Abstract
Diarrhea is a gastrointestinal symptom associated with systemic anaphylaxis and could be induced by increased colonic motility. We determined colonic motility and expulsion by measuring the intracolonic pressure (ICP) and expelled fluid weight in anesthetized rats during anaphylactic hypotension. Substantial systemic hypotension occurred in every sensitized rat after antigen injection. One min after antigen injection, ICP began to increase and remained elevated for 5 min, which was revealed to represent tonic contraction by the video-recording procedure, and was accompanied by increased colonic fluid expulsion. Parasympathectomy composed of subdiaphragmatic vagotomy combined with pelvic nerve transection reduced the duration of the tonic contraction, but not expelled colonic fluid. Furthermore, denervation of afferent parasympathetic nerves produced essentially the same effect as parasympathectomy. Sympathectomy did not significantly change any parameters. In conclusion, the colonic motility during anaphylactic hypotension is characterized by 5-min lasting tonic contraction which is associated with increased colonic fluid expulsion and is involved by parasympathetic nerves, especially their afferents, but not sympathetic nerves, in anesthetized rats.
Similar content being viewed by others
Introduction
Diarrhea is one of the gastrointestinal symptoms during systemic anaphylaxis [1] and is at least in part caused by increased colonic motility [2]. Concerning anaphylaxis-associated colonic motility change, the local antigen challenge was examined ex vivo in rat colon segments and in vivo in rats. In the ex vivo experiments, isolated proximal [3] and distal [4, 5] colon, both of which were excised from sensitized rats, showed prolonged or sustained contraction, when the antigen was locally challenged. However, colonic contractility was not well studied with direct measurement of the colonic intraluminal pressure in in vivo animals in response to the antigen, although administration of the ovalbumin antigen into the colon was reported to increase colonic myoelectric spike activity [6] and the rate of aboral colonic transit [5]. Furthermore, the responses of colonic motility and colonic intraluminal expulsion were not examined during anaphylactic hypotension or systemic anaphylaxis in anesthetized rats.
The autonomic nervous system can be divided into parasympathetic and sympathetic components. In animals, the nerves conveying the parasympathetic or sympathetic outflow to the large bowel vary across species [7, 8]. In rats, there is dual parasympathetic innervation in the distal colon by both the vagal nerve [9, 10] and the pelvic nerve [9, 11]. The sympathetic fibers to the distal colon are derived from the lumbar preganglionic outflow that finally runs to the hypogastric nerves and the lumbar colonic nerves [12]. The electrical stimulation of the parasympathetic and sympathetic nerves innervating the distal colon causes modulation of colonic motility: electrical stimulation of the vagal nerves or the pelvic nerves elicits significant contractions in the mid colon and distal colon, while the stimulation of the hypogastric nerve causes relaxations in the mid colon and distal colon in anesthetized rats [9]. However, it is not known whether the extrinsic autonomic nerves innervating the distal colon modulate the colonic motility during anaphylactic hypotension.
Thus, we determined the colonic motility and colonic fluid expulsion during anaphylactic hypotension with special reference to the roles of the extrinsic parasympathetic and sympathetic nerves innervating the distal colon in anesthetized rats sensitized with ovalbumin.
Materials and methods
Animals
Male Sprague–Dawley rats weighing 352 ± 6 g (n = 40) were used in this study. Rats were maintained at 23 °C and under pathogen-free conditions on a 12:12-h dark/light cycle and allowed food and water ad libitum. All experiments conducted in the present study were approved by the animal research committee of Kanazawa Medical University (No. 2017-99).
Sensitization
Two weeks before experiments, rats were actively sensitized by the subcutaneous injection of an emulsion made by mixing equal volumes of complete Freund’s adjuvant (0.5 ml) with 1 mg ovalbumin (grade V, Sigma-Aldrich, St. Louis, MO, USA) dissolved in physiologic saline (0.5 ml) [13, 14]. Non-sensitized rats were injected with the adjuvant and ovalbumin-free saline.
