Abstract
Cardiac amyloidosis is a manifestation of one of several systemic amyloidoses, and is characterized by increased left-ventricular (LV) wall thickness and normal or decreased LV cavity size. Congestive heart failure in cardiac amyloidosis is characterized by a predominant diastolic LV dysfunction, and systolic dysfunction occurs only in late-stage disease. Echocardiography is a noninvasive, reproducible method for assessing cardiac morphology and function in cardiac amyloidosis, and some echocardiographic indices are prognostic for amyloidoses. This review describes the advances in echocardiography and its role in the diagnosis and management of cardiac amyloidoses. Our review suggests that LV longitudinal function and the cyclic variation of myocardial integrated backscatter may be the best predictors of adverse outcomes. In the future, new echocardiographic techniques, such as fully automated echocardiogram interpretation, should provide further useful information for assessing cardiac function and prognosis in cardiac amyloidosis patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cardiac amyloidosis is a manifestation of one of several systemic amyloidoses. It affects multiple organs and tissues with extracellular deposition of pathogenic, insoluble, fibrillar proteins. Cardiac involvement is the predominant feature and its relative predominance varies with the type of amyloidosis [1, 2]. The main forms of amyloidosis that affect the heart are as follows (Table 1):
-
(1)
Light-chain amyloidosis (AL: amyloid-light-chain or primary): this type of amyloidosis is caused by immunoglobulin light-chain genes and usually associated with plasma cell dyscrasia, but not multiple myeloma. It affects the heart, kidney, liver, peripheral/autonomic nerves, soft tissue, and the gastrointestinal system. Heart involvement occurs in one-third to the half of AL amyloidosis. Heart failure tends to progress rapidly once the heart is affected and patients have a poor prognosis. Major treatment options for this disease are chemotherapy, immunomodulatory drugs, and proteasome inhibitors.
-
(2)
Transthyretin-associated amyloidoses (ATTR). There are two types of ATTR.
-
Variant-type ATTR (ATTRv), also called “familial-type amyloid polyneuropathy (FAP)”, caused by a variant form of transthyretin produced in the liver. It affects peripheral/autonomic nerves and the heart. This disease is autosomal dominant and amyloid deposition is derived from a mixture of mutant and wild-type transthyretin (ATTRwt). Treatments include liver transplantation and drugs (diflunisal and tafamidis) to stabilize transthyretin [3]. Previous studies reported that V30M-type ATTRv patients rarely suffered from cardiac amyloidosis in endemic areas; however, it appears that some Japanese patients with V30M-type ATTRv develop the disease at a later age and do have severe cardiac amyloidosis. Most originated from nonendemic areas [4, 5].
-
Another type of ATTR is associated with ATTRwt and is also known as senile systemic amyloidosis (SSA). The main organ of involvement is the heart. Carpal tunnel syndrome is a common initial symptom of ATTRwt. This type is almost exclusively found in elderly men (male:female ratio 20:1 and 50:1). Symptoms are slowly progressive. The mean survival period is 5 years after onset of congestive heart failure.
-
-
(3)
Secondary amyloidosis: this type of amyloidosis is caused by serum amyloid A and is also called AA amyloidosis. It is seen in association with rheumatoid arthritis and other rheumatic disorders such as ankylosing spondylitis, as well as with inflammatory bowel disease. Hepatic and renal amyloid deposition is the dominant clinical feature, and clinical heart disease related to cardiac amyloidosis is rare (5%). Treatment is based on healing of the underlying inflammatory process.
The purpose of this review was to describe the advances in echocardiographic assessment, and to discuss their crucial role in the diagnosis and management of cardiac amyloidoses.
M-mode echocardiography
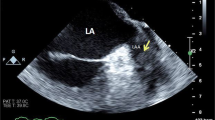
The m-mode echocardiography characteristics of cardiac amyloidosis were first reported by Chew et al. [6]. This three-case report suggested that the features of cardiac amyloidosis were normal left-ventricular (LV) dimensions in diastole, diminished amplitude of excursion, increased systolic dimensions, and pericardial effusion. Child et al. reported the following basic findings: (1) symmetric LV hypertrophy in the absence of hypertension or aortic valvular disease, (2) hypokinesia and decreased systolic thickening of the interventricular septum and LV posterior wall, and (3) small-to-normal LV cavity size. In addition, the characteristics of infiltrate cardiomyopathy include septal/posterior free-wall thickness ratio < 1.3, increased maximal left atrial (LA) transverse dimension, reduced mitral valve closure (E–F) slope, and preserved ejection fraction (EF) > 60% [7] (Fig. 1).
Right-ventricular (RV) anterior wall thickness is reportedly significantly increased in patients with clinically significant amyloid infiltrative cardiomyopathy, and this is consistent with pathologic findings [8, 9]. In conjunction with LV abnormalities, this finding differentiates cardiac amyloidosis from constrictive pericardial disease. Furthermore, Child et al. reported that the mitral E point to ventricular septal separation and its ratio to the LV end-diastolic dimension accurately differentiates normal individuals from those with abnormal LV function, irrespective of LV cavity size [9].
