Abstract
Aluminum (Al) is the most widely distributed metal in the environment and is extensively used in human daily life without any known biological function. It is known that exposure to high concentrations of Al impacts negatively on serum testosterone levels, testicular histomorphometry, and sperm parameters; however, no information is available about the effects of low exposure levels on reproduction. International organizations have established the Al concentration tolerated in drinking water as 3.35 × 10−4 mg/kg. Therefore, we aimed to compare the effects of long-term exposure to low and high concentrations of Al on male reproductive functions, focusing on testis, epididymis, and sperm parameters. Adult Wistar rats were exposed to aluminum chloride (AlCl3) at 6.7 × 10−5, 3.35 × 10−4, 10, and 40 mg/kg for 112 days by gavage. Al-exposed animals presented low values of testis and epididymis weight, and serum testosterone levels when compared to controls. The stereology of Leydig cells, epididymis histomorphometry, sperm motility, and structural integrity of sperm membranes changed depending on the Al concentration. In regard to epididymis histomorphometry, the initial segment and caput regions were more affected by Al exposure than distal regions. Otherwise, the histology of testis and epididymis did not alter after the Al exposure, as well as sperm morphology. In summary, we concluded that the consequences of Al exposure at low levels were as negative as high levels on reproductive parameters, suggesting adverse impact on male fertility.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aluminum (Al) is the third most abundant metal on earth, being found in minerals, rocks, and soil [1, 2]. In addition, human beings might be exposed to Al through the use of intravenous solutions, lipid emulsions, dialysis fluids, medicines, food, toothpaste, and cosmetic products. Drinking water can also be source of Al exposure since it is added to water for purification purposes [3,4,5].
Aluminum levels vary according to the levels found in the source water and whether Al coagulants are used during water treatment. The contribution of drinking water to the total oral exposure to Al is less than 5% of the total intake in humans. However, the relative risk for the development or acceleration of onset of Alzheimer disease from its exposure is above 100 μg/L [6]. In this context, the Al concentration tolerated in drinking water is 3.35 × 10−4 mg/kg [6], and no information is available about its toxicity in humans. It is well known that the intestinal absorption of Al is very low (0.1–1.0%) [7]. Indeed, the gastrointestinal epithelium works as an impervious barrier to the passage of this metal, and its permeability is dependent on the chemical form, pH, and Al concentration [8, 9]. Moreover, organic dietary components, such as phosphorus, magnesium, iron, calcium, and vitamin D, may act as potential chelators of Al and facilitate its absorption by membranes [10]. This fact contributes to the chronic Al bioaccumulation in the central nervous system [11, 12], muscles, and reproductive organs [13].
Despite the exposure to low concentrations of Al has not been investigated in male reproductive organs, studies have reported that high concentrations of this metal cause degeneration of seminiferous tubules, presence of edema in the intertubular compartment of the testis [14], presence of immature spermatocytes in the epididymal lumen [15], and reduction in serum testosterone [16]. Sperm motility and concentration were altered in rats exposed to Al as well [17]. Furthermore, epithelial cells from the epididymis might change their structure and function after Al exposure [14, 18]. The Al-induced toxicity on reproductive organ functions may be involved in oxidative stress and nitric oxide production [19, 20].
Based on that, the aim of the present study was to evaluate the long-term oral exposure to low concentrations of Al, such as the concentration tolerated in drinking water (3.35 × 10−4 mg/kg) and 1/5 of it (6.7 × 10−5 mg/kg), on reproductive outcomes. Moreover, we aimed to compare them with the exposure to high Al concentrations (10 and 40 mg/kg) in order to clarify if these concentrations may cause similar damages on the reproductive organs functionality. Thus, here, we focused on testis and epididymis biometry and histomorphometry, serum testosterone level, and sperm analyses.
Materials and Methods
Animals
Sexually mature male Wistar rats (n = 25, 70 days old, 264.33–367.6 g) were provided by the Central Animal Facility of the Center of the Biological and Health Sciences of the Federal University of Viçosa (UFV). Animals were housed individually in polypropylene cages, under controlled photoperiod (12-h light/dark) and temperature (21 °C). The provision of feed was controlled, being 25 g of balanced rat feed (Socil®) per day, whereas drinking water was provided ad libitum. All experimental procedures were in accordance with the ethical principles in animal research adopted by the animal ethics committees (CEUA protocol number 19/2011).