Surgical preparation
After an overnight fast with free access to water, rats were anesthetized with ketamine hydrochloride (50 mg/kg, i.m.), followed by α-chloralose (40 mg/kg, i.v.), and placed supinely on a heating pad with body temperature maintained at 36–37 °C. The trachea was cannulated to facilitate spontaneous breathing. Polyethylene catheters were inserted into the left femoral vein and the right carotid artery for a continuous infusion of saline (20 ml/kg/h) and injections of drugs and for measurement of the systemic arterial pressure (SAP), respectively.
The motility of the distal colon was measured continuously according to the previous studies [15, 16]. After an abdomen midline incision, the distal colon was exposed and cut at the splenic flexure. The oral cut end was cannulated with polyethylene tubing, the end of which was kept open outside. The distal cut end was also cannulated with the inflow stainless steel cannula, which was connected via polyethylene tubing to a Mariotte bottle filled with warm saline (37–39 °C) to perfuse the colon at a constant pressure. The outflow stainless steel cannula was inserted through the anus and fixed at the colorectal junction, and this cannula was connected to the outflow tubing. The intracolonic pressure (ICP) was measured using a pressure transducer (TP-400T, Nihon-Kohden, Tokyo, Japan) via the side-arm from the outflow tubing, and was maintained at 1–2 mmHg by adjusting the height of the Mariotte bottle and the outflow tubing. The intracolonic fluid was expelled through a one-way valve set at the end of the outflow tubing and was collected drop by drop in a plastic cup, the weight of which was continuously and cumulatively measured with a force transducer (SB-1T, Nihon-Kohden) for determining the colonic effluent (CE).
To determine the roles of the extrinsic parasympathetic and sympathetic nerves innervating the distal colon in the responses of ICP and CE to the antigen, we performed separately parasympathectomy or sympathectomy. The distal colon is supplied by dual parasympathetic innervation, namely the vagal and pelvic nerves [7, 9]. To ablate parasympathetic nerves to the distal colon, after the abdomen midline incision, denervation of both the subdiaphragmatic vagi and the pelvic nerves was performed by directly cutting the dorsal and ventral bundles of the vagi around the esophagus [17] and bilateral pelvic nerves [18]. Furthermore, to investigate whether parasympathectomy exerted its action via parasympathetic afferent pathways, we examined the effect of parasympathetic afferent denervation using the sensory neurotoxin capsaicin: the nerve bundles of the subdiaphragmatic vagi and the pelvic nerves were tied loosely with a cotton thread soaked in 1% capsaicin dissolved in the vehicle containing 10% ethanol, 10% Tween-80, and saline for 30 min [19]. For colonic sympathectomy, the lumbar splanchnic nerves were cut carefully [18]. The non-sensitized control rats and sensitized rats for the anaphylaxis group, as described below, underwent sham operations that did not include the above-mentioned denervation. In the preliminary study, we confirmed that sham maneuvers did not affect the response of the intact sensitized rats to the antigen by comparing the response of intact sensitized rats with that of the sham-operated sensitized rats (data not shown).
Experimental protocol
After surgery, the baseline was measured for at least 30 min prior to an intravenous injection of the ovalbumin antigen (0.6 mg, i.v.). The SAP, heart rate (HR), ICP, and CE were continuously monitored for 60 min after antigen injection. These variables were recorded at 40 Hz by PowerLab (AD Instruments, Bella Vista, NSW, Australia).
Sensitized rats were assigned to the following groups: (1) anaphylaxis (n = 8), (2) parasympathectomy (n = 8), (3) parasympathetic deafferentation, i.e., parasympathetic afferent denervation (n = 8), and (4) sympathectomy (n = 8). The non-sensitized rats were served as the control group (n = 8).
Statistical analysis
Results are expressed as mean ± SEM. Intragroup comparison was performed by two-way repeated-measures ANOVA and a P value less than 0.05 was considered significant. When a significant difference was obtained, Dunnett’s post hoc test was performed. Between-group comparison was performed by two-way ANOVA followed by the Bonferroni post hoc test.
