Abstract
Rectal distension (RD) is known to induce intestinal dysmotility. Few studies were performed to compare effects of RD, colon distension (CD) and duodenal distension (DD) on small bowel motility. This study aimed to investigate effects and underlying mechanisms of distensions in these regions on intestinal motility and slow waves. Eight dogs chronically implanted with a duodenal fistula, a proximal colon fistula, and intestinal serosal electrodes were studied in six sessions: control, RD, CD, DD, RD + guanethidine, and CD + guanethidine. Postprandial intestinal contractions and slow waves were recorded for the assessment of intestinal motility. The electrocardiogram was recorded for the assessment of autonomic functions. (1) Isobaric RD and CD suppressed intestinal contractions (contractile index: 6.0 ± 0.4 with RD vs. 9.9 ± 0.9 at baseline, P = 0.001, 5.3 ± 0.2 with CD vs. 7.7 ± 0.8 at baseline, P = 0.008). Guanethidine at 3 mg/kg iv was able to partially block the effects. (2) RD and CD reduced the percentage of normal intestinal slow waves from 92.1 ± 2.8 to 64.2 ± 3.4 % (P < 0.001) and from 90 ± 2.7 to 69.2 ± 3.7 % (P = 0.01), respectively. Guanethidine could eliminate these inhibitory effects. (3) DD did not induce any changes in small intestinal contractions and slow waves (P > 0.05). (4) The spectral analysis of the heart rate variability showed that both RD and CD increased sympathetic activity (LF) and reduced vagal activity (HF) (P < 0.05). Isobaric RD and CD could inhibit postprandial intestinal motility and impair intestinal slow waves, which were mediated via the sympathetic pathway. However, DD at a site proximal to the measurement site did not seem to impair small intestinal contractions or slow waves.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clinically, patients with chronic idiopathic constipation often complain about the upper abdominal symptoms such as bloating, abdominal distension, pain, cramps, nausea, and vomiting [1]. These symptoms are related to the impaired gastrointestinal myoelectrical activity, delayed gastric emptying, and slow small bowel transit [1–7]. It is reported that abnormalities of upper gut motility occur frequently in 70 % of the patients with slow-transit constipation [1].
In chronic slow-transit constipation, stasis of the rectal and colonic contents may result in rectal and/or colonic distension. The intestinal tumor may result in small intestinal, colonic, or rectal distension. The current study was designed to investigate the effect of rectal, colonic, and duodenal distension on small intestinal motility, aiming at elucidating the possible existence of recto-enteric reflexes and colon-enteric reflexes and its mechanisms involving the extrinsic nerves.
Rectal distension (RD) with balloon may mimic fecal stasis in rectum in patients with constipation. RD was reported to reduce jejunal and ileal pressures in humans by an inhibitory reflex called “recto-enteric reflex” [8]. It was shown that RD inhibited intestinal transit and contraction in canine and human [6, 9]. It was proposed that RD and distal colon distension with fecal stasis directly caused reflexive inhibition of proximal gastrointestinal motility [10–12], possibly mediated via the neural reflex involving either the nociceptive or non-nociceptive afferent pathway [13]. However, it is not clear whether sympathetic and vagal activities are involved or not.
Like RD, distension of the distal colon has been frequently reported. However, few studies are found in the literature on proximal colonic distension due to difficulties in accessing the proximal colon. Clinically, proximal colon distension (CD) is commonly seen in patients with colon tumor, fecal stasis, intestinal tuberculosis, and inflammatory bowel disease, such as Crohn’s disease and ulcerative colitis. In a previous study, we found that proximal colon distension significantly decreased the compliance and tone of the rectum, and guanethidine could abolish the effect [14]. However, no literature is available on the effect of proximal colonic distension (CD) on small intestinal motor and myoelectrical activities.
Duodenal distension (DD) mainly occurs in patients with duodenal stasis diseases such as Crohn’s disease, and local intestinal tumors such as lymphoma. However, little is known on possible effects of DD on small intestinal motor and myoelectrical activities. In one previous study, DD was found to induce intestinal myoelectrical dysrhythmia that could be normalized with intestinal pacing [15].