Using computer-assisted analyses of m-mode echocardiograms, patients with cardiac amyloids show characteristic quantitative echocardiographic findings, including normal or small LV cavity size, decreased peak diastolic cavity filling rate, prolonged isovolumic relaxation, decreased fractional shortening and peak circumferential fiber shortening rate, and decreased peak rates of both systolic thickening and diastolic thinning of the septum and posterior LV wall [10]. Interestingly, reports indicate 75% reduction in diastolic thinning compared to 50% systolic thickening, indicating greater impairment of diastolic regional function. Thus, simple m-mode echocardiography provides useful information to differentiate cardiac amyloidosis from the other causes of LV hypertrophy, especially when symptoms of congestive heart failure are present.
Two-dimensional echocardiography
Two-dimensional echocardiographic findings in cardiac amyloidosis were first reported by Siqueira-Filho et al. [11]. Two-dimensional echocardiography reveals additional features such as thickened papillary muscles, thickened valves, better appreciation of the thickened RV wall, and a characteristic “granular sparkling” appearance of the thickened cardiac walls. Mohty et al. examined the prevalence and prognostic impact of left-sided valve thickening in 150 systemic AL amyloidosis patients [12]. They found that 42% had mitral and/or aortic valve thickening (> 3 mm). Left heart valve thickening was significantly associated with reduced 5-year survival. In multivariate analysis, left-ventricular valve thickening remained significantly associated with higher all-cause mortality. Carroll et al. evaluated 14 systemic amyloidosis patients using electrocardiographic voltage and echocardiographic muscle cross-sectional areas [13]. Electrocardiographic voltage tended to be low and the echocardiographic muscle cross-sectional area tended to be increased. When these two techniques were combined, an inverse correlation between voltage and muscle cross-sectional area (r = − 0.79) was observed in amyloidosis patients. Moreover, a marked derangement of the voltage/cross-sectional area was associated with clinical symptoms and mortality (Fig. 2).
Simons et al. compared the relative sensitivity of noninvasive tests (two-dimensional echocardiography, computerized tomography, magnetic resonance imaging, electrocardiography, and nuclear scintigraphy) in 15 patients with proven cardiac amyloidosis [14]. The sensitivity of both myocardial technetium uptake and echocardiographic observation of sparkling was low; however, voltage/mass relation diagrams appeared to represent a promising noninvasive cardiac amyloidosis diagnostic method. The combination of increased LV thickness and a low-voltage electrocardiographic pattern is highly specific for cardiac amyloidosis, and was found in 3/30 (10%) and 13/24 (54%) of secondary and AL amyloidosis patients, respectively.
Echocardiography is a sensitive test of cardiac involvement in amyloidosis, in both symptomatic and asymptomatic patients [15]. In one study, 58/196 patients undergoing endomyocardial biopsy due to clinical suspicion of cardiac amyloidosis were confirmed to have the condition. Multivariate analysis revealed that a combination of low voltage and increased LV thickness produced the most statistically useful model [16]. One model showed a low-voltage and interventricular septal thickness of > 1.98 cm, and diagnosis of cardiac amyloidosis could be made with sensitivity of 72% and specificity of 91% with positive and negative predictive values of 79% and 88%, respectively.
Myocardial texture characterization
Bhandari et al. performed myocardial texture characterization using two-dimensional echocardiography in 181 patients with various cardiac disorders, including seven with amyloidosis, and 24 controls [17]. Cardiac amyloidosis was characterized by the presence of very bright or highly refractile echoes in the myocardium. While all amyloidosis patients showed this pattern, it was also observed in patients with uncomplicated ventricular hypertrophy, chronic renal failure, hypertrophic cardiomyopathy, Pompe disease, hemochromatosis, and left heart hypoplastic syndrome, indicating that this finding is non-specific for cardiac amyloidosis.
Among 15 patients with changes in myocardial wall echogenicity on two-dimensional echocardiography, a positive gingival biopsy result for amyloidosis was observed in 11 patients [18]. Increased myocardial echogenicity has a reported sensitivity of 87% and specificity of 81% [19], whereas the combination of increased myocardial echogenicity and increased atrial thickness showed 60% sensitivity and 100% specificity for amyloidosis diagnosis.
