkekoglu and Kocer-Gumusel 2016). In both animals and humans, EDCs are the primary causes of male infertility (Skakkebaek et al. 2001). Globally, rising rates of hypospadias, cryptorchidism, testicular germ cell cancer, and decreased sperm count, and fertility rates have prompted concerns about the effects of EDCs on male reproductive health (Sharpe and Skakkebaek 1993; Acerini and Hughes 2006; Nordkap et al. 2012).
Heavy metals are bio-persistent toxic pollutants that accumulate in the top-of-food-chain organisms (Scheifler et al. 2006). Apart from natural causes such as seepage from rocks, volcanic activity, and forest fires, the main sources of heavy metals in the environment are anthropogenic-industrial activities and motor vehicles (INSA 2011). Humans exposed to polluted air, water, and/or food with heavy metals have neurodegenerative and cardiovascular diseases, increased risk of cancer, developmental disorders, and reproductive dysfunction (EEA 2005). Lead(Pb), mercury(Hg), arsenic (As), and chromium (Cr) are the most common heavy metal contaminants that have been linked to endocrine disruption and reproduction (Amadi et al. 2017).
Hexavalent Chromium [Cr(VI)]: A Heavy Metal Environmental Pollutant and EDC
Cr(VI) is one of the top ten pollutants of the environment (Pure earth 2015) that contaminates land and water in the environment (Wuana and Okieimen 2011). Cr toxicity in humans normally occurs from exposures to environmental pollution via soil and water contamination or occupational exposure (Garg et al. 2012). Cr concentrations in the environment have risen dramatically as a result of rapid industrialization (Bhattacharya et al. 2019). The high concentration of Cr in the environment is due to an inappropriate discharge of Cr-contaminated effluents from a variety of industries such as tanning, electroplating, steel production, ore refining, automobile manufacturing, alloy, ammunition, and wood treatment (De Flora, 2000; Bagchi et al. 2002; Fernandez et al. 2018). Industrial products (pigments for textile dyes, glassware cleaning solutions, metal finishing, paints, inks, and plastics) and their waste are the prominent sources for the presence of Cr in the soil and water (Ferreira et al. 2019). Cigarette smoke and automobile emissions are also additional sources for the contamination of the environment through the release of Cr(VI) (Zhu et al. 2020).
Total Cr exposure limits have been set at 5 mg/m3 per typical working day due to substantial health risks, while the limit is 100 mg/l in drinking water (Wilbur et al. 2012). However, Cr(VI) concentrations in groundwater have been found to be higher than the permitted limit in many countries (Homa et al. 2016; Ahmed and Mokhtar 2020; Khan et al. 2020; Paithankar et al. 2021).
Mechanism of Toxicity of Cr(VI)
Cr is the world’s seventh most abundant element (Cervantes et al. 2001), and exists in –2 to + 6 oxidation state, albeit only Cr(III) and Cr(VI) are commonly found within the pH and redox potential ranges seen in environmental systems (Shupack 1991). Cr(VI), the most toxic form, is found as oxyanions like chromate (CrO42−) or dichromate (Cr2O72−), whereas Cr(III) is found as oxides, hydroxides, sulfates, or organically bound in the soil and aquatic environments (Zayed and Terry 2003).
Due to its structural resemblance to the tetrahedral sulfate and phosphate anions, Cr(VI) is easily absorbed by cells via sulfate transporters; the chromate anion (CrO42−) also enters the cell via active sulfate transporters (Collins et al. 2010). Cr(III) is not actively transported, but enters cells through slow diffusion; once Cr(VI) is absorbed into the cell, it is reduced to Cr(III), via low molecular weight thiols such as glutathione and cysteine and antioxidants such as ascorbate; all cell types can take up Cr(VI) and reduce it to Cr(III) with the help of intracellular reductants like ascorbate and glutathione (Costa 2003; Xu et al. 2004, 2005; McCarroll et al. 2010, Zhitkovich 2011; Sun et al. 2015). The intracellularly reduced Cr(III) binds directly to DNA, forming Cr(III)-DNA adduct and causes DNA–protein cross-linkages to form stable DNA adducts (Cohen et al.1993; Costa et al. 1997), leading to mutagenesis. In addition, the reduction of Cr(VI) within the cell leads to the formation of unstable Cr(V) and Cr(IV) and thiol radicals; Cr(VI) also reacts with oxygen to form reactive oxygen species (ROS), such as hydroxyl radicals and hydrogen peroxide radicals (Borges et al. 1991; Lay and Levina 1998;. Zhitkovich 2011) Intermediate Cr forms catalyze Fenton reaction in the presence of H2O2 and generate highly reactive OH° radicals (Shi and Dalal 1990; Zhitkovich 2011. The production of Cr(VI) intermediates and ROS cause damage to DNA and mutagenesis (McNeill and McLean 2012). Thus, oxidative stress-induced genomic instability and epigenetic changes are considered as the primary causes of Cr-induced health adversity (Wise et al. 2008; Chen et al. 2019).
Within the cell, glutathione, ascorbate, cysteine, lipoic acid, NADH, fructose, and ribose are all possible enzymes and non-enzymatic cellular reductants capable of reducing Cr(VI) (Quievryn et al. 2003). Glutathione and cysteine may be the most critical cofactors for Cr(VI) reduction in the cell, but ascorbic acid in the presence of NAD/NADH, microsomal cytochrome P450, and proteins like hemoglobin and glutathione reductase may also be involved (Dayan and Paine 2001). Cr(VI) in the blood is taken up into red blood cells, where it undergoes reduction and forms stable complexes with hemoglobin and other intracellular proteins (USEPA 2010).
Male Reproductive Toxicity of Cr(VI): Epidemiological Studies
Epidemiological studies have proven a negative correlation between exposure to EDCs and male reproductive health (Thankamony et al. 2009; Bornehag et al. 2012). The deleterious effect of EDCs on reproductive health includes delayed pubertal timing, the development of testicular dysgenesis syndrome-particularly a rise in germ cell malignancies in Caucasian populations around the world (Parent et al. 2015; Skakkebaek 2016). Cr, a heavy metal EDC, can be absorbed and passed to fetuses through the placenta and to newborns through breast milk, and it is primarily eliminated in urine (USEPA 2005). Shmitova (1980) found that pregnant women working in a dichromate manufacturing factory in Russia had abortions by 12 weeks; Cr levels in umbilical cord blood, placenta, breast milk, and the aborted fetuses were all significantly higher in these mothers. The above study also reported complications during pregnancy and labor in 20/26 exposed women who had high levels of Cr in their blood and urine. Collectively, these reports suggest that Cr can cause developmental abnormalities. Increased incidences of birth and developmental defects among children born to mothers living around tanneries, chrome, and leather industries in the developing world may attest to the adverse effect of Cr on reproduction (Blacksmith report 2007).
Sperm Anomalies with Cr(VI) Exposure
Disruption of spermatogenesis in men can reduce total sperm count, increase aberrant sperm count, affect sperm chromatin integrity, or damage sperm DNA at any stage of cell differentiation (Pizent et al. 2012). Worsening of the semen quality is one of the main contributing factors in declining human fertility (Danadevi et al. 2003; Murawski et al. 2007). The epidemiological testimonies attest abnormal sperm parameters and infertility due to occupational Cr exposure. Among 3119 metal workers, specifically, welders who were investigated for sperm quality showed inferior sperm quality compared with their non-welder counterparts (Mortensen 1988). Bonde (1990a) examined the semen quality of 35 stainless steel welders, 46 mild steel welders, and 54 non-welding metal workers and electricians, and found unaltered sperm concentration either in mild steel or stainless-steel welders; however, sperm count per ejaculate, the proportion of normal sperm, the degree of sperm motility and linear penetration rate were significantly decreased. Subfertility in 673 welders from the Danish population in the same geographical area showed a higher prevalence of delayed conception in 79% of wives of the welders (Bonde et al. 1990b). Poor semen quality, sperm abnormalities, and infertility in workers exposed to Cr(VI) in the mild steel welding industry were attributed to developmental problems including cancer in children (Bonde et al. 1993). The workers exposed to Cr(VI) in an electroplating factory in Henan, China, had decreased sperm count (by 47%) and motility, albeit no change in semen and liquefaction time observed (Li et al. 2001). The study also revealed augmentation of FSH, decreased concentration of zinc as well as reduced activities of lactate dehydrogenase (LDH-x) and total LDH in the seminal plasma of exposed workers. Since there was no data on the amount and type of Cr(VI) to which the subjects were exposed to, the validity of the findings of Li et al. as an outcome of Cr(VI) exposure was questioned (Duffus 2002). The occupational Cr exposure to 57 welding workers in welding/ electroplating industries in India revealed severe semen abnormalities and low sperm concentration (by 67%) (Danadevi et al. 2003). Specifically, the exposed workers’ sperm germline was shown to be damaged by welding fumes, demonstrating a definite link between poor semen quality and infertility in men with occupational exposure to Cr (Danadevi et al. 2003). These epidemiological investigations suggest that Cr(VI) exposure in occupational setup adversely affects the quality of sperm leading to subfertility/infertility. Though these studies pointed out the involvement of Cr in male fertility, none of them was able to prove a definitive association between Cr exposure and infertility as the involvement of other factors such as heat, and stress was not ruled out.
Cr exposure was found to cause multiple levels of sperm damage. Kumar et al. (2005) investigated sixty-one subjects occupationally exposed to Cr in a Cr2(SO4)3 manufacturing industry and fifteen control subjects (unexposed to Cr compounds); the outcome showed a positive correlation between the percentages of abnormal sperm morphology and blood Cr levels. Wirth et al. (2007) also reported an association between Cr and reduced semen quality in rodents and humans. Blood collected from 219 men and women with fertility issues in Michigan (USA) showed increased blood Cr level and serum prolactin (Meeker et al. 2009), and a non-linear decrease in serum inhibin β (Meeker et al. 2009) after adjusting for age, BMI, and smoking status, or other metals in both studies. These investigations entrench a link between multilevel sperm damage and hormonal changes with occupational Cr exposure.
Altered Sex Ratio with Cr(VI) Exposure
The sex ratio is an indicator of reproductive hazards (James 1995). Heavy metal pollutant Cr is known to alter the sex ratio. An epidemiological investigation showed alteration in sex ratio with reduced male births due to paternal Cr exposure (Cr + Ni fumes) among male workers in an Italian mint (Figa-Talamanca and Petrelli 2000). This is the only epidemiological report that showed occupational exposure to Cr modify sex ratio of progeny, albeit no evidence of the causal link between sex ratio and Cr exposure is known. James (1995) opined that sex ratio may be influenced by hormonal concentration, probably a boost in gonadotropin and diminished testosterone level favoring the birth of females. Nevertheless, more research is needed to fully comprehend the skewed males’ biased sex ratio as a result of Cr exposure.
Male Reproductive Toxicity of Cr(VI): Experimental Studies
Chromium has become a potential threat to human reproductive health (Remy et al. 2017). Many experimental studies have demonstrated Cr is an endocrine disruptor and reproductive toxicant (Aruldhas et al. 2000; 2004, 2005, 2006; Cheng et al. 2002; Subramaniam et al. 2006; Banu et al. 2008; Sekhar et al. 2011; Marouani et al. 2012; Kumar et al. 2017; Navin et al 2017, 2021; Shobana et al. 2020). Cr have adverse effects on development, and spermatogenesis, and steroidogenesis.