Accordingly, the aim of this study was to systematically explore the effects of RD, proximal CD, and DD on small intestinal motor and myoelectrical activities and the possible mechanisms involving the sympathetic/vagal pathway.
Materials and Methods
Animals
Eight healthy female hound dogs (2–3 years old, 23–33 kg) were recruited in the study. They were fasted overnight before surgery. Anesthesia was performed using a previously established method [16–18]. 5 mg/kg thiopental sodium (Abbott Laboratories, North Chicago, Ill, USA) was intravenously infused and maintained on 1.5 % isoflurane inhalation in 1:1 oxygen-nitrous oxide carrier gases delivered from a ventilator following endotracheal intubation. Tongue color, pulse rate, and breath rate of the dog were closely monitored. One pair of 28-gage cardiac pacing wires (A&E Medical, Farmingdale, NJ, USA) was implanted on the serosal surface of small bowel 50 cm beyond the pylorus. The two electrodes were 1 cm apart. They penetrated the subserosa and affixed to the serosa by non-absorbable sutures. The electrode wires were subcutaneously tunneled through the anterior abdominal wall along the right trunk and exited through the skin at the right hypochondria for the measurement of intestinal myoelectrical activity. Two cannulas were placed in each dog, one in the duodenum about 20 cm distal to the gastric pylorus, and the other one in the ascending colon about 5 cm distal to the cecum using a previously established method [19]. The duodenal cannula was used for placing a manometric catheter to the lumen of the small bowel to record the intestinal manometry. It was also used for the insertion of a duodenal distension (DD) balloon and for injecting the phenol red down toward the small bowel for evaluation of intestinal transit. The colon cannula was used for observing the outflow of phenol red and also for the insertion of a colon distension (CD) balloon. The study was initiated after the dogs completely recovered from the surgery (at least 2 weeks or later). All experiments were performed in the conscious state with the dog standing on an experimental table and slightly restrained. All the dogs were free from any drugs within 1 week and fasted overnight prior to the experiments. On the day of experiment, one bottle of enema liquid (Fleet enema; C.B. Fleet Co. Inc., Lynchburg, VA, USA) was used for clearing the rectum before performing the experiment in the rectal distension (RD) session. The washout time between two sessions for each dog was at least 1 week apart. The protocol was approved by the Animal Use and Care Committee of the University of Texas Medical Branch at Galveston, Texas.
Experimental Protocol
All experiments were composed of a 30 min baseline recording, a 30 min recording during rectal, colon or duodenal balloon distension (30 psi), and a 30 min recording of recovery.
The study consisted of six experimental sessions: control, RD, CD, DD, RD + guanethidine, and CD + guanethidine. All experiments were performed in the postprandial state with a meal containing 375 g chopped chicken (Pedigree; Mars Inc, Vernon California, USA). Heart rate variability, intestinal myoelectrical activity, and small bowel manometry were recorded simultaneously throughout each experiment. In the session with guanethidine, guanethidine 3 mg/kg was intravenously administrated immediately after the meal and before the experiment.
Rectal, Colonic, and Duodenal Distension
One balloon was tightly fixed to the tip of a catheter with a thread. Polyester film was twined above the thread to strengthen the sealing. The sealing of balloon was further confirmed by inflating with air and immersing in water. Then the balloon was completely deflated. Before the experiment, the balloon was inserted. For RD, the balloon was inserted into the rectum and positioned so that the caudal pole of the balloon laid 8 cm from the anal verge. For CD, the balloon was inserted into the colon through the colon cannula and the caudal pole of the balloon was positioned 18 cm from the outside verge of the colon cannula. For DD, the balloon was inserted into the duodenum via the duodenum cannula and the balloon caudal pole was positioned 18 cm from the outside verge of the cannula. In each experiment, after the 30 min baseline recording, the balloon was inflated and maintained at a pressure of 30 psi by a computed barostat device (Distender Series IIR; G & J Electronics Inc., Willowdale, Ontario, Canada) connected with the catheter with balloon. All dogs tolerated the rectal distension at a pressure of 30 psi without adverse behaviors indicative of pain, gasping for breath, or writhing.