Experimental Design
The animals were weighed and randomly divided into five groups (n = 5 animals/group). Rats from the control group received distilled water, whereas the others were exposed to Al, in the form of aluminum chloride (AlCl3; Sigma-Aldrich Co., St. Louis, MO), at the concentrations of 6.7 × 10−5 mg/kg BW (0.02 mg/L), 3.35 × 10−4 mg/kg BW (0.1 mg/L), 10 mg/kg BW (3.381 mg/L), and 40 mg/kg BW (13.6 mg/L) (based on ions of Al). The lower concentration used in the present study corresponds to 1/5 of the concentration tolerated in drinking water by international organizations, which is 3.35 × 10−4 mg/kg [6]. In addition, 10 and 40 mg/kg correspond respectively to 1/40 and 1/10 lethal dose 50 (LD50) of Al [21]. The animals were provided 1-mL solutions daily by gavage for 112 days. This exposure period was determined using the duration of two cycles of the seminiferous epithelium in rats [22].
On the 113th day of exposure, the animals were weighed, sedated with xylazine hydrochloride (10 mg/kg/intraperitoneally), anesthetized with ketamine hydrochloride (150 mg/kg/intraperitoneally), and euthanized [23, 24]. Testes and epididymides were removed, dissected, and weighed. The right testes and epididymides were used for histology, whereas the left epididymides were used for sperm analyses.
Determination of Serum Testosterone
During anesthesia, blood was collected by cardiac puncture and centrifuged at 419g for 15 min. The serum was then stored in microtubes and frozen at −20 °C [24]. Quantification of serum testosterone was determined by chemiluminescence immunoassay using the Access testosterone reagent kit (number 33560; Beckman Coulter, Brea, CA), suitable for the Access 2 Immunoassay System (Beckman Coulter, Brea, CA). The analytical sensitivity was 0.1 ng/mL.
Sperm Evaluation
Freshly dissected portions from epididymis cauda region were cut three times and placed in a petri dish containing 500 μL of Tris-citric acid-fructose (Tris 3.025 g, citric acid 1.7 g, fructose 1.25 g, distilled water 100 mL) to enable release of spermatozoa. The fluid was collected for evaluation of sperm motility, morphology, as well as structural integrity of sperm membranes.
Sperm motility was assessed using 10 μL epididymal fluid placed between the slide and coverslip, previously heated to 37 °C, and approximately 100 sperm were examined under the microscope (Bioval L-1000B, Brazil) at a magnification of 400×. Spermatozoa were classified as either motile or immotile, and motility was expressed as percentage [25].
For the sperm morphology analysis, epididymal fluid (50 μL) was fixed in 100 μL 4% buffered formaldehyde. Two hundred cells were examined under phase-contrast microscopy (1000× magnification; Bioval L-1000B, Brazil), being classified as defects in the head, midpiece, and tail [26]. Results were expressed as percentages.
Moreover, sperm samples (10 μL) were incubated in a solution of 4% buffered formaldehyde (10 μL) plus buffer citrate (10 μL), carboxyfluorescein diacetate (CFDA; 20 μL), and propidium iodide (PI; 10 μL) for 8 min at 37 °C [27]. Two hundred sperm were evaluated by epifluorescence microscope at magnification of 400× and filter of 480 at 610 nm, being classified into two categories: intact membranes (CFDA+/PI−), and non-intact membranes (CFDA−/PI+). Results were expressed as percentages.
Histological Processing
Testes were immersed in Karnovsky fixative (2.5% glutaraldehyde, 4% paraformaldehyde in 0.1 M pH 7.2 sodium phosphate buffer) for 24 h. The albuginea was removed and weighed. Its weight was subtracted of the testicular weight to calculate the weight of the testicular parenchyma. Epididymides were also immersed in Karnovsky fixative for 24 h and subsequently segmented into four regions: initial segment, caput, corpus, and cauda [28].
Fragments from testis and epididymal regions were dehydrated in crescent ethanol series (70, 80, 90, and 100%) and embedded in 2-hydroxyethyl methacrylate (Historesin®, Leica Microsystems, Nussloch, Germany). Sections with a thickness of 3 μm were obtained using a rotary microtome (RM 2255, Leica Biosystems, Nussloch, Germany) and stained with toluidine blue-sodium borate (1%). Histological sections of the testis and epididymis were analyzed using Olympus CX40 (Tokyo, Japan) optical microscope.
Testis Histomorphometry and Stereology
The gonadosomatic index was obtained using the following formula: GSI = GW/BW × 100, where GW = total gonadal weight and BW = body weight [29]. Once testis is divided into two compartments, tubular and intertubular, the volumetric ratios of these two were obtained by counting 2660 points projected onto 10 images captured from histological slides of each animal, at a magnification of 100×, using the photomicroscope (Olympus BX-53, Tokyo, Japan) equipped with a digital camera (Olympus DP73, Tokyo, Japan) and assessed with Image-Pro Plus 4.5 (Media Cybernetics Inc., Silver Spring, MD). Coincident points over the tunica propria, seminiferous epithelium, and lumen (tubular compartment), and the points on the intertubular space were recorded.