Accumulation of Cr
An early study in rat Leydig cells showed that Cr accumulates in the testicular interstitial cells (Danielsson et al.1984). Subsequent studies also established the uptake of Cr by the testis and the reduction of Cr(VI) to Cr(III), which remains in the tissue for a prolonged period (Sipowicz et al. 1997; Sutherland et al., 2000). Recently, our group has reported that prenatal exposure to 50, 100, and 200 ppm Cr(VI) through drinking water leads to accumulation of Cr (CrIII) in the testis of F1 progeny rats (Kumar et al. 2017; Shobana et al. 2020; Navin et al. 2021). These studies suggest that mammalian testis is a direct target of the heavy metal Cr.
Chromium Toxicity and Spermatogenesis
Mammalian testis consists of seminiferous tubules contribute to spermatogenesis (de Kretser and Kerr, 1988). Germ cells give rise to spermatozoa originated from spermatogonial stem cells in the seminiferous tubules; Sertoli cells (SCs) support spermatogenesis, and Leydig cells (LCs) secrete androgens, and peptide hormone such as insulin-like 3 (INSL3) to regulate the development of the male reproductive tract, the descent of testis and the spermatogenesis (de Kretser and Kerr 1988). SCs are essential for the assembly of the testis cords particularly during the fetal and neonatal ages, while in adult testis, SCs maintain spermatogenesis (Rebourcet et al. 2014, 2016; Smith et al. 2015). The elimination of SCs in newborn mice testis resulted in hampered development of adult Leydig cells, whereas the same in adult testis led to loss of germ cells (Rebourcet et al. 2014). The number of SCs in rodents and humans increases dramatically during fetal life, slows after birth, and approaches adult levels in early puberty (Sharpe et al. 2003; O’Shaughnessy et al. 2007; Guo et al. 2020; Tan et al. 2020).
Cr(VI) is known to be converted to Cr(III) once within the cells, passing through intermediary forms such as Cr(V), (IV), and Cr(III). Intermediate Cr forms produce extremely reactive OH radicals in the presence of H2O2 (Shi et al. 1990; Zhitkovich 2011). The intermediate forms of Cr(VI) have also been reported to be reprotoxic. Male mice exposed to high doses (1000, 2000, 5000 mg/L) of Cr(III) (CrCl3) through drinking water for 12 weeks recorded decreased weight of testis, seminal vesicles, and preputial gland in a dose-dependent manner, whereas females exposed to the same doses of Cr(III) showed fetal loss (Elbetieha and Al-Hamood 1997). Male rats exposed to 40 mg Cr(III) through drinking water for 12 weeks exhibited altered sexual behavior like reduction in the number of mounts, increased post-ejaculatory interval, decreased rate of ejaculation and aggressive behavior towards other males, and decreased absolute weight of testis, seminal vesicles, and preputial glands; male fertility indices were normal after exposure to Cr(III), although the untreated females mated with treated males exhibited increased resorptions (Bataineh et al. 1997). Cheng et al. (2002) reported increased serum corticosterone and glucose levels, and IGF-BP1 expression in the ten-week-old offspring of mice with pre-conceptional exposure to 1 mmol/kg Cr(III) (CrCl3) for two weeks before mating.
Cr(VI) exposure during fetal, neonatal, pubertal, and adulthood have a variety of negative consequences on spermatogenesis. Rabbits given 0.7 mg Cr(VI)/kg/bw intraperitoneally (i.p.) for six weeks, recorded significant edema of interstitial tissues, blood vessel congestion, and the complete absence of spermatocytes in the seminiferous tubules (Behari et al. 1978). Male rats administered with 2 or 3 mg Cr(VI)/kg/bw (i.p.) for 69 days showed decreased sperm count and motility, disturbed spermatogenesis with decreased late-stage spermatids and germ cell number at stage VII, and altered levels of testicular enzymes like sorbitol dehydrogenase, lactate dehydrogenase, λ-glutamyl transpeptidase and glucose-6-phosphate dehydrogenase (Saxena et al. 1990b). Mice fed with Cr(III) [(Cr2(SO4)3] and Cr(VI) (K2Cr2O7) at concentrations of 100, 200, and 400 ppm in the diet for 35 days which resulted in an ambiguous level of degeneration in the outermost cellular layers of the testicular seminiferous tubules, reduced spermatogonia per tubule, arrest of spermatogenesis in the resting spermatocytes stage, reduced sperm count in the epididymis, and increased percentage of morphologically abnormal sperm in all animals given Cr2(SO4)3, irrespective of the dose (Zahid et al. (1990). Testicular atrophy and reduced epididymal sperm count and motility were observed in rats administered high doses (0.5 mg/kg.bw) of Cr(VI) (i.p.), five days a week for eight weeks, which were reversed after eight weeks of the unexposed period (Ernst 1990; Ernst and Bonde 1992). Ultrastructural studies of the testis of adult rats given 2 mg Cr(VI)/kg bw/day (i.p.) for 15 days revealed leaking of SC tight junctions in seminiferous tubules, cytoplasmic vacuolization, and degeneration of mitochondria in the seminiferous epithelium and disruption of mitochondrial sheaths in the tail and midpiece of spermatids (Murthy et al. 1991a, b). Feeding of Na2CrO4 (20, 40 and 60 mg/kg daily for 90 days) to rats impaired spermatogenesis in a dose-dependent manner, coupled with reduced testicular protein, DNA, and RNA level (Chowdhury 1995); though the number of spermatogonia was not affected by the treatment, the number of resting and pachytene spermatocytes and stage-7 spermatids significantly decreased (Chowdhury 1995). A single dose (i.p.) of CrO3 (CrVI), 1.0 mg/kg.bw to Swiss mice showed significantly decreased sperm count and increased rate of sperm abnormality accompanied by the diminished activity of testicular antioxidant enzymes superoxide dismutase (SOD), peroxidase and catalase (CAT) and concentration of ascorbic acid with a concomitant increase in the level of lipid peroxidation (LPO) and H2O2 from 5 to 8th week (Acharya et al. 2006). Chowdhuri et al. (2001) reported a dose-dependent decrease in the number of sperm attaching to the ova and the content of DNA, RNA, and protein under sperm-zona binding conditions in mice exposed to Pb and Cr. Male rabbits exposed to chemical mixtures of As, Cr, Pb, chloroform, phenol, and trichloroethylene through drinking water from day 20 of pregnancy till weaning resulted in subnormal sperm quality and defective LC function (Veeramachaneni et al. 2001). Rabbits exposed to Cr(VI) (5 mg K2Cr2O7/kg/bw, given orally for 10 weeks) showed decreased testosterone level, body weight, the relative weight of testis and epididymis, and subnormal semen quality (Yousef et al. 2006). The above study also revealed increased levels of thiobarbituric acid reactive substances, and decreased activities of glutathione-s-transferase, transaminases, and phosphatases in the seminal plasma were observed as well; folic acid supplementation effectively protected Cr-induced reproductive toxicity. Chandra et al. (2007) reported reduced sperm count, serum testosterone level, and decreased accessory sex organs weight and reduced SOD and catalase activities but enhanced lipid peroxidation in adult rats administered 0.2, 0.4, and 0.6 mg/kg/bw (i.p.) Cr(VI) for 13 and 26 days. Male Wistar rats (puberal) given daily i.p. injection of K2Cr2O7 (1 or 2 mg/kg/bw) for 15 consecutive days recorded decreased testis weight and increased seminal vesicles and prostate weights (Marouani et al. 2012). Additionally, increased FSH, whereas decreased LH and testosterone titer in a dose-dependent manner were noticed along with decreased sperm motility and numerous abnormal spermatozoa in the epididymis of in Cr(VI) exposed rats. These studies indicate that Cr(VI) attacks at multiple levels to engender spermatogenic abnormalities.
Cr(VI) in drinking water, even at sub-lethal doses turn to be a male reproductive toxicant. A series of reports emanated from our laboratory (Aruldhas et al. 2000, 2004, 2005, 2006; Subramaniam et al. 2006) on a non-human primate (Macaca radiata) model entrenched the male reproductive toxicity of sublethal doses of Cr(VI) given through drinking water for a chronic period of six months. Adult monkeys exposed to 50,100, 200, and 400 ppm Cr(VI) (K2Cr2O7) in drinking water for six months experienced decreased sperm count, and sperm forward motility from the second month onwards leading to azoospermia by the sixth month (Aruldhas et al. 2000, 2005). Ultrastructural studies indicated the presence of multi-nucleated hypertrophied germ cells undergoing degeneration in the testicular and epididymal lumens of these monkeys (Aruldhas et al. 2004), which was attributed to oxidative stress (Aruldhas et al. 2005). Azoospermia in Cr(VI) treated monkeys was associated with free radical toxicity in the semen, which was reversed by supplementation of vitamin C (0.5, 1, 2 mg/L) (Subramanian et al. 2006). Cr(VI) treatment decreased sperm count, sperm forward motility, and the specific activities of antioxidant enzymes, SOD and CAT, and the concentration of reduced glutathione in both, seminal plasma, and sperm in a dose- and duration-dependent manner. The quantum of H2O2 in the seminal plasma/sperm from monkeys increased with increasing dose and duration of Cr(VI) exposure. All these changes were reversed after 6 months of the Cr-free exposure period. Simultaneous supplementation of vitamin C prevented the development of Cr-induced oxidative stress (Subramanian et al. 2006). Thus, induction of oxidative stress in the testis and its ancillary parts appears to be the possible mechanism underlying the reproductive toxicity of Cr(VI) (Aruldhas et al. 2005). Electron microscopic pictures of the testis of monkeys with chronic exposure to Cr(VI) revealed the pre-mature release of spermatocytes and round spermatids into the lumen, which underwent degeneration beginning with hypertrophy. Epididymis of monkeys exposed to Cr(VI) revealed microcanalization as an adaptive mechanism to avoid the extravasation of sperm (Aruldhas et al. 2004). The abundance of basal cells and intraepithelial macrophages and the content of LF (lipofuscin) material in these cell types of the epididymis increased in Cr(VI)-treated monkeys (Aruldhas et al. 2006). The principal cells in the epididymis phagocytosed the dead sperm from the lumen resulting from Cr(VI) exposure and processed them partially into lipofuscin (LF) material, which was acquired by the basal cells and intraepithelial macrophages and processed further (Aruldhas et al. 2006). In this study, the LF material–laden basal cells and intraepithelial macrophages appeared to leave the epithelium, accompanied by recruitment of fresh basal cells and intraepithelial macrophages. These investigations revealed that sublethal doses of Cr(VI) can reduce sperm count and sperm forward motility leading to azoospermia due to elevated oxidative stress; these effects of Cr(VI) are reversible and preventable as six months of Cr-free exposure reversed the outcome, whereas simultaneous supplementation of antioxidant vitamins protected the testis and epididymis from the toxic effects of Cr(VI).