Small Bowel Manometry
Small bowel manometry was recorded as previously described [9]. The catheter connected to the pressure transducer was passed through the duodenal cannula. In RD and CD, the catheter was inserted to the depth of 35 cm. In DD, the catheter was inserted to the depth of 50 cm so that there was enough space for duodenal distension balloon at its proximal site in the intestine. The catheter has four side holes spaced at 5-cm intervals (MedKinetic Inc., Ningbo, China). The catheter was infused by a low compliance perfusion system (MedKinetic Inc., Ningbo, China). The pressure transducer converted the pressure signal into the electrical signal. The recorded manometric signal was amplified by a multichannel manometric system (MedKinetic Inc., Ningbo, China). The recording tracing was displayed on a monitor and saved on the computer for further analysis. The contraction activity was evaluated by using the mean area under the curve (AUC) per second, a parameter called contractile index (CI) that was computed with the software provided by the manufacturer (MedKinetic Inc., Ningbo, China).
Recording and Analysis of Small Bowel Slow Waves
Small bowel slow waves were recorded by connecting the serosal electrode wires implanted in small bowel with a Biopac system (EOG 100A; Biopac Systems, Inc., Santa Barbara, Calif., USA) as described previously. Dominant frequency (DF), Dominant power (DP), the percentage of normal intestinal slow waves (N %), the percentage of bradyintestria (B %), tachyintestria (T %), and arrhythmia (A %) were analyzed by the adaptive spectral analysis software [20]. DF refers to the mean frequency of intestinal slow waves (17–22 cpm in dogs) [21]. DP refers to the power of intestinal slow waves at the dominant frequency. N % is the percentage of time during which regular normal intestinal slow waves (17–22 cpm in dogs) are present over certain period. The normal slow wave frequency range of regular 17–22 cpm in dogs was established based on our previous studies [21, 22]. In the meantime, intestinal slow wave with a frequency less than 17 cpm and more than 22 cpm were, respectively, regarded as bradyintestria and tachyintestria. It was categorized as arrhythmia if the slow wave did not show a regular rhythm [21, 22].
Recording of ECG and Analysis of Heart Rate Variability
The electrocardiogram (ECG) signal was recorded and analyzed using a previous described method [23]. A special amplifier (model 2283 Fti Universal Fetrode Amplifier, UFI, Morro Bay, CA) with a cutoff frequency of 50 Hz was used to record the ECG signal. The heart rate variability (HRV) signal was derived from the original ECG recording by using a special validated program developed in our laboratory [24]. The recorded ECG signal was sampled at a frequency of 6000 Hz and down-sampled to 500 Hz. The program can identify R waves, calculate and interpolate R–R interval data at 100 Hz, and finally down-sample the interpolated data to a frequency of 1 Hz. The sympathovagal parameters, low frequency (LF) and high frequency (HF), can be extracted from overall power spectral analysis. LF is defined as AUC in the frequency range of 0.04–0.15 Hz, and reflects mainly sympathetic or adrenergic activity. HF is defined as AUC in the frequency range of 0.15–0.50 Hz and reflects purely parasympathetic or vagal activity. The ratio of LF/HF represents the balance between sympathetic activity and vagal activity.
Statistical Analysis
Data are reported as mean ± standard error. Student’s t test was used to analyze the difference between two sessions. The ANOVA was used to analyze the differences among three or more sessions. If the result of ANOVA revealed a significant difference, further Tukey’s test was used to reveal the differences between any two sessions among the three or more sessions.
Results
Effect of RD, CD, and DD on Small Intestinal Contraction in Fed State
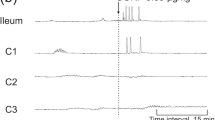
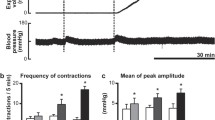
RD and CD suppressed intestinal motility (Fig. 1). When RD was used, the intestinal contractions were decreased immediately. The CI was significantly reduced from 9.9 ± 0.9 at baseline to 6.0 ± 0.4 with RD (P = 0.001), and the CI recovered to 7.3 ± 0.5 (vs. 9.9 ± 0.9 at baseline, P = 0.009) after the distension was terminated. When CD was used, the intestinal CI was reduced from 7.7 ± 0.8 at baseline to 5.3 ± 0.2 with CD (P = 0.008), and the CI was recovered to 6.0 ± 0.1 (vs. 7.7 ± 0.8 at baseline, P = 0.08).