Results
Colonic motility during anaphylactic hypotension
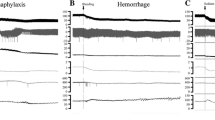
Figure 1a shows a representative example of the colonic responses to an intravenous injection of antigen in a sensitized rat, and Fig. 2a shows the time-course changes in SAP and HR after antigen in all groups studied. Systemic hypotension and an increase in HR were observed in all sensitized group rats (Fig. 2). In the anaphylaxis group, at 34 ± 5 s after antigen injection, SAP began to decrease from the baseline of 105 ± 4 mmHg, reaching a nadir of 36 ± 2 mmHg at 10 min, and then gradually returned to 63 ± 8 mmHg at 60 min (Fig. 2a). The HR increased significantly at 6 min after antigen injection and thereafter remained elevated throughout the experimental period (Fig. 2b).
Representative recordings of the systemic arterial pressure, heart rate, colonic effluent weight, and intracolonic pressure before and after intravenous injections of the ovalbumin antigen in sensitized rats of the anaphylaxis (a), parasympathectomy (b), and sympathectomy (c) groups. The horizontal bar with an asterisk indicates the period of tonic colonic contraction. The horizontal bar with double asterisks in a indicates the 10-min interval after the cessation of tonic contraction for evaluation of the frequency and amplitude of phasic increases in intracolonic pressure
Summarized data of the time-course changes in the systemic arterial pressure (a) and heart rate (b) after antigen injection in the control (circle, n = 8), anaphylaxis (square, n = 8), parasympathectomy (inverted triangle, n = 8), sympathectomy (triangle, n = 8), and parasympathetic deafferentation (diamond, n = 8) groups. Values are expressed as mean ± SEM. Open symbols, P < 0.05 versus the baseline; #P < 0.05 versus the anaphylaxis group
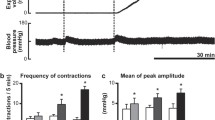
As shown in Fig. 1, in the anaphylaxis group, ICP before antigen injection showed spontaneous and phasic increase, the sinusoidal pulsation. In contrast, at 50 s after antigen injection, following anaphylactic hypotension, ICP sustainedly increased and kept elevated until 7 min 40 s (single asterisk in Fig. 1a). The video recording revealed that this sustained increase in ICP represented tonic contraction of the colon, as shown in the Supplemental Video. Actually, tonic contraction occurred after a time lag of 56 ± 4 s following antigen injection. Figure 3a, b shows ICP at baseline and during tonic contraction, and duration of the tonic contraction in each group. In the anaphylaxis group, ICP during tonic contraction significantly increased from the baseline of 1.2 ± 0.1 to 1.8 ± 0.2 mmHg with the duration of tonic contraction being 377 ± 54 s. Following tonic contraction, irregular phasic increases in ICP, which seemed different from the basal rhythmical ones, were observed, as shown in Fig. 1a. We analyzed the frequency (Fig. 3c) and amplitude (Fig. 3d) of the phasic ICP at a 10-min interval at baseline and after antigen, when the measurement started from the cessation of tonic contraction. The period of the first 10-min measurement was marked with double asterisks in Fig. 1a. In the anaphylaxis group, the frequency of phasic ICP increased only for first 10 min after tonic contraction (Fig. 3c), whereas the amplitude did not change significantly (Fig. 3d).