Sekar et al. (2011) reported a temporal response of testosterone and estradiol in rats exposed to 50 and 100 ppm Cr(VI) during the gestational and lactational period. Gestational exposure to Cr(VI) increased testosterone level in prepubertal rats, but the trend was reversed by PND60, and by PND120 its level was more than that of the coeval controls; while serum estradiol level decreased in PND30, it was elevated in PND60 rats but decreased by PND120. A similar trend was noticed in rats exposed to Cr(VI) during the lactational period but for a consistent increase in both steroids in rats at PND30 and PND60, which were exposed to 50 ppm Cr(VI), but it was decreased in 100 ppm Cr(VI) treated rats. By PND90, testosterone remained elevated or normal, but by PND120 its level increased in both, 50 ppm and 100 ppm treated rats. On the contrary, serum estradiol in these rats was low by PND90 and became normal by PND 120 (Sekar et al. 2011). Cr(VI) on sex steroids may be temporal and may vary in a dose dependent manner. Besides, hypertrophy and disintegration of the prematurely released germ cells in the lumen were also observed by these authors, which may be attributed to damage to Blood-Testis Barrier (BTB). This may be deduced from the finding of the disruption of Sertoli-Sertoli tight junction and high vacuolization of SCs, round spermatids, and LCs in rats with Cr(VI) exposure during prenatal or early postnatal period (Sekar 2005; Sekar et al. 2011). In another study, gestational exposure to 50, 100, and 200 ppm Cr(VI) manifested atrophy of seminiferous tubules and interstitial edema with distorted tubular morphology and increased interstitial spaces, mainly in F1 rats exposed to 100 ppm and 200 ppm (Kumar et al. 2017). Tubular lumen showed a reduced number of spermatozoa, perturbed GCs with evidence of sloughing of spermatocytes and round spermatid into the lumen along with degenerating multinucleated GCs. Though, the changes were drastic with granulomatous tissues filling the lumen of seminiferous tubules whose epithelial lining appeared shriveled in the rats exposed to 200 ppm Cr(VI). As a result of these research, it appears that Cr(VI) exposure during pregnancy and lactation has a significant negative impact on hormonal status, as well as structural and morphological characteristics of the testis, at various ages. Outstandingly, the spermatogonial stem cells were spared by detrimental effects of Cr on the testis, and the animals were able to revert to normal spermatogenesis and steroidogenesis after withdrawal of Cr, ascertaining the reversibility of Cr(VI)-induced adverse changes in the testicular structure and functions.
In a mammalian testis, the BTB is formed by a specific junction between adjacent SCs at the basement membrane in the seminiferous tubule (Wu et al. 2019). Cr(VI) is known to target BTB and induce its disruption by attacking specific proteins associated with it. The intermediate form Cr(V) affects the functional integrity of the BTB leading to testicular injury. Pereira et al. (2002) administered 0.5 ml Cr(V) [Cr(V)-BT]2−, (BT: bis(hydroxyethyl) Amino tris (hydroxymethyl) methane) to adult mice for 5 days resulted in disruption of BTB as evident from permeability check where Cr(V) permeated to intracellular space, intercellular junctions between the neighboring SCs and reached the adluminal compartment of SC. The findings suggest that Cr(V) may be involved in the bio-toxic process of testis damage by disrupting the BTB. Further investigations revealed seminiferous epithelium abnormalities including intraepithelial vacuolation and degeneration of SCs, spermatocytes, and spermatids accompanied by the premature release of germ cells into the tubular lumen of [Cr(V)-BT]2− exposed mice (Pereira et al. 2005). Additionally, a reduction in sperm acrosome integrity was also observed, albeit sperm motility and density were unaltered. These studies witness that Cr species in intermediate oxidation states are the potential reproductive hazards.
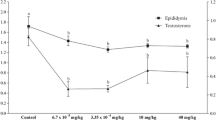
Cr(VI) exposure to twenty-one-day-old rats caused delocalization of the gap junction protein connexin 43 from the membrane to the cytoplasm of SCs (Carettte et al. 2013). Exposure to low concentrations of Cr(VI) (10 μg/l) increased the trans-epithelial resistance without altering claudin-11 and N-cadherin proteins, indicating Cr(VI)-induced alteration of connexin-43 indirectly mediates the effect of this metal on the dynamics of BTB. In another study, TM4 SC lines challenged with 0, 3.125, 6.25, 12.5, 25 or 50 μ M Cr(VI) for 24 h affected the differentiation and self-renewal mechanisms of spermatogonial stem cells (SSCs) along with decreased tight junction (TJ) signaling and cell receptor molecules like tight junction protein-1 (Tjp1), occluding (Ocln) and vimentin (Vim) that impaired secretory functions (Das et al. 2015). All reports on the response of testicular architecture at light microscopic and electron microscopic level, that emanated from our laboratory established the TJ between SCs and that between SC and gem cells undergo distortion due to Cr(VI) exposure, irrespective of the period of exposure, either during prenatal, early postnatal or adulthood (Kumar et al. 2017; Shobana et al. 2020). We have reported that gestational exposure to 50, 100, and 200 ppm Cr(VI) lead to atrophy of seminiferous tubules and disruption of SCs TJ by diminishing the levels of TJ proteins, occludin and claudin-11 (Kumar et al. 2017) (Fig. 1). Besides, the above study also revealed decreased FSHR and AR levels in SCs of postnatal rats with transient gestational exposure to Cr(VI). In a recent study we attempted to understand the molecular mechanism by which transient gestational exposure to Cr(VI) diminishes AR and FSHR in SCs of postnatal rats by testing the response of specific transcription factors that control the expression of Ar and Fshr in prepubertal rats. This study attributed diminished expression of FSHR and AR to attenuation of their specific transcriptional regulators (USF-1, USF-2, SF-1, c-fos, c-jun, GATA 1, Sp-1, ARA54, SRC-1, and CBP) and their interaction with the respective promoter by Cr(VI) (Shobana et al. 2020). Thus, Cr(VI) exposure can disrupt BTB in SCs leading to impaired spermatogenesis by diminishing the expression of Ar and Fshr by its adverse effect on specific transcription factors that control the expression of the gene through interference with the protein interaction of these transcription factors with the promoter region of Ar and Fshr. One of the major causative factors for this adverse effect of Cr(VI) on SC appears to be the induction of oxidative stress (Fig. 2).
Cr(VI)-induced oxidative stress disrupts spermatogenesis through altering gene expression: Cr(VI) enters the cell through sulfate/ phosphate anion channels located on the plasma membrane, gets reduced to Cr(III) by passing through intermediate products such as Cr(V), Cr(IV) along with generation of large quantity of ROS that damages lipid, proteins, and dissolve DNA. Cr(III), the stable metabolite of Cr(VI), binds with DNA to form Cr(III)-DNA adduct; severe cellular injuries cause cell cycle arrest leading to apoptosis. The increase in ROS induces a decrease in antioxidants (SOD, CAT, GPx), nutrients status (retinoic acid, tocopherol, lactate, and pyruvate), albeit ascorbic acid increased; SCs specific AMH and inhibin hormones level, and receptors of androgen and estrogen decreased, leading to cellular homeostatic disruption. In addition, Cr(VI)-induced oxidative stress adversely affects ectoplasmic specialization around a sperm head [Fig. EM (A)], damages mitochondria and vacuolated cytoplasm with enriched electron dense bodies in SCs [Fig. EM (B)]; and prominently damages BTB in SCs [Fig. EM (C) & (D)], all of which lead to disruption of spermatogenesis (Aruldhas et al. 2004, 2005, 2006, Subramaniam et al. 2006, Zhitkovich et al. 2005, 2011a; Sekar et al. 2005, 2011; Das et al. 2015; Kumar et al. 2017; Shobana et al. 2020, Navin et al. 2017, 2021). Abbreviations: OH°, Hydroxyl radicals; SOD, Superoxide dismutase; ) O2-, superoxide radicals, GPx, Glutathione Peroxidase, CAT, Catalase, ROS, Reactive oxygen species; BTB, Blood–Testis Barrier, DS DNA, Double stranded DNA; SS DNA, Single stranded DNA; PKA, Protein kinase A; AMH, Anti-Mullerian hormone; TJ proteins, Tight junction proteins; FSH, Follicle-stimulating hormone; FSHR, FSH receptor; AR, Androgen receptor; ER, Estrogen receptor; EM, Electron microscopy
Cr(VI) affects interaction of transcription factors with the Fshr promoter: The interaction of USF-1 and USF-2, the transcription factors of Fshr, with their specific response element in the promoter region is inhibited by Cr(VI)-induced oxidative stress and Cr(III), resulting in interference with transcription initiation and attenuation of Fshr. Reduced FSHR reduces the tight junction proteins occludin-1 and claudin-11 and disrupts BTB in SCs, resulting in impairment of spermatogenesis (Kumar et al. 2017; Shobana et al. 2020). Abbreviation: ROS, Reactive oxygen species; USF-1, Upstream stimulatory factor-1; USF-2, Upstream stimulatory factor-2; E-box, Enhancer box; BTB, Blood-Testis Barrier; SCs, Sertoli cells; FSHR, Follicle-stimulating hormone
Developmental toxicity of Cr
Cr(VI)-exposure is detrimental to embryos and fetuses in the animals (Junaid et al 1996a, b; Marouani et al. 2011). Trivedi et al. (1989) exposed mice to 250, 500, and 1000 ppm Cr(VI) daily through drinking water during the entire gestational period and found decreased fetal weight, increased resorption, and increased abnormalities (tail kinking and delayed cranium ossification), as well as the complete absence of uterine implantation in the high-dose group in Cr(VI)-exposed mice. Cr levels in the placenta and fetal tissue of rats and mice exposed to Cr(VI) in drinking water during pregnancy have been found to be elevated, implying that Cr can pass through the placenta (Saxena et al. 1990a). From gestational day 6 to14, pregnant female Swiss albino mice given drinking water containing 250, 500, or 750 ppm K2Cr2O7 experienced fetal growth retardation, reduction in the number of fetuses per dam in general, increased incidence of stillbirths, and post-implantation loss in mothers treated with 500 and 750 ppm Cr (Junaid et al. 1996a, b). Al-Hamood et al. (1998) found that gestational or lactational exposure to 1000 ppm Cr(III) (CrCl3)/CrVI (K2Cr2O7) led to the lower weight of male and female reproductive organs, delayed sexual development, and a reduction in the number of implantation and fetuses in adult male and female mice. On oocyte fertilization and subsequent embryo development, Cr(VI)-treated sperm have shown a deleterious effect. In an in vitro investigation, sperm from mice exposed to Cr(VI) concentrations of 0, 3.125, 6.25, 12.5, 25, or 50 M for 3 h showed decreased sperm viability and acrosome reaction when used for IVF of oocytes (Yoisungnern et al. 2016). With increasing levels of Cr(VI) exposure, IVF success was lowered by increasing developmental arrest at the two-cell stage of embryos and delaying blastocyst formation. These studies witness the amount of toxicity of Cr on the development.
Chromium Toxicity and Steroidogenesis
Steroid hormones regulate critical phases of development and are essential for physiological homeostasis. LCs of the testis are responsible for producing T, which is essential for spermatogenesis and maintenance of secondary sexual functions. Steroidogenesis is a multi-step biochemical process involved in the production of steroid hormones from cholesterol (Savchuk 2017). Endocrine disruptors, including Cr, can influence T production.