The volume of each testicular component, expressed in mL, was calculated from the percentage of the testis they occupy and from the volume of the testicular parenchyma. As the density of the testis is around 1 [30], the weight of the testis was considered equivalent to its volume. The volume of the epithelium, in turn, was calculated by considering the percentage represented by the seminiferous epithelium and the weight of the testicular parenchyma.
The tubule somatic index (TSI) was calculated to quantify the investment in seminiferous tubules in relation to body mass by the formula TSI = STV/BW × 100, where STV = seminiferous tubule volume [29].
The average tubular diameter per animal was obtained by measuring 30 randomly transverse sections of seminiferous tubules that displayed the most circular shape regardless of the cycle stage [31]. The same sections used for measuring the tubular diameter were used for determining the seminiferous epithelium height from the tunica propria to tubular lumen. The value found for epithelium height of each tubule represented the average of two measurements obtained in a diametrically opposite angle.
The total length (TL) of the seminiferous tubules, per testis, was estimated from previous knowledge of the volume occupied by these structures within the parenchyma, as well as from the mean tubular diameter: STV / πr 2 (STV = seminiferous tubule volume; πr 2 = transverse section area of the seminiferous tubule; r = tubular diameter / 2). The total length of the seminiferous tubules per gram of testis (TL/g) was determined using the formula: TL/g = TL / TW, where TW = testicular weight [32].
Moreover, the volumetric proportion of intertubular components was obtained by counting 1000 points per animal projected onto intertubular images of the testis. Coincident points over intertubular components were recorded: nucleus and cytoplasm of the Leydig cell, blood vessels, lymphatic space, macrophages, and connective tissue. To calculate these proportions, we used the following formula: volumetric proportion of each intertubular component (%) = number of points of the intertubular component × 100 / 1000 total points. To calculate the relation between nucleus and cytoplasm of the Leydig cells, the percentage occupied by nucleus was divided by the percentage occupied by cytoplasm. The volume (mL) of each intertubular component by the testis was calculated using the following formula: proportion of the element on the testis / (100 × parenchymal mass of one testis) [32].
The average diameter of the Leydig cell nucleus was obtained by counting 30 cells per animal, choosing the ones with the most spherical nuclei and evident nucleoli, using the software Image-Pro Plus®. The nuclear volume was obtained by using the mean nuclear diameter and the formula 4/3πR 3, where R = nuclear diameter / 2. The cytoplasm volume was estimated by multiplying the percentage of cytoplasm by the nuclear volume, divided by the nuclear percentage. The single-cell volume was estimated by adding the nuclear and cytoplasmic volumes. These values were expressed in cubic micrometer [32].
The number of Leydig cells per testis was estimated from the Leydig cell individual volumes and the total volume occupied by Leydig cells in the testicular parenchyma. This value was divided by the gonadal weight to estimate the number of Leydig cells per gram of testis, which allows comparisons between different species. The Leydig somatic index (LSI) was calculated by the ratio of the volume occupied by Leydig cells on the testis and the body mass, the result being multiplied by 100 [32].
Epididymal Histomorphometry
For morphometric analysis, digital images of each epididymal region (initial segment, caput, corpus, and cauda) were obtained using a light microscope (Olympus BX53, Tokyo, Japan) equipped with a digital camera (Olympus DP73, Tokyo, Japan) and analyzed with the Image-Pro Plus 4.5 (Media Cybernetics, Silver Spring, MD) software. The mean tubular diameter of each epididymal region was obtained by randomly measuring 20 tubular cross sections, as circular as possible, per animal. These sections were also used to measure the luminal diameter and epithelium height, which was obtained from the lamina propria to tubular lumen. The epithelium height for each tubule was the average of four diametrically opposite measurements [24].
The volumetric proportion of the 4 epididymal regions was obtained by counting 2660 points projected onto 10 images captured in histological slides per animal. Coincident points were registered in tubular components (lamina propria, epithelium, lumen with sperm, and lumen without sperm) and intertubular components (blood vessels, connective tissue, and smooth muscle). The percentage of points in each component was calculated using the following formula: volumetric proportion (%) = (number of points in the component / 2660 total points) × 100 [24].
Statistical Analysis
The results were analyzed by one-way analysis of variance (ANOVA) followed by the post hoc Student-Newman-Keuls test. In regard to sperm analyses, we used arcsine square root transformation for percentage data in order to meet the assumptions of normality. Those data were analyzed by ANOVA and Tukey test. Differences were considered significant when p < 0.05. Results were expressed as mean ± standard error mean (SEM).
Results
Biometric Parameters
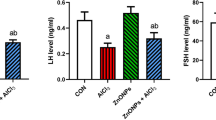
The animals exposed to Al at the four concentrations presented lower values of the testis and its parenchyma weight than the control animals (Table 1). No significant differences were observed between the control and Al-exposed groups in body weight, albuginea weight, and GSI (Table 1). Further, the epididymis weight was lower in all Al-exposed animals when compared to that in the control animals (Fig. 1).