Summarized data of the intracolonic pressure at baseline and during tonic contraction (a) and the duration of tonic contraction (b) in the control (white bars, n = 8), anaphylaxis (black bars, n = 8), parasympathectomy (stripe bars, n = 8), sympathectomy (gray bars, n = 8) and parasympathetic deafferentation (dark gray bars, n = 8) groups. Values are expressed as mean ± SEM. Tonic contraction was not detected in the control group (N.D.); *P < 0.05 versus the baseline; #P < 0.05 versus the anaphylaxis group. Summarized data of the time-course changes in the colonic contraction frequency (c) and mean colonic contraction amplitude (d) after antigen injection in the control (circle, n = 8), anaphylaxis (square, n = 8), parasympathectomy (inverted triangle, n = 8), sympathectomy (triangle, n = 8) and parasympathetic deafferentation (diamond, n = 8) groups. Values are expressed as means ± SEM. Open symbols, P < 0.05 versus the baseline
For measurement of CE, we measured the expelled fluid weight at 5-min intervals. Following antigen injection, CE increased soon after the start of the initial ICP increase, tonic contraction, as shown in Fig. 1a. Actually, during first 5 min after antigen, CE significantly increased fourfold as compared with baseline (Fig. 4). However, thereafter, it did not increase but rather decreased. In the non-sensitized control rats, antigen injection did not evoke any significant changes in the SAP, HR, ICP, or CE.
Summarized data of the time-course changes in the colonic effluent after antigen injection in the control (circle, n = 8), anaphylaxis (square, n = 8), parasympathectomy (inverted triangle, n = 8), sympathectomy (triangle, n = 8), and parasympathetic deafferentation (diamond, n = 8) groups. Values are expressed as mean ± SEM. Open symbols, P < 0.05 versus the baseline
Effects of parasympathectomy, sympathectomy, or parasympathetic deafferentation
Figure 1b, c shows representative responses to an antigen challenge in a parasympathectomized rat and a sympathectomized rat, respectively. As shown in Fig. 2, in both the parasympathectomy and sympathectomy groups, anaphylactic hypotension and tachycardia occurred in a manner similar to that in the anaphylaxis group. The basal ICP, or the frequency or amplitude of phasic ICP at baseline in either the parasympathectomy or sympathectomy group was not significantly different from those of the anaphylaxis group (Fig. 3a, c, d), suggesting that either parasympathectomy or sympathectomy did not affect basal colonic motility. After antigen injection, ICP in both the parasympathectomized and sympathectomized rats changed qualitatively in the same manner as that of the anaphylaxis group: ICP showed sustained increases followed by somewhat irregular phasic changes. Of note, as shown in Fig. 1b, the duration of tonic contraction of the parasympathectomy group was significantly shorter by 46% (202 ± 37 s) than that of the anaphylaxis group (377 ± 54 s), while that of the sympathectomy group (415 ± 33 s) was comparable to that of the anaphylaxis group (Fig. 3b). The frequency of phasic ICP increased transiently in the parasympathectomy, sympathectomy, and parasympathetic deafferentation groups during first 10 min after cessation of tonic contraction, although that of the latter group did not reach statistical significance (Fig. 3c). No significant changes in the amplitude of phasic ICP after antigen were found in denervation groups (Fig. 3d). Similar to the anaphylaxis group, in the parasympathectomy and sympathectomy group, CE increased transiently in accordance with tonic contraction only for first 5 min after antigen (Figs. 1, 4). Finally, we determined the role of parasympathetic afferent nerves. Parasympathetic afferent denervation with capsaicin caused essentially the same effects as parasympathectomy: as compared with the anaphylaxis group, the duration of tonic contractions was shorter in the deafferentation group, and other variables were not significantly different from those in the parasympathectomy group, as shown in Figs. 2, 3, and 4.
Discussion
In the present study, we demonstrated that short-lasting tonic contraction accompanied by transient increases in intracolonic fluid expulsion was observed during anaphylactic hypotension in anesthetized rats. In addition, the duration of anaphylaxis-associated tonic contraction was shortened by parasympathectomy of the subdiaphragmatic vagotomy combined with the pelvic nerve transection. Furthermore, this effect of parasympathectomy could be ascribed to that of parasympathetic afferent denervation, because deafferentation produced the same effects as those of parasympathectomy. This is the first study to demonstrate that tonic colonic contraction and the subsequent transient colonic content expulsion develop during anaphylactic hypotension in in vivo anesthetized rats and that the parasympathetic nerves are partly involved in the tonic contraction.