Cr(VI) exposure [Na2CrO4 (20, 40 and 60 mg/kg daily for 90 days)] to rats decreased LCs population with altered nuclear diameter, 3β-hydroxysteroid dehydrogenase (3β-HSD) activity, and sub-normal serum testosterone level, whereas testicular cholesterol concentration increased (Chowdhury 1995). Neonatal rats (PND30) of mothers exposed to 50 ppm Cr(VI) during gestational period exhibited enlarged LCs with huge vacuoles harboring cell debris within smooth endoplasmic reticulum (SER), whereas 100 ppm Cr(VI) treated rats showed disorganized LCs (Sekar et al. 2011). Abundant lipid inclusions, swollen mitochondria with collapsed cristae, and vacuoles in the SER were also reported in the above paper. In the pubertal rats (PND60) large vacuoles containing cell debris were present only in LCs of experimental rats exposed to 50 ppm and 100 ppm Cr(VI), whereas LCs of adult animals (PND90) had normal organization. However, in the F1 progeny of mothers that were exposed to 50 ppm and 100 ppm Cr(VI) during gestation, transmission electron microscopy revealed LCs with damaged mitochondria and macrophages with phagocytosed cell debris, although the LCs appeared fully normal by PND120. Sekar et al. (2011) also investigated the impact of lactational exposure to Cr(VI) on the testis and observed by PND30 nascent ALCs containing fewer mitochondria in 50 ppm Cr(VI) treated rats, whereas rats with 100 ppm Cr(VI) exposure showed LCs with collapsed mitochondrial cristae. In PND60 old rats with lactational exposure to 50 ppm Cr(VI) LCs contained abundant lipid inclusions, whereas by PND90 rats exposed to 100 ppm Cr(VI) during the lactational period had large vacuoles in the LCs. However, in PND120 old rats with lactational exposure to Cr(VI) at 100 ppm concentration, the LCs were normal (Sekar et al. 2011). Recently we reported disruption of steroidogenic apparatus in LCs of puberal rats with transient prenatal exposure to Cr(VI) (Navin et al. 2021). Prenatal exposure to 50, 100 and 200 mg/L Cr(VI) disrupted the steroidogenic pathway in the F1 rats by decreasing steroidogenic acute regulatory protein (StAR), cytochrome P450 11A1 (CYP11A1), cytochrome P450 17A1 (CYP17A1), 3β- and 17β-hydroxysteroid dehydrogenases (3β-HSD and 17β-HSD), cytochrome P450 aromatase and 5α reductase proteins leading to diminished testosterone level (Navin et al. 2021). The investigations also revealed decreased specific activity of key steroidogenic enzymes 3β-HSD and 17β-HSD, and increased LH, FSH, and E2, and diminished PRL, and T levels with the reduction in LHR, FSHR, AR, and ERα and augmentation in ERβ. The diminution of LHR and FSHR led to testicular resistance to gonadotropins, while AR and ERα disrupted local regulatory network in the steroidogenic machinery of LCs (Fig. 3). In an in vitro study, Das et al. (2015) challenged TM3 LC lines with 0, 3.125, 6.25, 12.5, 25 or 50 μM Cr(VI) for 24 h and found disruption of steroidogenic machinery in LCs by diminishing Cyp11a1 and 3βHsd, and augmenting Star and Cyp17a1 gene expression.
The potential sites of action of Cr(VI) on the steroidogenic machinery of Leydig cells (LCs): Cr(VI) passes through the cell membranes which on internalization get reduced to the stable Cr(III), which in turn affects androgen biosynthesis by repressing LHR and PRLR, attenuating cholesterol transport by StAR protein, and represses the expression and/or activity of different steroidogenic enzymes (e.g. CYP11A1, 3βHSD, CYP17A1, 17βHSD). Cr(VI) promotes testicular resistance to LH due to diminished LHR. Attenuation of functioning of these target proteins by Cr(VI) leads to subnormal steroidogenesis in LCs and impairment of androgen-dependent physiological processes. Resistance of SCs FSHR to FSH leads to decreased synthesis of paracrine factors (activin, inhibin, IGF-1, and ABP) controlling steroidogenesis in LCs, resulting in hypoandrogenism (Navin et al. 2021). Abbreviations: LH, luteinizing hormone; LHR, luteinizing hormone receptor; PRL, prolactin; PRLR, prolactin receptor; FSH, follicle stimulating hormone; FSHR, follicle stimulating hormone receptor; AC, adenylate cyclase; cAMP, cyclic AMP; PKA, protein kinase A; StAR, Steroidogenic acute regulatory protein; arom, P450aromatase; 5αR-1, 5α reductase-1; DHT, dihydrotestosterone; AR, Androgen receptor, ERα, Estrogen receptor α, ERβ, Estrogen receptor β; IGF-1, Insulin-like growth factor1; ABP, Androgen binding protein
Fetal development is a critical period as dynamic interaction occurs between the maternal/external environments and the developing organs and tissues (Drago et al. 2020). Neonatal rats (PND1) of mothers with transient gestational exposure to 50, 100, and 200 ppm Cr(VI) during the gestational period augmented StAR and CYP11A1 proteins, and diminished 3β-HSD and CYP17A1 proteins that led to compromised steroidogenic functions in LCs (Navin et al. 2017). Besides, the study also noticed histopathological changes such as shrunken and dispersed tubules with fewer gonocytes, extensive vacuolization of seminiferous cord accompanied by damaged epithelium, and shrunken LCs present in large interstitial spaces and loose compaction of cells revealing hampered fetal differentiation of testicular cells. In another study, female pregnant rats, gavaged with Cr(VI) [potassium dichromate (0, 3, 6, and 12 mg/kg)] exhibited biphasic effects on fetal LCs development; while low dose (3 mg/kg) stimulated testosterone production, high dose (12 mg/kg) inhibited it (Zheng et al. 2018). In addition, Cr(VI)-exposure reduced LCs size and cytoplasmic size with a concomitant decrease in Lhcgr, Hsd17b3, and Igf1 mRNA at high dose, whereas low dose of the metal augmented Lhcgr, Scarb1, and Hsd3b1 mRNA. Overall, these investigations entrench that Cr(VI) can bring about structural changes and interfere with both, enzymatic and non-enzymatic proteins of the steroidogenic apparatus, culminating in altered testosterone status.
Neuroendocrine Toxicity of Cr: An Indirect Effect on Male Reproduction
Many chemicals discharged into the environment as a result of industrial activities are known to impair the endocrine signaling programs. Thus, gametes, pregnant women, and developing fetuses are especially vulnerable to the damaging effects of environmental toxins (Caserta et al. 2011; Shah and Balkhair, 2011; Vecoli et al. 2016). For example, Cr is a known endocrine disruptor (Kumar et al. 2017; Shobana et al. 2017, 2020; Navin et al. 2017, 2021; Banu et al. 2018) and is emitted by industrial plants, adversely affects both male and female reproductive health by acting on the hypothalamic-pituitary—gonadal axis. Shreds of evidence show that Cr can alter the hormonal balance by affecting the neuroendocrine system, interrupting the secretion of androgens from LCs and inhibin β from SCs (Jensen et al. 2006). Quinteros et al. (2007) found that Cr(VI) accumulated in the pituitary and hypothalamus, resulting in oxidative stress and a reduction in serum prolactin (PRL). The preceding investigation revealed oxidative stress in the pituitary, which resulted in apoptosis, as evidenced by nuclear fragmentation and caspase 3 activations, both in vivo (adult male Wistar rats were subjected to 500 ppm Cr(VI) for 30 days) and in vitro. The study suggests that the pituitary could be the target for Cr(VI) toxicity, and Cr accumulation in the hypothalamic-pituitary axis might have an adverse influence on the neuroendocrine control of reproductive function. In another study, rats were given 100 ppm Cr(VI) (K2Cr2O7) in their drinking water for 30 days, which caused oxidative stress and elevated Cr concentration in the hypothalamus and pituitary gland (Nudler et al. 2009). The authors reported increased lipid peroxidase (LPO) in both the hypothalamus and pituitary, whereas it augmented the same only in the pituitary and glutathione reductase (GR) activity in the hypothalamus alone. Besides, the expression of SOD and other markers of oxidative stress also showed organ-specific variations; while hemeoxigenase-1 (HO-1) expression increased in both hypothalamus and pituitary, metallothionein-1 (MT-1) and MT-3 expression increased in anterior pituitary and hypothalamus, respectively. Together, the investigations established that chronic exposure to Cr(VI) produces excessive oxidative stress on the hypothalamus and pituitary gland, which negatively impact the reproductive neuroendocrine axis in male rats. In a recent study performed in our laboratory (Navin 2018) it was shown that prenatal exposure to Cr(VI) diminished hypothalamic GnRH, whereas it augmented GnRH receptor in the pituitary. The decrease in hypothalamic GnRH was accompanied by a parallel diminution of its transcription activator Kisspeptin1 and its receptor GPR54 and Pit-1 and GATA-4; it also diminished ERα, AR, aromatase and 5α reductase. Interestingly, increased immunopositivity of GnRH receptor in the pituitary was accompanied by a boost in ERα, ERβ, AR, aromatase and 5α reductase proteins in the pituitary, whereas diminished inhibin β. Taken together, the above study and our previous report (Navin 2018) point out that prenatal Cr(VI) exposure disrupts the network of hypothalamic -pituitary–testicular axis and thus the negative feedback loop of testicular steroids on gonadotropins.
Conclusion
Environmental contamination with heavy metal Cr adversely affects the testicular cells and impairs spermatogenesis and steroidogenesis leading to subfertility/ infertility. Various sperm abnormalities due to the Cr(VI) exposure are the defined causes of subfertility/infertility in the male. Cr(VI) also targets BTB which contributes to the impairment of spermatogenesis. Besides, the vulnerability of steroidogenic machinery in the LCs to Cr(VI) exposure that alters T levels also adds to the adversity of male reproduction. Cr(VI) adversely affects the development of the embryos and fetuses as well. The available data indicate Cr(VI) disrupts the hypothalamus-pituitary–testicular axis, and thus impairment of spermatogenesis and steroidogenesis. There are many more lacunae in our knowledge on the impact of Cr(VI) on specific functions of testicular cell types that control spermatogenesis and steroidogenesis.
References
Acerini, C.L., and I.A. Hughes. 2006. Endocrine disrupting chemicals: A new and emerging public health problem? Archives of Disease in Childhood. 91: 633–641. https://doi.org/10.1136/adc.2005.088500.
Acharya, U.R., M. Mishra, R.R. Tripathy, and I. Mishra. 2006. Testicular dysfunction and antioxidative defense system of swiss mice after chromic acid exposure. Reproductive Toxicology. 22: 87–91. https://doi.org/10.1016/j.reprotox.2005.11.004.
Ahmed, M.F., and M.B. Mokhtar. 2020. Assessing cadmium and chromium concentrations in drinking water to predict health risk in Malaysia. International Journal of Environmental Research and Public Health. 17(8): 2966. https://doi.org/10.3390/ijerph17082966.
Al-Hamood, M.H., A. Elbetieha, and H. Bataineh. 1998. Sexual maturation and fertility of male and female mice exposed prenatally and postnatally to trivalent and hexavalent chromium compounds. Reproduction Fertility Development. 10: 179–183. https://doi.org/10.1071/R97001.
Ali, A., Y. Ma, J. Reynolds, J.P. Wise Sr., S.E. Inzucchi, and D.L. Katz. 2011. Chromium effects on glucose tolerance and insulin sensitivity in persons at risk for diabetes mellitus. Endocrinology Practice. 17: 16–25. https://doi.org/10.4158/EP10131.OR.