Testosterone concentration (ng/dL) and epididymal weight (g) of Wistar rats exposed to low concentrations (6.7 × 10−5 and 3.35 × 10−4 mg/kg BW) and high concentrations (10 and 40 mg/kg BW) of aluminum (AlCl3). Values are mean ± SEM. Different letters (a, b) indicate significant differences between the groups (p < 0.05)
Serum Testosterone
The animals exposed to Al at the four concentrations presented lower values for testosterone concentration when compared to the control animals (Fig. 1).
Sperm Evaluation
The animals exposed to 40 mg/kg Al presented low percentage of motile sperm when compared to the other groups (F4,20 = 2.957; p = 0.045; Fig. 2). Furthermore, there were no differences among the groups for the percentage of normal spermatozoa (F4,20 = 1.093; p = 0.387) and abnormal sperm with head (F4,20 = 1.019; p = 0.421), midpiece (F4,20 = 1.494; p = 0.242), and tail (F4,20 = 1.725; p = 0.184) defects (Fig. 2). The distal cytoplasmic droplet is shown in a spermatozoa labeled with CFDA (Fig. 3), which is one of the sperm tail defects identified in the control and Al-exposed animals. The percentage of sperm with intact plasma and acrosomal membranes was lower in the Al-exposed animals at 3.35 × 10−4, 10, and 40 mg/kg when compared to that in the control animals (Fig. 3). Sperm with non-intact membranes were labeled by PI (Fig. 3).
Percentage (mean ± SEM) of sperm with intact plasma and acrosomal membranes labeled with carboxyfluorescein diacetate (CFDA; green) from Wistar rats exposed to low concentrations (6.7 × 10−5 and 3.35 × 10−4 mg/kg BW) and high concentrations (10 and 40 mg/kg BW) of aluminum (AlCl3). Different letters (a, b) indicate significant differences between the groups (p < 0.05). Sperm with non-intact membranes were labeled in the head with propidium iodide (PI; red)
Testicular Histology and Histomorphometry
No histological alterations were observed in the tissue architecture of testes from the Al-exposed animals, with the seminiferous epithelium composed by Sertoli cells, germ cells in different stages of development (spermatogonia, spermatocytes, round and elongated spermatids), and lumen with spermatozoa (Figs. 4 and 5). Moreover, intertubular compartment presented normal lymphatic space, macrophages (Figs. 4 and 5), and Leydig cells arranged next to blood vessels (Fig. 6).
Light microscopy of the testis and epididymis from control and exposed rats to aluminum at low concentrations (AlCl3; 6.7 × 10−5 and 3.35 × 10−4 mg/kg BW). Histological sections from testis show the tubular compartment composed of a seminiferous epithelium (Se) and lumen (L), whereas the intertubular compartment (IC) is composed of connective tissues with different cell types and lymphatic space (*). Sections from the initial segment, caput, corpus, and cauda epididymal regions showing the epithelium (E), lumen with sperm (L), and connective tissue (CT). Toluidine blue. Scale bars = 20 μm
Light microscopy of the testis and epididymis from control and exposed rats to aluminum at high concentrations (AlCl3; 10 and 40 mg/kg BW). Sections from the testis showing the tubular compartment composed of seminiferous epithelium (Se) and lumen (L), whereas intertubular compartment (IC) is composed of connective tissues with different cell types and lymphatic space (*). Sections from the initial segment, caput, corpus, and cauda epididymal regions showing the epithelium (E), lumen with sperm (L), and connective tissue (CT). Toluidine blue. Scale bars = 20 μm
Histological sections of the intertubular compartment in testes from the control rats and under the effect of aluminum (AlCl3) at low concentrations (6.7 × 10−5 and 3.35 × 10−4 mg/kg BW) and high concentrations (10 and 40 mg/kg BW). Note the presence of Leydig cells (arrow) arranged next to blood vessels (BV) showing round nucleus with nucleoli, and large amounts of dark-staining peripheral heterochromatin. Toluidine blue. Scale bars = 10 μm
Leydig cells exhibited nucleus with one to three prominent nucleoli, and large amounts of dark-staining peripheral heterochromatin (Fig. 6). In general, the histology of this cell type was similar between the control and Al-exposed animals. However, the histomorphometry showed that animals exposed to Al at the four concentrations presented lower values for their nuclear diameter and nuclear volume when compared to control animals. Animals exposed to 40 mg/kg Al showed higher percentage of cytoplasm than control animals. Further, cytoplasmic volume and cell volume of Leydig cells were lower in animals exposed to 10 mg/kg Al compared to those in the control animals (Table 2).