The inhibitory effects of both RD and CD on intestinal motility were blocked by guanethidine. In RD + guanethidine session, the CI was 11.9 ± 0.5 at the baseline, and remained at the similar level of 11.7 ± 0.2 during RD, and 11.8 ± 0.8 with that in the recovery period (ANOVA, P = 1.9). Similarly, in CD + guanethidine session, the CI was 10.1 ± 0.5 at the baseline, remained at the similar level of 9.8 ± 0.3 during CD, and 10.0 ± 0.7 in the recovery period (ANOVA, P = 1.4). These data demonstrated that RD or CD was no longer ineffective in inhibiting intestinal motility at the presence of guanethidine.
Interestingly, DD showed no effects on intestinal motility. The CI was 8.6 ± 1.4 at baseline, 7.8 ± 1.3 during DD, and 7.9 ± 1.3 at recovery (ANOVA P = 0.89) (Fig. 1).
Effect of RD, CD, and DD on Small Intestinal Slow Waves
RD impaired small intestinal slow waves (Figs. 2, 3). In RD session, the percentage of normal slow waves was 92.1 ± 2.8 % at baseline and significantly reduced to 64.2 ± 3.4 % during RD (P < 0.001). This was attributed to an increase in bradyintestria from 1.7 ± 1.0 % to 28.2 ± 4.5 % (P < 0.001). The percentage of normal slow waves was rapidly recovered to 82.6 ± 3.4 % (vs. 92.1 ± 2.8 % at basement, P = 0.14) after RD was terminated. RD also affected the dominant frequency (DF) of the slow waves. The DF was 18.9 ± 0.3 cycles/min (CPM) at baseline, reduced to 17.6 ± 0.3 CPM during RD (P = 0.009), and recovered to 18.5 ± 0.2 CPM (vs. 18.9 ± 0.3 CPM at basement, P = 0.1) after RD was terminated (Fig. 3). RD did not alter the dominant power of the intestinal slow waves (P = 0.98, among baseline, distension, and recovery, ANOVA).
CD also impaired small intestinal slow waves (Fig. 2, 3). In CD session, the percentage of normal slow waves was 90 ± 2.7 % at baseline and significantly reduced to 69.2 ± 3.7 % during CD (P = 0.01, Fig. 2). This was also attributed to the increase of bradyintestria from 5 ± 2.2 to 25.8 ± 2.8 % (P = 0.004). Similar to RD, CD also reduced the dominant frequency (DF) of the slow waves. The DF was 18.4 ± 0.2 at baseline, reduced to 17.4 ± 0.2 during RD (P = 0.02), and recovered to 18.2 ± 0.2 % (vs. 18.4 ± 0.2 % at basement, P = 0.1) after RD was terminated (Fig. 3). CD did not alter the dominant power (P = 0.18 among baseline, distension, and recovery, ANOVA).
Similarly, guanethidine abolished the inhibitory effects of RD and CD on intestinal slow waves. In guanethidine + RD session and guanethidine + CD session, the percentage of normal intestinal slow waves and the dominant frequency remained unchanged among the three periods (baseline, RD or CD, and recovery) (ANOVA P = 0.15) (Fig. 2, 3).
Similar to its effect on intestinal contractions, DD did not alter intestinal slow waves. In DD session, the percentage of normal intestinal slow waves and dominant frequency remained unaltered among the three periods (ANOVA P = 0.28).
Effects of RD, CD, and DD on Vagal Activity
RD increased sympathetic activity (LF) (0.47 ± 0.08 during RD vs. 0.31 ± 0.08 at baseline, P = 0.02), reduced vagal activity (HF) (0.23 ± 0.09 vs. 0.53 ± 0.06 at baseline, P = 0.04), and increased the ratio of LF/HF (0.69 ± 0.11 vs. 0.29 ± 0.1, P = 0.009). When RD was terminated, the LF, HF, and LF/HF were recovered to their baseline values (P > 0.05 vs. each baseline) (Fig. 4).