Anaphylaxis may cause different effects on gut motility depending on the region of the gastrointestinal tract, although the isolated gut segments derived from the stomach [20], small intestine [21], and colon [3,4,5] showed exclusively constrictive responses to local antigen challenge. On the other hand, local anaphylaxis evoked by direct antigen challenge to the relevant site in in vivo rats produced different motor responses in various gastrointestinal organs such as stomach, jejunum, and colon: in the stomach, anaphylaxis resulted in a transient reduction in gastric antral contractions [20, 22] and delayed gastric emptying [20, 22, 23]; in the small intestine, interruption of normal migrating motor complexes, initiation of a succession of aborally propagating bursts of spike activity, and an increased rate of aboral transit [24,25,26,27]; in the colon, increased colonic myoelectric spike activity [6] and an increased rate of aboral colonic transit [5]. Here, we, for the first time, demonstrated that in in vivo sensitized rats, anaphylactic hypotension is accompanied by short-lasting tonic colonic contraction along with colonic intraluminal propulsion.
It was reported that local challenge of the antigen caused prolonged or sustained contraction of colon smooth muscles excised from rats sensitized with ovalbumin [3,4,5]. The present study has shown that the similar pattern of tonic contraction also occurred in vivo in anesthetized rats during anaphylactic hypotension. The above-mentioned previous studies reported that anaphylaxis-induced colonic contraction was induced by mediators produced by local anaphylactic reactions such as platelet-activating factor [4, 5], leukotriene D4 [4, 5], and metabolites of the lipoxygenase [4, 5] and cyclooxygenase [3,4,5]. In the present in vivo study, in response to the antigen injected intravenously, these anaphylactic mediators may be generated in the colon or other remote tissues, and produce tonic contraction.
The duration of anaphylaxis-induced tonic contraction of the distal colon was shortened by parasympathectomy. This finding suggests that parasympathetic nerves of the vagal nerve and/or pelvic nerve augment anaphylaxis-induced colonic contraction. Parasympathectomy disrupts both efferents and afferents. In the present study, denervation of parasympathetic afferents with capsaicin produced the same effects as parasympathectomy. This finding indicates that the parasympathectomy-induced shortening of the duration of tonic contraction evoked by systemic anaphylaxis is presumably due to parasympathetic deafferentation. Fargeas et al. [28] reported that in the intestinal anaphylaxis of conscious ovalbumin-sensitized Hooded Lister rats, the duration of the antigen-induced alterations in jejunal motility is shortened by pretreatment with capsaicin which causes denervation of sensory afferent nerves, suggesting that capsaicin-sensitive afferent nerve endings are involved in anaphylaxis-induced intestinal motility alterations. Furthermore, Castex et al. [29] using the same anaphylaxis models demonstrated that vagal afferent denervation by perivagal capsaicin treatment significantly reduced both brain stem expression of c-fos, a marker of neuron activation, and intestinal motility dysfunction. Similar interactions between the afferent sensory vagal or pelvic nerves and the central nervous system may occur for the colon during systemic anaphylaxis. On the other hand, the role of parasympathetic efferents is not known, but seems to be little for the following evidence: c-fos positive neurons were not seen in the dorsal motor nucleus, the center of vagal efferents, during rat systemic anaphylaxis [29]; vagal efferents might not be activated during anaphylaxis. Actually, we have recently demonstrated that the activity of vagal efferents innervating the stomach did not change during systemic anaphylaxis in anesthetized rats [30].
CE increased significantly and exclusively for first 5 min after antigen in accordance with tonic contraction in every sensitized rat group, as shown in Figs. 1 and 4. This finding suggests that intraluminal colonic contents were expelled by tonic contraction. Of note, the duration of tonic contraction of the parasympathectomy group was 46% shorter than that of the anaphylaxis group, whereas increased CE was similar between these two groups, suggesting that the amount of colonic expelled contents does not depend on the duration of tonic contraction. Furthermore, the anaphylaxis-increased propulsion was only fourfold baseline and was limited within 5 min after antigen. Based on this finding, in this anaphylactic hypotension model, the antigen-induced increase in colonic motility seems to be small and may not so much contribute to colonic content propulsion. Thus, the colonic lesions in the present rat anaphylaxis model may not account for the anaphylaxis-induced diarrhea.