No significant differences were observed between the control and Al-exposed groups for the volumetric proportion and volume of the tubular and intertubular components, besides the tubular morphometry and length of seminiferous tubules (Supplementary information S1–S3).
Epididymal Histomorphometry
Epididymal sections from the control and Al-exposed animals showed normal tissue arrangement, with a pseudostratified columnar epithelium and spermatozoa in the lumen of all regions (Figs. 4 and 5). In addition, no significant differences were observed between the control and Al-exposed groups in histomorphometric parameters, such as volumetric proportions of lamina propria, lumen without sperm, blood vessels, connective tissue, and smooth muscle, regardless of the epididymal region analyzed (Supplementary information S4).
On the other hand, the epithelium height in the caput region was higher in animals exposed to 40 mg/kg Al than that in the animals from the other groups (Table 3). This region also presented tubular diameter lower in animals exposed to 40 mg/kg Al when compared to that in animals exposed to 6.7 × 10−5 mg/kg (Table 3). Further, the luminal diameter in the initial segment and caput regions was lower in animals exposed to 3.35 × 10−4 and 40 mg/kg (Table 3). The percentage of lumen with sperm was lower in animals exposed to 3.35 × 10−4, 10, and 40 mg/kg in the initial segment, whereas the same parameter was lower in animals exposed to 3.35 × 10−4 and 40 mg/kg in the caput region (Table 3). Furthermore, the percentage of epithelium in the caput region was higher in Al-exposed animals when compared to that in control animals. This percentage was also high in animals exposed to 3.35 × 10−4 and 40 mg/kg in the corpus region (Table 3). Finally, the cauda region did not show any changes on its morphometry in Al-exposed animals (Table 3).
Discussion
Our results showed that oral administration of Al caused alterations in testis and epididymis biometry and histomorphometry, testosterone production, and sperm parameters, without alterations on their histological features and sperm morphology. These alterations were dependent upon the concentration utilized.
The weight loss in the testis and epididymis has been described in rats exposed orally to AlCl3 at a concentration of 34 mg/kg [16, 17] and 20 mg/kg [14] for 70 days, 100 mg/kg for 3 days [33] or 60 days [34], and 128.36 and 256.72 mg/kg for 120 days [35]. The maintenance of gonadosomatic index after Al exposure indicated there was no alteration in the testicular mass. It was confirmed by the absence of differences between the control and Al-exposed animals for the volumetric proportion of the tubular compartment. It is known that this analysis reflects the efficiency of the testicular parenchyma in producing spermatozoa [31]. Moreover, other stereological parameters were not affected by Al exposure, such as length of seminiferous tubules, epithelium height, tubular diameter, and tubule somatic index. Thus, we can assume that the Al concentrations tested did not negatively alter the spermatogenesis. Maybe other factors are involved with the reduction in the testis and parenchyma weight, such as a reduction in the germ cell count or germ cell volume.
Furthermore, we did not observe any alteration in the volumetric proportion and volume of the intertubular components from the testes. However, Leydig cells presented lower nuclear diameter and volume in the Al-exposed animals, with a reduction in the cellular and cytoplasm volume in animals exposed to 10 mg/kg Al. Those alterations might be related to the low concentrations of serum testosterone [36]. In fact, we observed a decrease in the serum testosterone levels of Al-exposed animals. This reduction was also described in previous studies [14, 16, 17, 33, 37]. Guo et al. [38] reported that Al stimulates the activity of the nitric oxide synthase in mice exposed to AlCl3 at 34 mg/kg/IP, causing a subsequent increase of nitric oxide in the testis. It is known that the nitric oxide might be a suppressor of testosterone by controlling androgen synthesis [17, 20]. Moreover, Guo et al. [38] also suggested that excessive nitric oxide compounds might directly inhibit the main second messenger cAMP, which mediates gonadotropin action in the conversion of cholesterol to pregnenolone. The cAMP inhibition reduces the transport of cholesterol to the inner mitochondria membrane, where it is primarily converted to pregnenolone, causing its accumulation in the Leydig cell cytoplasm [38]. It might explain the high percentage of cytoplasm in Leydig cells from animals exposed to 40 mg/kg Al. In this way, Kumar and Singh [39] described an increase of cholesterol levels in the testes of mice exposed to AlCl3 at 100 mg/kg for 30 days.
Nevertheless, the serum testosterone concentration did not negatively influence the spermatogenesis and the testicular histomorphometry. Moselhy et al. [16] and Kumar and Sigh [39] reported mild degenerative changes in the seminiferous epithelium with disorganization and focal areas of necrosed germ cells, besides the sperm depletion in the lumen, and hyperplasia and vacuolization of Leydig cells. Indeed, the presence of testosterone in the tubular compartment is more important for the maintenance of spermatogenesis than serum testosterone [40]. Moreover, the testosterone in seminiferous tubules is 10–100 times higher than that in the serum [40], probably due to its bounding to the androgen-binding protein (ABP) produced by Sertoli cells [41, 42]. Therefore, we suggest that Leydig cells produced less testosterone when exposed to Al in the present study, but the maintenance of adequate testosterone concentration in the seminiferous tubules supported the sperm production.