CD also increased sympathetic activity (LF) (0.56 ± 0.14 vs. 0.45 ± 0.14 at baseline, P = 0.01), reduced vagal activity (HF) (0.52 ± 0.07 vs. 0.82 ± 0.09 at baseline, P = 0.03), and increased the ratio of LF/HF (0.92 ± 0.13 vs. 0.39 ± 0.11 at baseline, P = 0.002). Similarly, when RD was terminated, the LF, HF, and LF/HF recovered to their baseline values (P > 0.05 vs. each baseline) (Fig. 4).
Guanethidine eliminated the effects of RD and CD on sympathetic and vagal activities. In guanethidine + RD session and guanethidine + CD session, the LF, HF, and LF/HF had no difference among the three periods (baseline, RD or CD, and recovery) (ANOVA P > 0.05) (Fig. 4).
DD did not alter the sympathetic or vagal activity. In DD session, the sympathetic activity (LF), the vagal activity (HF), and sympathovagal balance (LF/HF) assessed by the spectral analysis of the HRV remained unchanged among baseline, during DD and recovery period (ANOVA, P = 0.11).
Discussion
In this study, we investigated the effects and mechanisms of RD, CD, and DD on small intestinal slow waves, contractions, and sympathetic-vagal activity. We found that RD and CD inhibited intestinal contractions, impaired small intestinal dysrhythmia, and enhanced sympathetic activity in dogs. The effects were blocked by guanethidine, suggesting a sympathetic pathway. Duodenal distension at the proximal site, however, did not affect small bowel contractions, intestinal slow waves or sympathetic-vagal activity.
Effects and Mechanisms of RD, CD, and DD on Intestinal Motility
Clinically, patients with chronic idiopathic constipation and constipation-dominant irritable bowel syndrome (IBS-C) often have upper gastrointestinal symptoms such as bloating, upper abdominal pain, and/or discomfort. These symptoms have been reported to be associated with impaired gastric motility, gastric dysrhythmia, delayed gastric emptying, and/or slow small bowel transit [3, 4, 25, 26]. The impaired gastrointestinal motility may be caused by fecal or gas distension. Rectal distension has been frequently used in studying visceral sensation [27], colorectal reflex [28], recto-anal inhibitory reflex (RAIR) [29, 30], anal sphincter function [31], and gastrointestinal motility [9, 32]. Our previous studies in animals showed inhibitory effects of RD on gastric tone, accommodation, and antral contractions, involving the adrenergic pathway and the nociceptive afferent pathway [32, 33]. In the small intestine, it was found that RD inhibited postprandial small intestinal motor activity in a distension volume-dependent manner in dogs, partially mediated via the alpha and beta adrenergic pathways [9]. A few clinical studies showed intestinal dysmotility in patients with constipation [6, 8, 34, 35]. The RD-induced suppression in jejunal and ileal contractions has been reported to be attributed to “recto-enteric reflux” in human healthy volunteers [8]. RD in the present study was shown to suppress small bowel contractions and guanethidine could block such inhibitory effect. The spectral analysis of HRV revealed the activation of sympathetic activity with RD. These findings demonstrate that RD inhibits small intestinal motility mediated via the sympathetic mechanism. These findings also provide an explanation of upper abdominal discomfort and other symptoms in patients with constipation or rectal tumors.
In previous studies with CD, the distension was usually performed at the distal colon or rectum-colon junction with few exceptions due to the difficulty in accessing the region. In one previous canine study, CD in the proximal colon decreased the tone and compliance of the rectum and guanethidine abolished the effects [18]. Shafik et al. found that the distension in the right colon decreased ileal pressure, providing an evidence of coloileal reflexes [36]. In the current study, we found the distension in the proximal colon suppressed the proximal small intestinal contractions and the effects were similar to RD [9]. To the best of our knowledge, this was the first study investigating the effect of proximal colonic distension on proximal intestinal motility and providing an evidence of colonic-jejunal inhibitory reflexes. These findings possibly explain why patients with Crohn’s disease often complain about upper abdominal bloating and vomiting.
Also, duodenal distension was reported to increase pyloric sphincter pressure and decreased antral pressure in mongrel dogs [9]. Ileal or Jejunal distension, respectively, decreased Jejunal or ileal pressure by “ileal-jejunal and jejuno-ileal inhibitory reflexes.” These reflexes appear to regulate chyme flow in the small intestine by creating a balance of chyme delivery between the jejunum and ileum [37]. In the current study, we found that the DD at a site proximal to the measurement site did not seem to impair small intestinal motility at a distal site.