Amadi, C.N., Z.N. Igweze, and O.E. Orisakwe. 2017. Heavy metals in miscarriages and stillbirth in developing nations. Middle East Fertility Society Journal. 22: 91–100. https://doi.org/10.1016/j.mefs.2017.03.003.
Aruldhas, M.M., P. Govindarajulu, and G.C. Hasan. 2000. Effect of chronic chromium exposure on sperm maturation and male fertility. In Final Technical Report of major research project sponsored by Council of Scientific and Industrial Research, Government of India, New Delhi (no. 60(0022)/EMR II/97).
Aruldhas, M.M., S. Subramanian, P. Sekhar, G. Vengatesh, G.C. Hasan, P. Govindarajulu, and M.A. Akbarsha. 2005. Chronic chromium exposure-induced changes in testicular histoarchitecture are associated with oxidative stress: Study in a non-human primate (Macaca radiata Geoffroy). Human Reproduction. 20: 2801–2813. https://doi.org/10.1093/humrep/dei148.
Aruldhas, M.M., S. Subramanian, P. Sekhar, G. Vengatesh, P. Govindarajulu, and M.A. Akbarsha. 2006. In vivo spermatotoxic effect of chromium as reflected in the epididymal epithelial principal cells, basal cells, and intraepithelial macrophages of a nonhuman primate (Macaca radiata Geoffroy). Fertility and Sterility. 86: 1097–1105. https://doi.org/10.1016/j.fertnstert.2006.03.025.
Aruldhas, M.M., S. Subramanian, P. Sekhar, G.C. Hasan, P. Govindarajulu, and M.A. Akbarsha. 2004. Microcanalization in the epididymis to overcome ductal obstruction caused by chronic exposure to chromium - a study in the mature bonnet monkey (Macaca radiata Geoffroy). Reproduction 128: 127–137. https://doi.org/10.1530/rep.1.00067.
Bagchi, D., M. Bagchi, and S.J. Stohs. 2002. Chromium (VI)-induced oxidative stress, apoptotic cell death and modulation of p53 tumor suppressor gene. Molecular and Cellular Biochemistry. 222: 149–158.
Banu, S.K., J.A. Stanley, J. Lee, S.D. Stephen, J.A. Arosh, P.B. Hoyer, and R.C. Barghardt. 2011. Hexavalent chromium-induced apoptosis of granulosa cells involves selective sub-cellular translocation of Bcl-2 members ERK1/2 and p53. Toxicology and Applied Pharmacology. 251: 253–266. https://doi.org/10.1016/j.taap.2011.01.011.
Banu, S.K., J.A. Stanley, J.A. Arosh, and M.M. Aruldhas. 2018. Environment and toxicology. Metals in female reproduction. In Encyclopedia of Reproduction, ed. T. Spencer and J. Flaws, 714–723. New York: Academic Press.
Banu, S.K., J.A. Stanley, K.K. Sivakumar, J.A. Arosh, R. Barhoumi, and R.C. Berghardt. 2015. Identifying a novel role for X-prolyl aminopeptidase (Xpnpep)2 in CrVI-induced adverse effects on germ cell nest breakdown and follicle development. Biology of Reproduction. 92: 67. https://doi.org/10.1095/biolreprod.114.125708.
Banu, S.K., J.A. Stanley, K.K. Sivakumar, J.A. Arosh, and R.C. Burghardt. 2016. Resveratrol protect ovary against chromium toxicity by enhancing endogenous antioxidant enzymes and enhancing metabolic clearance of estradiol. Toxicology and. Applied Pharmacology. 303: 65–78. https://doi.org/10.1016/j.taap.2016.04.016.
Banu, S.K., J.B. Samuel, J.A. Arosh, R.C. Berghardt, and M.M. Aruldhas. 2008. Lactational exposure to hexavalent chromium delays puberty by impairing ovarian development, steroidogenesis and pituitary hormone synthesis in developing Wistar rats. Toxicology and Applied Pharmacology. 232: 180–189. https://doi.org/10.1016/j.taap.2008.06.002.
Bataineh, H., M.H. Al-Hamood, E. Elbetieha, and I.B. Hani. 1997. Effect of long-term ingestion of chromium compounds on aggression sex behavior and fertility in adult male rat. Drug and Chemical Toxicology. 20: 133–149. https://doi.org/10.3109/01480549709003875.
Behari, J., S.V. Chandra, and S.K. Tandon. 1978. Comparative toxicity of trivalent and hexavalent chromium to rabbits: III. Biochemical and histological changes in testicular tissue. Acta Biologica Et Medica Germanica. 37: 463–468.
Bhattacharya, A., A. Gupta, A. Kaur, and D.K. MaliK. 2019. Alleviation of hexavalent chromium by using microorganisms: Insight into the strategies and complications. Water Science and Technology 79(3): 411–424. https://doi.org/10.2166/wst.2019.060.
Blacksmith Institute Polluted Places- India. Final report, 2007. January 2005- December 2007. Project implemented by Blacksmith Institute. Supported under Poverty and Environment Program (PEP), Asian Development Bank.
Bonde, J.P. 1990a. Subfertility in relation to welding. A case referent study among male welders. Danish Medical Bulletin. 37: 105–108.
Bonde, J.P. 1990b. Semen quality and sex hormones among mild steel and stainless-steel welders: A cross sectional study. British Journal of Industrial Medicine. 47: 508–514. https://doi.org/10.1136/oem.47.8.508.
Bonde, J.P. 1993. The risk of male subfecundity attributable to welding of metals. Studies of semen quality, infertility, fertility, adverse pregnancy outcome and childhood malignancy. International Journal of Andrology. 16: 1–29. https://doi.org/10.1111/j.1365-2605.1993.tb01367.x.
Borges, K.M., J.S. Boswell, R.H. Liebross, and K.E. Wetterhahn. 1991. Activation of chromium (VI) by thiols results in chromium-(V) formation, chromium binding to DNA and altered DNA conformation. Carcinogenesis 12: 551–561. https://doi.org/10.1093/carcin/12.4.551.
Bornehag, C.G., S. Moniruzzaman, M. Larsson, C.B. Lindstrom, M. Hasselgren, A. Bodin, L.B. von Kobyletzkic, F. Carlstedt, F. Lundin, E. Nanberg, B.A. Jonsson, T. Sigsgaard, and S. Janson. 2012. The SELMA study: a birth cohort study in Sweden following more than 2000 mother-child pairs. Paediatrics and Perinatal Epidemiology. 26: 456–467. https://doi.org/10.1111/j.1365-3016.2012.01314.x.
Calogero, E.A., M. Fiore, F. Giacone, M. Altomare, P. Asero, C. Ledda, G. Romeo, L.M. Mongioì, C. Copat, M. Giuffrida, E. Vicari, S. Sciacca, and M. Ferrante. 2021. Exposure to multiple metals/ metalloids and human semen quality: A cross sectional study. Ecotoxicology and Environmental Safety. 215: 112165. https://doi.org/10.1016/j.ecoenv.2021.112165.
Campbell. 2009. Evidence of the developmental and reproductive toxicity of chromium (hexavalent compounds) reproductive and cancer hazard assessment section office of environmental health hazard assessment California environmental protection agency.
Carette, D., M.H. Perrard, N. Prisant, J. Gilleron, G. Pointis, D. Segretain, and P. Durand. 2013. Hexavalent chromium at low concentration alters Sertoli cell barrier and connexin 43 gap junction but not claudin-11 and N-cadherin in the rat seminiferous tubule culture model. Toxicology and Applied Pharmacology. 268: 27–36. https://doi.org/10.1016/j.taap.2013.01.016.
Caserta, D., A. Mantovan, R. Marci, A. Fazi, F. Ciardo, C. La Rocca, C, et al. 2011. Environment and women’s reproductive health. Human Reproduction Update. 17: 418–433. https://doi.org/10.1093/humupd/dmq061.
Cervantes, C., J.C. García, S. Devars, F.G. Corona, H.L. Tavera, J.C.T. Guzmán, and R. Moreno-Sánchez. 2001. Interactions of chromium with microorganisms and plants. FEMS Microbiology Reviews. 25: 335–347. https://doi.org/10.1111/j.1574-6976.2001.tb00581.x.
Chandra, A., A. Chatterjee, R. Ghosh, M. Sarkar, and S. Chaube. 2007. Chromium induced testicular impairment in relation to adrenocortical activities in adult albino rats. Reproductive Toxicology 24(3–4): 388–396. https://doi.org/10.1016/j.reprotox.2007.07.009.
Chen, Q.Y., A. Murphy, H. Sun, and M. Costa. 2019. Molecular and epigenetic mechanisms of Cr(VI)-induced carcinogenesis. Toxicology and Applied Pharmacology. 15(377): 114636. https://doi.org/10.1016/j.taap.2019.114636.
Cheng, R.Y.S., W.G. Alvord, D. Powell, K.S. Kasprzak, and L.M. Anderson. 2002. Increased serum corticosterone and glucose in offspring of chromium (III)- treated male mice. Environmental Health Perspective 110: 801–804. https://doi.org/10.1289/ehp.02110801.
Chowdhuri, D.K., R. Narayan, and D.K. Saxena. 2001. Effect of lead and chromium on nucleic acid and protein synthesis during sperm-zona binding in mice. Toxicology in-Vitro. 15: 605–613. https://doi.org/10.1016/S0887-2333(01)00077-7.
Chowdhury, A.R. 1995. Spermatogenic and steroidogenic impairment after chromium treatment in rats. Indian Journal of Experimental Biology. 33: 480–484. https://doi.org/10.1007/s001289900194.
Choy, T.J., and M.L. Eisenberg. 2018. Male infertility as a window to health. Fertility and Sterility. 110: 810–814. https://doi.org/10.1016/j.fertnstert.2018.08.015.
Cohen, M.D., B. Kargacin, C.B. Klein, and M. Costa. 1993. Mechanisms of chromium carcinogenicity and toxicity. Critical Reviews in Toxicology. 23: 255–281. https://doi.org/10.3109/10408449309105012.
Colborn, T., and C. Clement. 1992. Chemically-induced alterations in sexual and functional development: The wildlife/human connection. Princeton: Princeton Scientific Publishing Co.
Collins, B.J., M.D. Stout, K.E. Levine, G.E. Kissling, R.L. Melnick, T.R. Fennell, R. Walden, K. Abdo, J.B. Pritchard, R.A. Fernando, T. Burka, and M.J. Hooth. 2010. Exposure to hexavalent chromium resulted in significantly higher tissue chromium burden compared to trivalent chromium following similar oral doses to male F344/N rats and female B6C3F1 mice. Toxicological Sciences. 118: 368.
Costa, M. 2003. Potential hazards of hexavalent chromate in our drinking water. Toxicology and Applied Pharmacology. 188: 15. https://doi.org/10.1016/s0041-008x(03)00011-5.
Costa, M. 1997. Toxicity and carcinogenicity of Cr (VI) in animal models and humans. Critical Reviews in Toxicology. 27: 431–442. https://doi.org/10.3109/10408449709078442.
Danadevi, K., R. Rozati, P.P. Reddy, and P. Grover. 2003. Semen quality of Indian welders occupationally exposed to nickel and chromium. Reproductive Toxicology. 17: 451–456. https://doi.org/10.1016/s0890-6238(03)00040-6.