On the other hand, the decrease in serum testosterone levels may explain the low epididymis weight observed in the Al-exposed animals. The epididymis is recognized as an androgen-dependent organ [43], and three factors contribute to its weight, the presence of fluid and sperm in the luminal duct, as well as the action of testosterone on the epididymal epithelial cells [40]. Overall, a reduction in the luminal fluid and sperm concentration causes changes on the epididymis histomorphometry [44]. In this way, we observed a reduction in the luminal diameter and the percentage of lumen with sperm in the epididymis of the animals exposed to 3.35 × 10−4 and 40 mg/kg Al, especially in the initial segment and caput regions. In fact, most part of these alterations was present in proximal regions of the epididymis, which are more sensitive to variations on testosterone concentrations [45]. Moreover, the increase in the epithelium height and percentage of the epithelium observed in those Al-exposed animals might be one more factor responsible for that reduction of the luminal diameter.
In the present study, the morphometric analysis detected differences among the groups in the epididymis tissue components and Leydig cells that were not easily observed by qualitative assessment under light microscopy. This fact highlights the importance and sensitivity of morphometric and stereological analyses, which are able to detect non-evident alterations in organ morphology that could trigger severe consequences [46].
Moreover, the sperm morphology did not change after Al exposure, and the percentage of motile sperm decreased only in animals exposed to 40 mg/kg Al. Several studies have reported low sperm motility and high percentage of abnormal sperm morphologies in animals exposed orally to Al chloride [14, 18, 33, 39]. The Al effects were attributed to the nitric oxide production that suppresses the testosterone production, the low fructose level in seminal plasma that decreases the sperm metabolism and subsequently their motility [18, 38], and the reactive oxygen species generation that causes lipid peroxidation and DNA damage [16]. In this context, lipid peroxidation might be related to the membrane damages that could explain the low values for structural integrity of sperm membranes observed in animals exposed to 3.35 × 10−4, 10, and 40 mg/kg. Furthermore, low levels of serum testosterone may also be involved in our sperm findings, as it is required for the maintenance of epididymal epithelium functions. Principal cells, the most abundant cell type lining the epididymis, are particularly sensitive to the presence of androgens and play an active role in the formation of luminal fluid [44, 45, 47, 48]. Their proteins secreted in the intraluminal compartment interact with spermatozoa, modifying its surface proteins or other plasma membrane components, whereas others are incorporated into the sperm subcellular domains [49]. Thus, an inadequate protein production and secretion by principal cell may alter the sperm maturation [50], for example, reducing sperm viability by losses of the functional and structural integrity of their membranes as observed herein. It is known that the structural integrity of sperm membranes is an important requirement for sperm capacitation, acrosome reaction, and sperm-egg interaction, which are greatly related to the sperm fertility [51].
Conclusion
Aluminum exposures at 6.7 × 10−5 and 3.35 × 10−4 mg/kg were enough to cause a reduction in the serum testosterone levels, and testis and epididymis weight as observed to high concentrations. In addition, the concentration of 3.35 × 10−4 mg/kg was able to negatively influence the number of sperm with intact membranes as 10 and 40 mg/kg of Al. Moreover, exposure to Al for 112 days produced alterations in Leydig cell stereology and epididymis histomorphometry depending on its concentration. These findings suggest Al may interfere negatively on the reproductive potential of exposed male rats to low concentrations.