Effects and Mechanisms of RD, CD, and DD on Small Bowel Slow Waves
In addition to its inhibitory effect on intestinal contractions, RD in the present study also impaired small intestinal slow waves: decreased the frequency of intestinal slow waves and reduced the percentage of normal intestinal slow waves. The effects were eliminated by guanethidine, suggesting a sympathetic mechanism. Previous studies have reported the effect of rectal distension on gastric slow waves but rarely on intestinal slow waves. In one study, a minor effect was noted with rectal distension at a constant volume of 120 mL on intestinal slow waves in dogs: increase in the variation of slow wave frequency but not the percentage of normal slow waves [4].
The effects of proximal colon distension to intestinal slow waves were similar to that with rectal distension, i.e., reduction in the percentage of normal intestinal slow waves and increase in variation of slow wave frequency. To the best of our knowledge, the literature is silent on the effects of CD in the proximal colon on small intestinal slow waves.
Interestingly, distension of the proximal site of the small intestine did not alter intestinal slow waves nor intestinal contractions in the distal site of the small intestine. These findings seem to suggest the inhibitory reflexes may be more in the backward direction rather than forward direction. Further studies are needed to provide explanations such a difference. In one previous study, however, duodenal distension was reported to impaired intestinal myoelectrical activity in the proximal jejunum: reduced percentage of normal slow wave and increased minute-by-minute variation of slow wave frequencies [15]. The difference might be attributed to the distance between the distension site and the recording set: it was 30 cm in the previous study but 15 cm in the current study. The reason possibly is that the location of distension and the position of measurement were too close in the current study.
Autonomic Mechanisms
The inhibitory effects of RD and CD on intestinal contractions and slow waves were accompanied with increased sympathetic activity (LF) and decreased vagal activity (HF). Moreover, these inhibitory effects were blocked by guanethidine. These findings suggest the autonomic mechanisms involved in the inhibitory effects of RD and CD on intestinal motility. Similar autonomic mechanisms were reported in previous studies [32, 38, 39]. In dogs, RD was reported to inhibit contractions and slow waves of the stomach and concurrently increase sympathetic activity; the effects were also blocked by guanethidine, demonstrating the adrenergic pathways similar to the present study [32].
Although there have been isolated studies investigating effects and mechanisms of gastrointestinal distension on gastrointestinal motility, the present study is systematic and comprehensive: on the same animals, the comprehensive measurements of intestinal motility, including both mechanical and myoelectrical activities, were conducted and distension of different organs, duodenum, colon, and rectum was performed.
Conclusion and Prospects
In the current study, we systematically and comprehensively explored the effects and autonomic mechanisms of distension at duodenum, colon, and rectum on proximal small intestinal contractions and slow waves in dogs. The findings of the study indicate that distension at a location in the gut distal but not proximal to the measurement site suppresses small intestinal contractions and impair intestinal slow waves, mediated via the autonomic mechanisms. These findings may also be used to explain why patients with constipation or Crohn’s disease often complain of upper abdominal discomforts.
References
Mollen, R. M., Hopman, W. P., Kuijpers, H. H., & Jansen, J. B. (1999). Abnormalities of upper gut motility in patients with slow-transit constipation. European Journal of Gastroenterology and Hepatology, 11, 701–708.
Boccia, G., Buonavolonta, R., Coccorullo, P., Manguso, F., Fuiano, L., & Staiano, A. (2008). Dyspeptic symptoms in children: the result of a constipation-induced cologastric brake? Clinical Gastroenterology and Hepatology, 6, 556–560.
Bassotti, G., Stanghellini, V., Chiarioni, G., et al. (1996). Upper gastrointestinal motor activity in patients with slow-transit constipation. Further evidence for an enteric neuropathy. Digestive Diseases and Sciences, 41, 1999–2005.
Abo, M., Kono, T., Wang, Z., & Chen, J. D. (2000). Impairment of gastric and jejunal myoelectrical activity during rectal distension in dogs. Digestive Diseases and Sciences, 45, 1731–1736.