Danielsson, B.R., L. Dencker, A. Lindgren, and H. Tjalve. 1984. Accumulation of toxic metals in male reproduction organs. Archives of Toxicology. 7: 177–180. https://doi.org/10.1007/978-3-642-69132-4_26.
Das, J., M.H. Kang, E. Kim, D.N. Kwon, Y.J. Choi, and J.H. Kim. 2015. Hexavalent chromium induces apoptosis in male somatic and spermatogonial stem cells via redox imbalance. Scientific Reports. 5: 13921. https://doi.org/10.1038/srep13921.
Dayan, A.D., and A.J. Paine. 2001. Mechanisms of chromium toxicity, carcinogenicity and allergenicity? Review of the Literature from 1985 to 2000. Human Experimental Toxicology. 20: 439–451. https://doi.org/10.1191/096032701682693062.
de Kretser, D., and J. Kerr. 1988. The cytology of the testis. In The physiology of reproduction, ed. E. Knobil, J. Neill, L. Ewing, G. Greenwald, C. Markert, and D. Pfaff, 837–932. New York: Raven Press.
De Flora, S. 2000. Threshold mechanisms and site specificity in chromium (VI) carcinogenesis. Carcinogenesis 21: 533–541. https://doi.org/10.1093/carcin/21.4.533.
de Pereira, L., R.P. Das Neves, H. Oliveira, T.M. Santos, and J.P. de Jesus. 2005. Effect of Cr(V) on reproductive organ morphology and sperm parameters: an experimental study in mice. Environmental Health. 4: 9–14. https://doi.org/10.1186/1476-069X-4-9.
Diamanti-Kandarakis, E., J. Bourguignon, L.C. Giudice, R. Hauser, G.S. Prins, A.M. Soto, R.T. Zoeller, and A.C. Gore. 2009b. Endocrine-disrupting chemicals: An endocrine society scientific statement. Endocrinology Review. 30(4): 293–342. https://doi.org/10.1210/er.2009-0002.
Drago, G., S. Ruggieri, F. Bianchi, S. Sampino, and F. Cibella. 2020. Birth cohorts in highly contaminated sites: A tool for monitoring the relationships between environmental pollutants and children’s health. Frontiers in Public Health 8: 125. https://doi.org/10.3389/fpubh.2020.00125.
Duffus, J.H. 2002. Effect of Cr(VI) exposure on sperm quality. Annals of Occupational Hygiene. 46: 269–270. https://doi.org/10.1093/annhyg/mef041.
EEA. 2005. Vulnerability and adaptation to climate change impacts in Europe: a scoping report. EEA Technical report no 7/2005.
Elbetieha, A., and M.H. Al-Hamood. 1997. Long-term exposure of male and female mice to trivalent and hexavalent chromium compounds: Effect on fertility. Toxicology 116: 39–47. https://doi.org/10.1016/S0300-483X(96)03516-0.
Erkekoglu, P., and B. Kocer-Gumusel. 2016. Environmental effects of endocrine-disrupting chemicals: A special focus on phthalates and bisphenol-A. In Environmental health risk - hazardous factors to living species, ed. M.L. Larramendy and S. Soloneski, 155–190. London: InTech Open.
Ernst, E. 1990. Testicular toxicity following short-term exposure to tri- and hexavalent chromium: An experimental study in the rat. Toxicological Letters 51: 269–275. https://doi.org/10.1016/0378-4274(90)90069-x.
Ernst, E., and J.P. Bonde. 1992. Sex hormones and epididymal sperm parameters in rats following sub-chronic treatment with hexavalent chromium. Human Experimental Toxicology. 11: 255–258. https://doi.org/10.1177/096032719201100403.
Fernandez, P.M., S.C. Vinarta, A.R. Bernal, E.L. Cruz, and L.I.C. Figueroa. 2018. Bioremediation strategies for chromium removal: Current research, scale-up approach and future perspectives. Chemosphere 208: 139e148. https://doi.org/10.1016/j.chemosphere.2018.05.1669e.
Ferreira, L.M.R., T. Cunha-Oliveira, M.C. Sobral, P.L. Abreu, M.C. Alpoim, and A.M. Urbano. 2019. Impact of carcinogenic chromium on the cellular response to proteotoxic stress. International Journal of Molecular Sciences 20: 4901. https://doi.org/10.3390/ijms20194901.
Figa-Talamanca, I., and G. Petrelli. 2000. Reduction in male births among workers exposed to metal fumes. International Journal of Epedimiology. 29: 381–383. https://doi.org/10.1093/ije/29.2.381.
Gao, Y., D.D. Mruk, and C.Y. Cheng. 2015. Sertoli cells are the target of environmental toxicants in the testis - a mechanistic and therapeutic insight. Expert Opinion on Therapeutic Targets. 19: 1073–1090. https://doi.org/10.1517/14728222.2015.1039513.
Garg, S.K., M. Tripathi, and T. Srinath. 2012. Strategies for chromium bioremediation of tannery effluent. Reviews of Environmental Contamination and Toxicology. 217: 75–140. https://doi.org/10.1007/978-1-4614-2329-4_2.
Geoffroy-Siraudin, Cendrine, Anderson D. Loundou, Fanny Romain, Vincent Achard, Blandine Courbière, Marie-Hélène. Perrard, Philippe Durand, and Marie-Roberte. Guichaoua. 2012. Decline of semen quality among 10 932 males consulting for couple infertility over a 20-year period in Marseille. France. Asian Journal of Andrology. 14: 584–590. https://doi.org/10.1038/aja.2011.173.
Gore, A.C., V.A. Chappell, S.E. Fenton, J.A. Flaws, A. Nadal, G.S. Prins, et al. 2015. EDC-2: The endocrine society’s second scientific statement on endocrine-disrupting chemicals. Endocrine Review. 36(6): E1–E150. https://doi.org/10.1210/er.2015-1010.
Guo, J., X. Nie, M. Giebler, H. Mlcochova, Y. Wang, E.J. Grow, et al. 2020. The dynamic transcriptional cell Atlas of testis development during human puberty. Cell Stem Cell 26: 262-276.e4. https://doi.org/10.1016/j.stem.2019.12.005.
Homa, D., E. Haile, and A.P. Washe. 2016. Determination of spatial chromium contamination of the environment around industrial zones. International Journal of Analytical Chemistry. 2016: 7214932. https://doi.org/10.1155/2016/7214932.
Hotchkiss, A.K., C.V. Rider, C.R. Blystone, V.S. Wilson, P.C. Hartig, G.T. Ankley, P.M. Foster, C.L. Gray, and L.E. Gray. 2008. Fifteen years after ‘“wingspread”’—environmental endocrine disrupters and human and wildlife health: Where we are today and where we need to go. Toxicological Sciences. 105(2): 235–259. https://doi.org/10.1093/toxsci/kfn030.
INSA (Indian National Science Academy, New Delhi) (2011). Hazardous metals and minerals pollution in India, A position paper. New Delhi, Angkor Publishers (P) Ltd., Noida-201301, India.
James, W.H. 1995. Offspring sex ratio as an indicator of reproductive hazards associated with pesticides. Occupational and Environmental Medicine. 52(6): 429–430. https://doi.org/10.1136/oem.52.6.429-a.
Jensen, T.K., J.P. Bonde, and M. Joffe. 2006. The influence of occupational exposure on male reproductive function. Occupational Medicine. (lond.) 56: 544–553. https://doi.org/10.1093/occmed/kql116.
Jorgensen, N.M., R. Vierula, E. Jacobsen, A. Pukkala, H.E. Perheentupa, N.E. Skakkebaek. Virtanen, and J. Toppari. 2011. Recent adverse trends in semen quality and testis cancer incidence among Finnish men. International Journal of Andrology. 34: e37-48. https://doi.org/10.1111/j.1365-2605.2010.01133.x.
Jorgensen, N., A.G. Andersen, F. Eustache, D.S. Irvine, J. Suominen, J.H. Petersen, A.N. Andersen, J. Auger, E.H. Cawood, A. Horte, T.K. Jensen, P. Jouannet, N. Keiding, M. Vierula, J. Toppari, and N.E. Skakkebaek. 2001. Regional differences in semen quality in Europe. Human Reproduction. 16: 1012–1019. https://doi.org/10.1093/humrep/16.5.1012.
Junaid, M., R.C. Murthy, and D.K. Saxena. 1996a. Embryo- and fetotoxicity of chromium in pre-gestationally exposed mice. Bulletin of Environmental Contamination. 57: 327–334. https://doi.org/10.1007/s001289900194.
Junaid, M., R.C. Murthy, and D.K. Saxena. 1996b. Embryotoxicity of orally administered chromium in mice: Exposure during the period of organogenesis. Toxicology Letters. 84: 143–148. https://doi.org/10.1017/S0967199410000274.
Kavlock, R.J., G.P. Daston, C. DeRosa, P. Fenner-Crisp, L.E. Gray, S. Kaattari, G. Lucier, M. Luster, M.J. Mac, C. Maczka, R. Miller, J. Moore, R. Rolland, G. Scott, D.M. Sheehan, T. Sinks, and H.A. Tilson. 1996. Research needs for the risk assessment of health and environmental effects of endocrine disruptors: A report of the U.S. EPA-Sponsored Workshop. Environmental Health Perspective. 104: 714–740. https://doi.org/10.1289/ehp.96104s4715.
Khan, A., N. Michelsen, A. Marandi, R. Hossain, M.A. Hossain, K.E. Roehl, A. Zahid, M.Q. Hassan, and C. Schuth. 2020. Processes controlling the extent of groundwater pollution with chromium from tanneries in the Hazaribagh area, Dhaka Bangladesh. Science of the Total Environment. 710: 136213. https://doi.org/10.1016/j.scitotenv.2019.136213.
Krieg, S.A., L.K. Shahine, and R.B. Lathi. 2016. Environmental exposure to endocrine-disrupting chemicals and miscarriage. Fertility and Sterility. 106(4): 941–947. https://doi.org/10.1016/j.fertnstert.2016.06.043.
Kumar, M.K., M.M. Aruldhas, S.L. Banu, B. Sadasivam, G. Vengatesh, K.M. Ganesh, S. Navaneethabalakrishnan, A.K. Navin, F.M. Michael, S. Venkatachalam, J.A. Stanley, I. Ramachandran, S.K. Banu, and M.A. Akbarsha. 2017. Male reproductive toxicity of Cr(VI): in-utero exposure to Cr(VI) at the critical window of testis differentiation represses the expression of Sertoli cell tight junction proteins and hormone receptors in adult F1 progeny rats. Reproductive Toxicology. 69: 84–98. https://doi.org/10.1016/j.reprotox.2017.02.007.
Kumar, S., N.G. Sathwara, A.K. Gautam, K. Agarwal, B. Shah, P.K. Kulkarni, K. Patel, A. Patel, L.M. Dave, D.J. Parikh, and H.N. Saiyed. 2005. Semen quality of industrial workers occupationally exposed to chromium. Journal of Occupational Health. https://doi.org/10.1539/joh.47.424.
Philip, Landrigan J., R. Fuller, N.J.R. Acosta, O. Adeyi, R. Arnold, N.N. Basu, et al. 2018. The Lancet commission on pollution and health. Lancet 3: 462–512. https://doi.org/10.1016/S0140-6736(17)32345-0.