In conclusion, the systemic anaphylaxis in rats is characterized by initial short-lasting tonic contraction of the colon, which was associated with increased colonic fluid expulsion. Parasympathetic nerves, especially afferents, but not sympathetic nerves, are involved in systemic anaphylaxis-associated tonic colonic contraction.
References
Beyer K, Eckermann O, Hompes S, Grabenhenrich L, Worm M (2012) Anaphylaxis in an emergency setting—elicitors, therapy and incidence of severe allergic reactions. Allergy 67:1451–1456
Karaus M, Wienbeck M (1991) Colonic motility in humans—a growing understanding. Baillieres Clin Gastroenterol 5:453–478
Kadowaki H, Yamamoto T, Kageyama-Yahara N, Kurokawa N, Kadowaki M (2008) The pathophysiological roles of COX-1 and COX-2 in the intestinal smooth muscle contractility under the anaphylactic condition. Biomed Res 29:113–117
Oliver MR, Tan DT, Scott RB (1995) Intestinal anaphylaxis: mediation of the response of colonic longitudinal muscle in rat. Am J Physiol 268:G764–G771
Tobin G, Giglio D, Lundgren O (2009) Muscarinic receptor subtypes in the alimentary tract. J Physiol Pharmacol 60:3–21
Oliver MR, Tan DT, Kirk DR, Rioux KP, Scott RB (1997) Colonic and jejunal motor disturbances after colonic antigen challenge of sensitized rat. Gastroenterology 112:1996–2005
Langley JN, Anderson HK (1985) On the innervation of the pelvic and adjoining viscera: part I. The lower portion of the intestine. J Physiol 18:67–105
Langley JN, Anderson HK (1896) The innervation of the pelvic and adjoining viscera: part VII. Anatomical observations. J Physiol 20:372–406
Tong WD, Ridolfi TJ, Kosinski L, Ludwig K, Takahashi T (2010) Effects of autonomic nerve stimulation on colorectal motility in rats. Neurogastroenterol Motil 22:688–693
Berthoud HR, Carlson NR, Powley TL (1991) Topography of efferent vagal innervation of the rat gastrointestinal tract. Am J Physiol 260:R200–R207
Luckensmeyer GB, Keast JR (1998) Projections of pelvic autonomic neurons within the lower bowel of the male rat: an anterograde labelling study. Neuroscience 84:263–280
Corman ML (2005) Colon and rectal surgery, 5th edn. Lippincott Williams and Wilkins, Philadelphia
Shibamoto T, Cui S, Ruan Z, Liu W, Takano H, Kurata Y (2005) Hepatic venoconstriction is involved in anaphylactic hypotension in rats. Am J Physiol Heart Circ Physiol 289:H1436–H1441
Mukai K, Kuda Y, Shibamoto T, Tanida M, Kurata Y, Yokoyama H (2018) Renal response to anaphylaxis in anesthetized rats and isolated perfused rat kidneys: roles of nitric oxide. J Physiol Sci 68:689–697
Shimizu Y, Chang EC, Shafton AD, Ferens DM, Sanger GJ, Witherington J, Furness JB (2006) Evidence that stimulation of ghrelin receptors in the spinal cord initiates propulsive activity in the colon of the rat. J Physiol 576:329–338
Bogeski G, Shafton AD, Kitchener PD, Ferens DM, Furness JB (2005) A quantitative approach to recording peristaltic activity from segments of rat small intestine in vivo. Neurogastroenterol Motil 17:262–272
Tanida M, Satomi J (2011) Effects of intragastric injection of glutamate on efferent sympathetic nerve activity in rats. Neurosci Lett 491:211–215
Maruyama S, Okabe S, Endo M, Sato K, Iwai T (2003) The role of the rectal branches of pelvic plexus in defecation and colonic motility in a canine model. J Med Dent Sci 50:275–284
Sabbatini ME, Rodríguez MR, Dabas P, Vatta MS, Bianciotti LG (2007) C-type natriuretic peptide stimulates pancreatic exocrine secretion in the rat: role of vagal afferent and efferent pathways. Eur J Pharmacol 577:192–202
Catto-Smith AG, Tan D, Gall DG, Scott RB (1994) Rat gastric motor response to food protein-induced anaphylaxis. Gastroenterology 106:1505–1513