Reynolds, J. C., Ouyang, A., Lee, C. A., Baker, L., Sunshine, A. G., & Cohen, S. (1987). Chronic severe constipation. Prospective motility studies in 25 consecutive patients. Gastroenterology, 92, 414–420.
van der Sijp, J. R., Kamm, M. A., Nightingale, J. M., et al. (1993). Disturbed gastric and small bowel transit in severe idiopathic constipation. Digestive Diseases and Sciences, 38, 837–844.
Yin, J., & Chen, J. D. (2011). Electroacupuncture improves rectal distension-induced delay in solid gastric emptying in dogs. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology, 301, R465–R472.
Seidl, H., Gundling, F., Pehl, C., Pfeiffer, A., Schepp, W., & Schmidt, T. (2009). Small bowel motility in functional chronic constipation. Neurogastroenterol Motil, 21, 1278–e1122.
Shafik, A. (1998). Effect of duodenal distension on the pyloric sphincter and antrum and the gastric corpus: Duodenopyloric reflex. World Journal of Surgery, 22, 1061–1064.
Coremans, G., Geypens, B., Vos, R., et al. (2004). Influence of continuous isobaric rectal distension on gastric emptying and small bowel transit in young healthy women. Neurogastroenterology and Motility, 16, 107–111.
Kellow, J. E., Gill, R. C., & Wingate, D. L. (1987). Modulation of human upper gastrointestinal motility by rectal distension. Gut, 28, 864–868.
Bojo, L., & Cassuto, J. (1992). Gastric reflex relaxation by colonic distension. Journal of the Autonomic Nervous System, 38, 57–64.
Iwa, M., Strickland, C., Nakade, Y., Pappas, T. N., & Takahashi, T. (2005). Electroacupuncture reduces rectal distension-induced blood pressure changes in conscious dogs. Digestive Diseases and Sciences, 50, 1264–1270.
Luo, Z. H., Chen, J. H., & Tao, Z. Z. (2009). Hyoid suspension treatment of obstructive sleep apnea hypopnea syndrome. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi, 44, 877–880.
Li, F., Chen, J. H., Ma, S., Zhang, L., & Xiao, Y. H. (2009). Fang M [Antibacterial effects of a dental adhesive incorporating a quaternary ammonium monomer against Streptococcus mutans]. Zhonghua Kou Qiang Yi Xue Za Zhi, 44, 621–625.
Chen, J., Xing, J., & Chen, J. D. (2009). Effects of muscarinic receptor stimulation and nitric oxide synthase inhibition on gastric tone and gastric myoelectrical activity in canines. Journal of Gastroenterology and Hepatology, 24, 1130–1135.
Oliveira, H. M., Sallam, H. S., Espana-Tenorio, J., et al. (2009). Gastric and small bowel ileus after severe burn in rats: The effect of cyclooxygenase-2 inhibitors. Burns, 35, 1180–1184.
Song, J., Yin, J., Chen, J. D. (2013). Acute and chronic effects of desvenlafaxine on gastrointestinal transit and motility in dogs. Neurogastroenterol Motil, 25, 824–e637.
Chen, J. H., Lin, L., & Chen, J. D. Z. (2009). Colorectal and rectocolic reflexes in canines: involvement of tone, compliance and anal sphincter relaxation. Neurogastroenterology and Motility, 21, 20.
Chen, J., Stewart, W. R., & McCallum, R. W. (1993). Adaptive spectral analysis of episodic rhythmic variations in gastric myoelectric potentials. IEEE Transactions on BioMedical Engineering, 40, 128–135.
Lin, X., Peters, L. J., Hayes, J., & Chen, J. D. (2000). Entrainment of segmental small intestinal slow waves with electrical stimulation in dogs. Digestive Diseases and Sciences, 45, 652–656.
Lin, X., Hayes, J., Peters, L. J., & Chen, J. D. (2000). Entrainment of intestinal slow waves with electrical stimulation using intraluminal electrodes. Annals of Biomedical Engineering, 28, 582–587.
Ouyang, H., Yin, J., Wang, Z., Pasricha, P. J., & Chen, J. D. (2002). Electroacupuncture accelerates gastric emptying in association with changes in vagal activity. American Journal of Physiology. Gastrointestinal and Liver Physiology, 282, G390–G396.