References
Yokel RA, McNamara PJ (2001) Aluminium toxicokinetics: an updated minireview. Pharmacol Toxicol 88:159–167
Csuros M, Csuros S (2002) Environmental sampling and analysis for metals. CRC Press, Boca Raton
Fernandez-Lorenzo JR, Cocho JA, Rey-Goldar ML, Couce M, Fraga JM (1999) Aluminum contents of human milk, cow’s milk, and infant formulas. J Pediatr Gastroenterol Nutr 28:270–275
Popi’nska K, Kierku’s J, Lyszkowska M, Socha J, Pietraszek E, Kmiotek W, Ksiazyk J (1999) Aluminum contamination of parenteral nutrition additives, amino acid solutions, and lipid emulsions. Appl Nutr Investig 15:683–686
Ochmanski W, Barabasz W (2010) Aluminium-occurrence and toxicity for organisms. Przegl Lek 57:665–668
WHO World Health Organization. 2011. Guidelines for drinking water quality. 4th ed. 1 Geneva
Deng Z, Coudray C, Gouzoux L, Mazur A, Rayssiguier Y, Pépin D (2000) Effects of acute and chronic coingestion of AlCl3 with citrate or polyphenolic acids on tissue retention and distribution of aluminum in rats. Biol Trace Elem Res 76:245–256
Alfrey CA, Hegg M, Craswell P (1980) Metabolism and toxicity of aluminum in renal failure. Am J Clin Nutr 33:1509–1516
Rana SVS (2007) Environmental pollutions: health and toxicology. Alpha Science International, Oxford
Greger JL (1993) Aluminum metabolism. Annu Rev Nutr 13:43–63
Chen TJ, Hui HS, Wang DC, Chen SS (2010) The protective effect of rho-associated kinase inhibitor on aluminum-induced neurotoxicity in rat cortical neurons. Toxicol Sci 116:264–272
Erazi H, Sansar W, Ahboucha S, Gamrani H (2010) Aluminum affects glial system and behavior of rats. Comptes Rendus - Biol 333:23–27
Peto MV (2010) Aluminium and iron in humans: bioaccumulation, pathology, and removal. Rejuvenation Res 13:589–598
Hala AHK, Inas ZAA, Gehan MK (2010) Grape seed extract alleviate reproductive toxicity caused by aluminium chloride in male rats. J Am Sci 6:1200–1209
Hasseeb MM, Al-Hizab FA, Hussein YA (2011) A histopathologic study of the protective effect of grape seed extract against experimental aluminum toxicosis in male rat. Sci J King Faisal Univ (Basic Appl Sci) 12:283–299
Moselhy WA, Helmy NA, Abdel-Halim BR, Nabil TM, Abdel-Hamid MI (2012) Role of ginger against the reproductive toxicity of aluminium chloride in albino male rats. Reprod Domest Anim 47:335–343
Yousef MI, Salama AF (2009) Propolis protection from reproductive toxicity caused by aluminium chloride in male rats. Food Chem Toxicol 47:1168–1175
Yousef MI, El-Morsy AMA, Hassan MS (2005) Aluminium-induced deterioration in reproductive performance and seminal plasma biochemistry of male rabbits: protective role of ascorbic acid. Toxicology 215:97–107
Xie CX, Yokel RA (1996) Aluminum facilitation of iron mediated lipid peroxidation is dependent on substrate, pH and aluminum and iron concentrations. Arch Biochem Biophys 327:222–226
Dobashi M, Fujisawa M, Yamazaki T, Okuda Y, Kanzaki M, Tatsumi N, Tsuji T, Okada H, Kamidono S (2001) Inhibition of steroidogenesis in Leydig cells by exogenous nitric oxide occurs independently of steroidogenic acute regulatory protein (star) mRNA. Arch Androl 47:203–209
Krasovskiĭ GN, Vasukovich LY, Chariev OG (1979) Experimental study of biological effects of leads and aluminum following oral administration. Environ Health Perspect 30:47–51
Russel LD, Ettlin RA, Sinhafukjm AP, Clegg ED (1990) The classification and timing of spermatogenesis. In: Russell LD, Ettlin RA, Sinha Hikim AP, Clegg ED (eds) Histological and histopathological evaluation of the testis. Cache River Press, Clearwater, pp 1–40
Malaivijitnond S, Takenaka O, Sankai T, Yoshida T, Cho F, Yoshikawa Y (1998) Effects of single and multiple injections of ketamine hydrochloride on serum hormone concentrations in male Cynomolgus monkeys. Lab Anim Sci 48:270–274
Souza ACF, Marchesi SC, Ferraz RP, Lima GDA, Oliveira JA, Neves MM (2016) Effects of sodium arsenate and arsenite on male reproductive functions in Wistar rats. J Toxicol Environ Heal, Part A 79:274–286
Morakinyo AO, Achema PU, Adegoke OA (2010) Effect of Zingiber officinale (ginger) on sodium arsenite induced reproductive toxicity in male rats. Afr J Biomed Res 13:39–45
Filler R (1993) Methods for evaluation of rat epididymal sperm morphology. In: Chapin RE, Heindel JJ (eds) Male reproductive toxicology. Academic Press, San Diego, pp 334–343
Harrison RAP, Vickers SE (1990) Use of fluorescent probes to assess membrane integrity in mammalian spermatozoa. J Reprod Fertil 88:343–352