Scott RB, Gall DG, Maric M (1990) Mediation of food protein-induced jejunal smooth muscle contraction in sensitized rats. Am J Physiol 259:G6–G14
Catto-Smith AG, Patrick MK, Scott RB, Davison JS, Gall DG (1989) Gastric response to mucosal IgE mediated reactions. Am J Physiol 257:G704–G708
Kuda Y, Shibamoto T, Zhang T, Yang W, Tanida M, Kurata Y (2018) Gastric vascular and motor responses to anaphylactic hypotension in anesthetized rats, in comparison to those with hemorrhagic or vasodilator-induced hypotension. J Physiol Sci 68:253–260
Maric M, Gall DG, Scott RB (1989) The effect of IgE mediated intestinal anaphylaxis on intestinal transit. Can J Physiol Pharmacol 67:1437–1441
Scott RB, Diamant SC, Gall DG (1988) Motility effects of intestinal anaphylaxis in the rat. Am J Physiol 255:G505–G511
Diamant SC, Gall DG, Scott RB (1989) The effect of intestinal anaphylaxis on postprandial motility in the rat. Can J Physiol Pharmacol 67:1326–1330
Scott RB, Tan DT, Sharkey KA (2000) Effect of splanchnectomy on jejunal motility and fos expression in brain stem after intestinal anaphylaxis in rat. Am J Physiol Gastrointest Liver Physiol 279:G990–G997
Fargeas MJ, Fioramonti J, Bueno L (1993) Involvement of capsaicin-sensitive afferent nerves in the intestinal motor alterations induced by intestinal anaphylaxis in rats. Arch Allergy Immunol 101:190–195
Castex N, Fioramonti J, Fargeas MJ, Bueno L (1995) c-fos expression in specific rat brain nuclei after intestinal anaphylaxis: involvement of 5-HT3 receptors and vagal afferent fibers. Brain Res 688:149–160
Kuda Y, Tanida M, Chen F, Kurata Y, Shibamoto T (2019) Anaphylaxis stimulates afferent vagal nerve activity and efferent sympathetic nerve activity in the stomach of anesthetized rats. Am J Physiol Regul Integr Comp Physiol 317:R337–R345
Acknowledgements
This study was supported by JSPS KAKENHI Grant Number 19K09445, a Grant for Collaborative Research from Kanazawa Medical University (C2017-1) and a Grant for Kieikai Research Foundation.
Author information
Authors and Affiliations
Contributions
TS, the corresponding author, was responsible for the study concept, design, writing, and supervision; TZ and MT performed experiment: YuK and WY analyzed the data; YaK wrote the paper. All authors approved the manuscript.
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement on the welfare of animals
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Legends of the Supplemental video (Animation 1) This video is a representative recording of an example of experiment showing that colonic motility changes occurred as tonic contraction, which started at the anal side (the right side) at 50 s after an intravenous injection of the antigen. The time when the antigen was intravenously injected can be temporally identified by the “antigen injection” described in the video, and the time when contraction started can be identified by the “contraction start” in the video. For the direction in the video, the left side is oral side and the right side is anal side. The distal colon was cannulated with the stainless steel inflow cannula at the oral side. It could be easily observed that the tonic contraction were propagating from the anal side to the oral side, and finally distributed over the whole distal colon. Actually, the final width of the distal colon was much smaller than that before antigen injection. (MPG 28748 kb)
About this article
Cite this article
Zhang, T., Shibamoto, T., Tanida, M. et al. Tonic contraction develops in the colon during anaphylactic hypotension in anesthetized rats. J Physiol Sci 69, 953–960 (2019). https://doi.org/10.1007/s12576-019-00710-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12576-019-00710-8