Lay, P.A., and A. Levina. 1998. Activation of molecular oxygen during the reactions of chromium (VI/V/IV) with biological reductants: Implications for chromium-induced genotoxicities. Journal of American Chemical Society. 120: 6704–6714. https://doi.org/10.1021/ja974240z.
Li, H., Q. Chen, S. Li, W. Yao, L. Li, and X. Shi. 2001. Effect of CrVI exposure on sperm quality: Human and animal studies. Annals of Occupational and Hygiene 45: 505–511. https://doi.org/10.1016/S0003-4878(01)00004-7.
Loebenstein, Moshe, Jorgen Thorup, Dina Cortes, Erik Clasen-Linde, John M. Hutson, and Ruili Li. 2019. Cryptorchidism, gonocytes development, and the risks of germ cell malignancy and infertility: A systematic review. Journal of Pediatric Surgery. S0022–3468(19): 30450–30456. https://doi.org/10.1016/j.jpedsurg.2019.06.023.
Marouani, N., O. Tebourbi, M. Mokni, M.T. Yacoubi, M. Sakly, M. Benkhalifa, and K. Ben Rhouma. 2011. Embryotoxicity and fetotoxicity following intraperitoneal administrations of hexavalent chromium to pregnant rats. Zygote. 19(3): 229–235. https://doi.org/10.1017/S0967199410000274.
Marouani, N., O. Tebourbi, S. Mahjoub, M.T. Yacoubi, M. Sakly, M. Benkhalifa, and K.B. Rhouma. 2012. Effects of hexavalent chromium on reproductive functions of male adult rats. Reproductive Biology. 12: 119–133. https://doi.org/10.1016/S1642-431X(12)60081-3.
McCarroll, N., N. Keshava, J. Chen, G. Akerman, A. Kligerman, and E. Rinde. 2010. An evaluation of the mode of action framework for mutagenic carcinogens case study II: Chromium (VI). Environmental and Molecular Mutagenesis. 51: 89–111. https://doi.org/10.1002/em.20525.
McNeill, L., and J. McLean. 2012. State of the science of hexavalent chromium in drinking water. Water Research. Foundation, 6666 W. Quincy Ave. Denver, CO 80235.
Meeker, J.D., M.G. Rossano, B. Protas, M.P. Diamond, E. Puscheck, D. Daly, N. Paneth, and J.J. Wirth. 2009. Multiple metals predict prolactin and thyrotropin (TSH) levels in men. Environmental Research. 109: 869–873. https://doi.org/10.1016/j.envres.2009.06.004.
Mortensen, J. 1988. Risk for reduced sperm quality among metal workers, with special reference to welders. Scandinavian Journal of Work Environment and Health. 14: 27–30. https://doi.org/10.5271/sjweh.1954.
Murawski, M., J. Saczko, A. Marcinkowska, A. Chwiłkowska, M. Grybos, and T. Banas. 2007. Evaluation of superoxide dismutase activity and its impact on semen quality parameters of infertile men. Folia Histochemica Et Cytobiologica. 45(1): S123–S126.
Murthy, R.C., D.K. Saxena, S.K. Gupta, and S.V. Chandra. 1991a. Lead induced ultrastructural changes in the testis of rats. Experimental Pathology. 42: 95–100. https://doi.org/10.1016/s0232-1513(11)80054-x.
Murthy, R.C., D.K. Saxena, S.K. Gupta, and S.V. Chandra. 1991b. Ultrastructural observations in testicular tissue of chromium-treated rats. Reproductive Toxicology. 5: 443–447. https://doi.org/10.1016/0890-6238(91)90008-4.
Navin, A.K. 2018. An insight into the mechanism underlying the disruption of hypothalamic-pituitary-testicular axis in F1 progeny of rats with transient gestational exposure to hexavalent chromium. Ph.D. Thesis. University of Madras, Chennai (India).
Navin, A.K., M.M. Aruldhas, S. Navaneethabalakrishnan, M.K. Kumar, F.M. Michael, N. Srinivasan, and S.K. Banu. 2021. Prenatal exposure to hexavalent chromium disrupts testicular steroidogenic pathway in peripubertal F1 rats. Reproductive Toxicology. 101: 63–73. https://doi.org/10.1016/j.reprotox.2021.01.014.
Navin, A.K., N. Shobana, S. Venkatachalam, M.A. Akbarsha, S.K. Banu, and M.M. Aruldhas. 2017. Transient gestational exposure to hexavalent chromium (Cr(VI)) adversely affects testicular differentiation: A study in rat model. Journal of Endocrinology and Reproduction. 21: 67–76.
Nordkap, L., U.N. Joensen, M.B. Jensen, and N. Jorgensen. 2012. Regional differences and temporal trends in male reproductive health disorders: Semen quality may be a sensitive marker of environmental exposures. Molecular and Cellular Endocrinology. 355: 221–230. https://doi.org/10.1016/j.mce.2011.05.048.
Nudler, S.I., F.A. Quinteros, E.A. Miler, J.P. Cabilla, S.A. Ronchetti, and B.H. Duvilansi. 2009. Chromium VI administration induces oxidative stress in hypothalamus and anterior pituitary gland from male rats. Toxicology Letters. 185: 187–192. https://doi.org/10.1016/j.toxlet.2009.01.003.
O’Shaughnessy, P.J., P.J. Baker, and H. Johnston. 2007. The foetal Leydig cell differentiation, function and regulation. International Journal of Andrology. 29: 90–95. https://doi.org/10.1111/j.1365-2605.2005.00555.x.
Paithankar, J.G., S. Saini, S. Dwivedy, A. Sharma, and D.K. Chowdhury. 2021. Heavy metal associated health hazard: An interplay of oxidative stress and signal transduction. Chemosphere 262: 128350. https://doi.org/10.1016/j.chemosphere.2020.128350.
Parent, A.S., D. Franssen, J. Fudvoye, A. Gerard, and J.P. Bourguignon. 2015. Developmental variations in environmental influences including endocrine disruptors on pubertal timing and neuroendocrine control: Revision of human observations and mechanistic insight from rodents. Frontiers in Neuroendocrinology. 38: 12–36. https://doi.org/10.1016/j.yfrne.2014.12.004.
Pizent, A., B. Tariba, and T. Zivkovic. 2012. Reproductive toxicity of metals in men. Archives of Industrial Hygiene and Toxicology. 63: 35–46. https://doi.org/10.2478/10004-1254-63-2012-2151.
Pure Earth and Green Cross (Switzerland), The World’s Worst Pollution Problems Series -“The New Top Six Toxic Threats: a Priority List for Remediation”, 10th Report, 2015.
Quievryn, G., E. Peterson, J. Messer, and A. Zhitkovich. 2003. Genotoxicity and mutagenicity of chromium (VI)/ ascorbate generated DNA adducts in human and bacterial cells. Biochemistry 42: 1062–1070. https://doi.org/10.1021/bi0271547.
Quinteros, F.A., A.H.B. Poliandri, L.I. Machiavelli, J.P. Cabilla, and B.H. Duvilanski. 2007. In vivo and in vitro effects of chromium VI on anterior pituitary hormone release and cell viability. Toxicology and Applied Pharmacology. 218: 79–87. https://doi.org/10.1016/j.taap.2006.10.017.
Rebourcet, D., P.J. O’Shaughnessy, A. Monteiro, L. Milne, L. Cruickshanks, N. Jeffrey, F. Guillou, T.C. Freeman, R.T. Mitchell, and L.B. Smith. 2014. Sertoli cells maintain Leydig cell number and peritubular myoid cell activity in the adult mouse testis. PLoS ONE 9: e105687. https://doi.org/10.1371/journal.pone.0105687.eCollection.
Rebourcet, D., P.J. O’Shaughnessy, J.L. Pitetti, A. Monteiro, L. O’Hara, L. Milne, Y.T. Tsai, L. Cruickshanks, D. Riethmacher, F. Guillou, R.T. Mitchell, R. van Hof, T.C. Freeman, S. Nef, and L.B. Smith. 2014. Sertoli cells control peritubular myoid cell fate and support adult Leydig cell development in the prepubertal testis. Development 141: 2139–2149. https://doi.org/10.1242/dev.107029.
Rebourcet, D., J. Wu, L. Cruickshanks, E. Sarah, S. Laura, M. Anuruddika, J. Fernando Robert, D. Wallace Calum, W.F. Gray Patrick, T. Hadoke Rod, J. Mitchell Peter, O. Lee, and B. Smith. 2016. Sertoli cells modulate testicular vascular network development structure and function to influence circulating testosterone concentrations in adult male mice. Endocrinology. 157(6): 2479–2488. https://doi.org/10.1210/en.2016-1156.
Rehman, S., Z. Usman, S. Rehman, M.A. Draihem, N. Rehman, I. Rehman, and G. Ahmad. 2018. Endocrine disrupting chemicals and impact on male reproductive health. Translational Andrology and Urology. 7(3): 490–503. https://doi.org/10.21037/tau.2018.05.17.
Remy, L.L., V. Byers, and T. Clay. 2017. Reproductive outcomes after non-occupational exposure to hexavalent chromium, Willits California, 1983–2014. Environmental Health 16: 18. https://doi.org/10.1186/s12940-017-0222-8.
Savchuk, I. 2017. Diverse effects of endocrine-disruptive chemicals on Leydig and adrenocortical cell steroidogenesis in rodents and humans. Ph.D. Thesis. Karolinska Institutet, Stockholm, Sweden
Saxena, D.K., R.C. Murthy, B. Lal, R.S. Srivastava, and S.V. Chandra. 1990a. Effect of hexavalent chromium on testicular maturation in the rat. Reproductive Toxicology. 4: 223–228. https://doi.org/10.1016/0890-6238(90)90062-z.
Saxena, D.K., R.C. Murthy, V.K. Jain, and S.V. Chandra. 1990b. Fetoplacental-maternal uptake of hexavalent chromium administered orally in rats and mice. Bulletin of Environmental Contamination and Toxicology. 45: 430–435. https://doi.org/10.1007/BF01701168.
Scheifler, R., A. de Vaufleury, M. Coeurdassier, N. Crini, and P.M. Badot. 2006. Transfer of Cd, Cu, Ni, Pb, and Zn in a soil-plant-invertebrate food chain: A microcosm study. Environtal Toxicology and Chemistry. 25: 815–822. https://doi.org/10.1897/04-675r.1.
Schjenken, E.J., E.S. Green, T.S. Overduin, C.Y. Mah, D.L. Russell, and S.A. Robertson. 2021. Endocrine disruptor compounds—a cause of impaired immune tolerance driving inflammatory disorders of pregnancy? Frontiers in Endocrinology. 12: 607539. https://doi.org/10.3389/fendo.2021.607539.
Sekar, P., 2005. Impact of gestational and neonatal exposure to chromium on post-natal rat testis – an endocrine and ultrastructural study, Ph.D. Thesis, University of Madras, Chennai, India.
Sekar, P., G. Vengatesh, M.K. Kumar, S. Balaji, M.A. Akbarsha, and M.M. Aruldhas. 2011. Impact of gestational and lactational exposure to hexavalent chromium on steroidogenic compartment of post-natal rat testis. Journal of Endocrinology and Reproduction. 15: 15–26.
Shah, P.S., and T. Balkhair. 2011. Knowledge synthesis group on determinants of preterm/LBW births. air pollution and birth outcomes: A systematic review. Environment International. 37: 498–516. https://doi.org/10.1016/j.envint.2010.10.009.