Wang, Z. S., & Chen, J. D. Z. (2000). Robust ECG R-R wave detection using evolutionary-programming-based inference system (EPFIS) and its application to assessing brain-gut interaction. IEEE Proceedings-Science, Measurement and Technology, 147, 351–356.
Glia, A., & Lindberg, G. (1998). Antroduodenal manometry findings in patients with slow-transit constipation. Scandinavian Journal of Gastroenterology, 33, 55–62.
Preston, D. M., & Lennard-Jones, J. E. (1986). Severe chronic constipation of young women: ‘idiopathic slow transit constipation’. Gut, 27, 41–48.
Zagorodnyuk, V. P., Kyloh, M., Gregory, S. J., et al. (2011). Loss of visceral pain following colorectal distension in an endothelin-3 deficient mouse model of Hirschsprung’s disease. Journal of Physiology, 589, 1691–1706.
Qi, H., & Chen, J. D. (2006). Effects of intestinal electrical stimulation on postprandial small-bowel motility and transit in dogs. American Journal of Surgery, 192, e55–e60.
Xu, X., Pasricha, P. J., Sallam, H. S., Ma, L., & Chen, J. D. (2008). Clinical significance of quantitative assessment of rectoanal inhibitory reflex (RAIR) in patients with constipation. Journal of Clinical Gastroenterology, 42, 692–698.
Guinet, A., Jousse, M., Damphousse, M., et al. (2011). Modulation of the rectoanal inhibitory reflex (RAIR): Qualitative and quantitative evaluation in multiple sclerosis. International Journal of Colorectal Disease, 26, 507–513.
Bajwa, A., Thiruppathy, K., Trivedi, P., Boulos, P., & Emmanuel, A. (2010). Effect of rectal distension on voluntary external anal sphincter function in healthy subjects. Colorectal Disease, 13, 1173–1179.
Chen, J., Song, G. Q., Yin, J., Koothan, T., & Chen, J. D. (2008). Electroacupuncture improves impaired gastric motility and slow waves induced by rectal distension in dogs. American Journal of Physiology. Gastrointestinal and Liver Physiology, 295, G614–G620.
Lei, Y., Zhu, H., Xing, J., & Chen, J. D. (2005). Rectal distension modulates canine gastric tone and accommodation. Digestive Diseases and Sciences, 50, 2134–2140.
Rao, S. S., Kuo, B., McCallum, R. W., et al. (2009). Investigation of colonic and whole-gut transit with wireless motility capsule and radiopaque markers in constipation. Clinical Gastroenterology and Hepatology, 7, 537–544.
Zarate, N., Knowles, C. H., Yazaki, E., Lunnis, P. J., & Scott, S. M. (2009). Clinical presentation and patterns of slow transit constipation do not predict coexistent upper gut dysmotility. Digestive Diseases and Sciences, 54, 122–131.
Shafik, A., Shafik, A. A., & Ahmed, I. (2003). Effect of colonic distention on ileal motor activity with evidence of coloileal reflex. Journal of Gastrointestinal Surgery, 7, 701–705.
Shafik, A., Shafik, A. A., El, S. O., & Shafik, I. A. (2007). Study of the effect of ileal distension on the motor activity of the jejunum, and of jejunal distension on the motor activity of the ileum. Hepato-Gastroenterology, 54, 2007–2010.
Qi, H., Brining, D., & Chen, J. D. (2007). Rectal distension inhibits postprandial small intestinal motor activity partially via the adrenergic pathway in dogs. Scandinavian Journal of Gastroenterology, 42, 807–813.
Chen, J. H., Sallam, H. S., Lin, L., & Chen, J. D. (2010). Colorectal and rectocolonic reflexes in canines: Involvement of tone, compliance, and anal sphincter relaxation. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology, 299, R953–R959.
Conflicts of interest
No conflicts of interest are declared by the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, J., Yin, J. & Chen, J.D.Z. Inhibitory Effects and Sympathetic Mechanisms of Distension in the Distal Organs on Small Bowel Motility and Slow Waves in Canine. Cell Biochem Biophys 73, 665–672 (2015). https://doi.org/10.1007/s12013-015-0679-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-015-0679-4