Turner TT, Johnston DS, Jelinsky SA, Tomsig JL, Finger J (2007) Segment boundaries of the adult rat epididymis limit interstitial signaling by potential paracrine factors and segments lose differential gene expression after efferent duct ligation. Asian J Androl 9:565–573
Amann RP (1970) Sperm production rates. In: Johnson AD, Gomes WR, Vandemark NL (eds) The testis. Academic Press, New York, pp 433–482
Johnson L, Neaves WB (1981) Age-related changes in the Leydig cell population, seminiferous tubules, and sperm production in stallions. Biol Reprod 24:703–712
França LR, Russell LD (1998) Testis morphometric seminiferus epithelium cycle length, and daily sperm production on domestic cats (Felis catus). Biol Reprod 68:1554–1561
Russel LD, Ettilin RA, Hikim, APS, Clegg ED (1990) Histological and histopathological evaluation of the testis. Cache River Press, Clearwater, Florida,
Ige SF, Akhigbe RE (2012) Effect of aluminium and Allium cepa on reproduction. J Hum Reprod Sci 5:200–205
Kalaiselvi A, Ramalingam V (2016) Effect of Zingiber officinale on aluminium induced epidydimal sperm abnormalities in albino rats. World J Pharm Pharm Sci 5:1664–1680
Zhu YZ, Sun H, Fu Y, Wang J, Song M, Li M, Li YF, Miao LG (2014) Effects of sub-chronic aluminum chloride on spermatogenesis and testicular enzymatic activity in male rats. Life Sci 102:36–40
Russell LD (1996) Mammalian Leydig cell structure. In: Payne AH, Hardy MP, Russell LD (eds) The Leydig cell. Cache River Press, Vienna, pp 218–222
Muselin F, Cristina RT, Igna V, Dumitrescu E, Brezovan D, Trif A (2016) The consequences of aluminium intake on reproductive function in male rats: a three-generation study. Turk J Med Sci 46:1240–1248
Guo CH, Lin CY, Yeh MS, Hsu G-SW (2005) Aluminum-induced suppression of testosterone through nitric oxide production in male mice. Environ Toxicol Pharmacol 19:33–40
Kumar P, Singh P (2015) Protective role of Tribulus terrestris on aluminium chloride-induced reproducive toxicity in the male laboratory mouse. Int J Pharm Sci Res 6:2395–2405
Robaire B, Seenundun S, Hamzeh M, Lamour S-A (2007) Androgenic regulation of novel genes in the epididymis. Asian J Androl 9:545–553
Sharpe RM (2005) Sertoli cell endocrinology and signal transduction: androgen regulation. In: Skinner MK, Griswold MD (eds) Sertoli cell biology. Academic Press, San Diego, pp 199–216
Ma Y, Yang H-Z, Xu L-M, Huang Y-R, Dai H-L, Kang X-N (2015) Testosterone regulates the autophagic clearance of androgen binding protein in eat Sertoli cells. Sci Rep 5:8894
Robaire B, Hinton BT (2015) The epididymis. In: Plant TM, Zeleznik AJ (eds) Knobil and Neill’s physiology of reproduction. Elsevier, pp 691–771
Hamzeh M, Robaire B (2009) Effect of testosterone on epithelial cell proliferation in the regressed rat epididymis. J Androl 30:200–212
Robaire B, Hamzeh M (2011) Androgen action in the epididymis. J Androl 32:592–599
Predes FS, Diamante MAS, Dolder H (2010) Testis response to low doses of cadmium in Wistar rats. Int J Exp Path 91:125–131
Hermo L, Robaire B (2002) Epididymal cell types and their functions. In: Robaire B, Hinton BT (eds) The epididymis—from molecules to clinical practice: Kluwer Academic/Plenum Publisher, New York, pp. 81–102
Cheung K.-h, Leug GPH, Leug MCT, Shum WWC, Zhou W-l, Woug PYD (2005) Cell-cell interaction underlies formation of fluid in the male reproductive tract of the rat. J Gen Physiol 125:443–454
Sullivan R, Saez F (2013) Epididymosomes, prostasomes, and liposomes: their roles in mammalian male reproductive physiology. Reproduction 146:R21–R35
Berihu BA (2015) Histological and functional effect of aluminium on male reproductive system. Int J Pharm Sci Res 6:1122–1132
Jeyendran RS, Van der Ven HH, Perez-Pelaez M, Crabo BG, Zaneveld LJD (1984) Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J Reprod Fertil 70:219–228
Funding
This work was supported by the Fundação de Amparo à Pesquisa do Estado de Minas Gerais, FAPEMIG (APQ-04083-10).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experimental procedures were in accordance with the ethical principles in animal research adopted by the animal ethics committees (CEUA protocol number 19/2011).
Conflict of Interest
The authors declare that there are no conflicts of interest.
Electronic supplementary material
ESM 1
(DOCX 35 kb)
Rights and permissions
About this article
Cite this article
Mouro, V.G.S., Menezes, T.P., Lima, G.D.A. et al. How Bad Is Aluminum Exposure to Reproductive Parameters in Rats?. Biol Trace Elem Res 183, 314–324 (2018). https://doi.org/10.1007/s12011-017-1139-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-1139-3