Sharma, A., J. Mollier, R.W.K. Brocklesby, C. Caves, N. Jayasena, and S. Minhas. 2020. Endocrine-disrupting chemicals and male reproductive health. Reproductive Medicine and Biology. 19(3): 243. https://doi.org/10.1002/rmb2.12326.
Sharpe, R.M., and N.E. Skakkebaek. 1993. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet 341: 1392–1395. https://doi.org/10.1016/0140-6736(93)90953-e.
Sharpe, R.M., C. McKinnell, C. Kivlin, and J.S. Fisher. 2003. Proliferation and functional maturation of Sertoli cells and their relevance to disorders of testis function in adulthood. Reproduction. 125(6): 769–784. https://doi.org/10.1530/rep.0.1250769.
Shi, X.G., and N.S. Dalal. 1990. On the hydroxyl radical formation in the reaction between hydrogen peroxide and biologically generated chromium (V) species. Archives of Biochemistry and Biophysics. 277: 342–350. https://doi.org/10.1016/0003-9861(90)90589-Q.
Shmitova, L.A. 1980. Content of hexavalent chromium in the biological substrates of pregnant women and women in the immediate post-natal period engaged in the manufacture of chromium compounds. Gigiena Truda i Professional’nye Zabolevaniya 2: 33–35.
Shobana, N., M. Michael, A. Lalmuankimi, T. Ayyalu, L. Sadhasivam, B. Mani, K. Kumar, L. Alikhan, S. Banu, A.K. Navin, C. Mayilvanan, R. Ilangovan, and K. Balasubramanian. 2017. Transient gestational exposure to drinking water containing excess hexavalent chromium modifies insulin signaling in liver and skeletal muscle of rat progeny. Chemico-Biological Interactions. 277: 119–128. https://doi.org/10.1016/j.cbi.2017.09.003.
Shobana, N., M.K. Kumar, A.K. Navin, M.A. Akbarsha, and M.M. Aruldhas. 2020. Prenatal exposure to excess chromium attenuates transcription factors regulating expression of androgen and follicle stimulating hormone receptors in Sertoli cells of prepuberal rats. Chemico-Biological Interaction. 328: 109188. https://doi.org/10.1016/j.cbi.2020.109188.
Shupack, S.I. 1991. The chemistry of chromium and some resulting analytical problems. Environmental Health Perspective. 92: 15–11. https://doi.org/10.1289/ehp.91927.
Sipowicz, M.A., L.M. Anderson, W.E. Utermahlen Jr., H.J. Issaq, and K.S. Kasprzak. 1997. Uptake and tissue distribution of chromium (III) in mice after a single intraperitoneal or subcutaneous administration. Toxicology Letters. 93: 9–14. https://doi.org/10.1016/s0378-4274(97)00064-7.
Sivakumar, K.K., J.A. Stanley, J.A. Arosh, M.E. Pepling, R.C. Burghardt, and S.K. Banu. 2014. Prenatal exposure to chromium induces early reproductive senescence by increasing germ cell apoptosis and advancing germ cell cyst breakdown in F1 offspring. Developmental Biology. 388: 22–34. https://doi.org/10.1016/j.ydbio.2014.02.003.
Skakkebaek, N.E. 2016. A brief review of the link between environment and male reproductive health: Lessons from studies of testicular germ cell cancer. Hormone Research in Pediatrics. 86: 240–246. https://doi.org/10.1159/000443400.
Skakkebaek, N.E., E. Rajpert-De Meyts, and K.M. Main. 2001. Testicular dysgenesis syndrome: An increasingly common developmental disorder with environmental aspects. Human Reproduction. 16: 972–978. https://doi.org/10.1093/humrep/16.5.972.
Smith, L.B., P.J. O'Shaughnessy, and D. Rebourcet. 2015. Cell-specific ablation in the testis: what have we learned? Andrology. 3(6): 1035–1049. https://doi.org/10.1111/andr.12107.
Subramanian, S., G. Rajendran, P. Sekhar, C. Gowri, P. Govindarajulu, and M.M. Aruldhas. 2006. Reproductive toxicity of chromium in adult bonnet monkeys (Macaca radiata Geoffrey). Reversible oxidative stress in the semen. Toxicology and Applied Pharmacology. 215: 237–249. https://doi.org/10.1016/j.taap.2006.03.004.
Sun, H., J. Brocato, and M. Costa. 2015. Oral chromium exposure and toxicity. Current Environmental Health Reports. 2: 295–303. https://doi.org/10.1007/s40572-015-0054-z.
Sutherland, J.E., A. Zhitkovich, T. Kluz, and M. Costa. 2000. Rats retain chromium in tissues following chronic ingestion of drinking water containing hexavalent chromium. Biological Trace Element Research. 74: 41–53. https://doi.org/10.1385/BTER:74:1:41.
Tan, K., H.W. Song, and M.F. Wilkinson. 2020. Single-cell RNAseq analysis of testicular germ and somatic cell development during the perinatal period. Development. https://doi.org/10.1242/dev.183251.
Thankamony, A., K.K. Ong, D.B. Dunger, C.L. Acerini, and I.A. Hughes. 2009. Anogenital distance from birth to 2 Years: A population study. Environmental Health Perspective. 117: 1786–1790. https://doi.org/10.1289/ehp.0900881.
Trivedi, B., D.K. Saxena, R.C. Murthy, and S.V. Chandra. 1989. Embryotoxicity and fetotoxicity of orally administered hexavalent chromium in mice. Reproductive Toxicology. 3: 275–278. https://doi.org/10.1016/0890-6238(89)90022-1.
U.S. Environmental Protection Agency (USEPA). Code of Federal Regulations, 40 CFR 141.32.
USEPA (2005). Cost and Performance Summary Report in Situ Chemical Reduction at the Frontier Hard Chrome Superfund Site Vancouver, Washington. August 5, 2011.
USEPA (2010). Toxicoloical review of hexavalent chromium. EPA/635/R-10/004A www.epa.gov/iris, Washington, DC
Vecoli, C., L. Montano, and M.G. Andreassi. 2016. Environmental pollutants: Genetic damage and epigenetic changes in male germ cells. Environmental Science and Pollution Research Int. 23: 23339–23348. https://doi.org/10.1007/s11356-016-7728-4.
Veeramachaneni, D.N.R., J.S. Palmer, and R.P. Amann. 2001. Long-term effects on male reproduction of early exposure to common chemical contaminants in drinking water. Human Reproduction. 16: 979–987. https://doi.org/10.1093/humrep/16.5.979.
Von Burg, R., and D. Liu. 1993. Chromium and hexavalent chromium. Journal of Applied Toxicology. 5: 91–106. https://doi.org/10.1002/jat.2550130315.
Wilbur, S., H. Abadin, M. Fay, D. Yu, B. Tencza, L. Ingerman, J. Klotzbach, S. James. 2012. Toxicological Profile for Chromium. Agency for toxic substances and disease registry (ATSDR) Toxicological Profiles, Atlanta (GA).
William, J.H. 1996. Evidence that mammalian sex ratios at birth are partially controlled by parental hormone levels at the time of conception. Journal of Endocrinology. 180: 271–286. https://doi.org/10.1677/JOE-07-0446.
Wirth, J.J., M.G. Rossano, D.C. Daly, N. Paneth, E. Puscheck, and R.C. Potter. 2007. Ambient manganese exposure is negatively associated with human sperm motility and concentration. Epidemiology 18: 270–273. https://doi.org/10.1097/01.ede.0000253939.06166.7e.
Wise, S.S., A.A. Holmes, and J.P. Wise. 2008. Hexavalent chromium-induced DNA damage and repair mechanisms. Reviews of Environmental Health. 23(1): 39–57. https://doi.org/10.1515/reveh.2008.23.1.39.
World Health Organization. 2003. Chromium in drinking-water background document for development of WHO Guidelines for Drinking-water Quality.
Wu, S., M. Yan, R. Ge, and C.Y. Cheng. 2019. Crosstalk between Sertoli and germ cells in male fertility. Trends in Molecular Medicine. 26: 215–231. https://doi.org/10.1016/j.molmed.2019.09.006.
Wuana, R.A., and F.A. Okiemen. 2011. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecology 402647: 1–20. https://doi.org/10.5402/2011/402647.
Xu, X.-R., H.-B. Li, X.-Y. Li, and J.-D. Gu. 2004. Reduction of hexavalent chromium by ascorbic acid in aqueous solutions. Chemosphere. 57(7): 609–613. https://doi.org/10.1016/j.chemosphere.2004.07.031.
Xu, X.-R., H.-B. Li, J.-D. Gu, and X.-Y. Li. 2005. Kinetics of the reduction of chromium(VI) by vitamin C. Environmental Toxicology and Chemistry. 24(6): 1310. https://doi.org/10.1897/04-238R.1.
Yoisungnern, Ton, Joydeep Das, Yun-Jung. Choi, Rangsun Parnpai, and Jin-Hoi. Kim. 2016. Effect of hexavalent chromium-treated sperm on in vitro fertilization and embryo development. Toxicology and Industrial Health. 32: 1700–1710. https://doi.org/10.1177/0748233715579805.
Yousef, M.I., F.M. El-Demerdash, K.I. Kamil, and F.A. Elaswad. 2006. Ameliorating effect of folic acid on chromium (VI)-induced changes in reproductive performance and seminal plasma biochemistry in male rabbits. Reproductive Toxicology. 21: 322–328. https://doi.org/10.1016/j.reprotox.2005.09.005.
Zahid, Z., Z. Al-Hakkak, A. Kadhim, E. Elias, and I. Al-Jumaily. 1990. Comparative effects of trivalent and hexavalent chromium on spermatogenesis of the mouse. Toxicology and Environmental Chemistry. 25: 131–136. https://doi.org/10.1080/02772249009357512.
Zawatski, W., and M.M. Lee. 2013. Male pubertal development: Are endocrine-disrupting compounds shifting the norms? Journal of Endocrinology. 218: R1–R12. https://doi.org/10.1530/JOE-12-0449.
Zayed, A.M., and N. Terry. 2003. Chromium in the environment: Factors affecting biological remediation. Plant and Soil 249: 139–156. https://doi.org/10.1023/A:1022504826342.
Zheng, Wenwen, Fei Ge, Wu. Keyang, Xianwu Chen, Xiaoheng Li, Yong Chen, Yao Lv, Qingquan Lian, and Ren-Shan. Ge. 2018. In utero exposure to hexavalent chromium disrupts rat fetal testis development. Toxicology Letters. 299: 201–209. https://doi.org/10.1016/j.toxlet.2018.10.010.
Zhitkovich, A. 2011a. Chromium in drinking water: Sources, metabolism, and cancer risks. Chemical Research in Toxicology. 24: 1617–1629. https://doi.org/10.1021/tx200251t.
Zhu, Q., X. Li, and R.-S. Ge. 2020. Toxicological effects of cadmium on mammalian testis. Frontiers in Genetics. 11: 527. https://doi.org/10.3389/fgene.2020.00527.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Navin, A.K., Aruldhas, M.M. Hexavalent Chromium and Male Reproduction: An Update. Proc Zool Soc 74, 617–633 (2021). https://doi.org/10.1007/s12595-021-00417-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12595-021-00417-y