Falk et al. reported a correlation between cardiac arrhythmias and echocardiographic abnormalities, as well as heart failure, in systemic amyloidosis [20]. There were four sudden deaths, all in patients with abnormal echocardiograms and complex ventricular arrhythmias. Cueto-Garcia et al. showed that clinical congestive heart failure strongly correlated with greater wall thickness and many other echocardiographic abnormalities, and that survival was negatively influenced by greater wall thickness and reduced systolic function (fractional shortening), indicating that echocardiographic examination is an important tool for identifying cardiac amyloid involvement and may be useful in estimating prognosis [21]. Low-voltage pattern on electrocardiography and LVEF allows the identification of patients at high risk of death [22]. Dubrey et al. demonstrated that late potentials were more frequent in patients with echocardiographic evidence of cardiac amyloidosis (31%) compared to patients with normal echocardiograms (9%, p < 0.003) [23], and abnormal and signal-averaged electrocardiograms were both predictive of all-cause death (p < 0.0001) and sudden cardiac death (p < 0.0001). Thus, two-dimensional echocardiographic findings suggestive of advanced-stage cardiac amyloidosis may represent adverse prognostic factors.
Myocardial scintigraphy and echocardiography
Eriksson et al. reported that only 4/12 FAP patients with echocardiographic abnormalities (highly refractile myocardial echoes, thickened heart valves, and increased heart wall thickness) had abnormal myocardial uptake of technetium-99 m-pyrophosphate. Thus, cross-sectional echocardiography is superior to technetium-99 m-pyrophosphate scintigraphy in detecting cardiac involvement in FAP [24]. Furthermore, the scintigraphy uptake index clearly differs between echocardiographic and non-detectable cardiac amyloidosis [25].
Sperry et al. examined 54 ATTR patients (31% hereditary ATTR) using technetium 99 m-pyrophosphate (TcPYP). The heart-to-contralateral lung (H/CL) ratio was calculated on planar images, and LV uptake was determined in each of the 17 segments using SPECT. A measure of apical–sparing of myocardial TcPYP uptake, termed the apical sparing ratio, was calculated as basal + mid/apical counts. There was increased TcPYP uptake in basal and mid LV segments, and an apical–sparing LS pattern on echocardiography. Similarly, the RV showed greater uptake in basal segments. Apical–sparing ratio of TcPYP uptake was associated with age-adjusted all-cause mortality with worse prognosis seen at levels < 2.75. Global longitudinal strain (LS) was also prognostic, whereas H/CL ratio and total LV uptake indexed to blood pool were not [26].
Intracardiac thrombus in cardiac amyloidosis
Dubrey et al. reported three rare cases of extensive cardiac amyloidosis with large atrial thrombi in the LA during sinus rhythm [27]. Doppler studies showed no A wave on mitral inflow. The authors concluded that severe atrial and ventricular infiltration by amyloids may have resulted in mechanical atrial standstill with resultant thrombus formation, and that such patients may require anticoagulation therapy when atrial function is impaired.
Feng et al. reported on 116 autopsy or explanted cases of cardiac amyloidosis (55 AL, 61 others) examined for intracardiac thrombus [28]. Intracardiac thrombosis was identified in 38 hearts (33%). The AL group had significantly more intracardiac thrombi and fatal embolic events, despite being younger and having less atrial fibrillation. Multivariate analysis demonstrated that AL type and LV diastolic dysfunction were independently associated with the risk of thromboembolism. In another study by the same group, transthoracic and transesophageal echocardiograms of 156 cardiac amyloidosis patients (80 AL, 76 others) were studied [29]. Fifty-eight intracardiac thrombi were identified in 42 patients (27%). Multivariate analysis showed that atrial fibrillation, poor LV diastolic function, and lower LA appendage emptying velocity were independent risk factors for intracardiac thrombosis, whereas anticoagulation was associated with a significantly decreased risk.
Doppler echocardiography
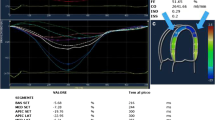
Klein et al. first performed Doppler echocardiographic assessment of LV diastolic function in 64 patients with primary systemic amyloidosis [30]. In the early amyloidosis (mean wall thickness 12–15 mm), relaxation was abnormal, with decreased peak early velocity, increased late velocity, decreased early/late velocity ratio, and prolonged isovolumic relaxation time. In advanced amyloidosis (mean LV wall thickness ≥ 15 mm), a restrictive filling pattern with a markedly shortened deceleration time, decreased pulmonary vein peak systolic flow, and increased diastolic flow velocity were observed. Abnormal RV diastolic function by Doppler ultrasound measurements of RV inflow, superior vena cava, and hepatic vein flow velocities with respiratory monitoring have also been reported [31] (Fig. 3).
Patients with advanced-stage disease show a markedly shortened deceleration time (restrictive pattern). Serial pulsed wave Doppler studies of LV inflow were performed over 12.6 ± 4.9 months in 41 patients with typical two-dimensional echocardiographic features of cardiac involvement [32]. Seven patients in the early group (mean LV thickness < 15 mm) showed changes from abnormal relaxation or “normal” patterns to restriction patterns, which coincided with worsened symptoms in six patients. Fifteen of seventeen patients in the advanced group (mean LV thickness ≥ 15 mm) did not show significant changes during the follow-up; however, these patients had already shown a restrictive pattern.
Dubrey et al. reported a comparison of echocardiographic and electrocardiographic features in FAP (n = 12) and AL (n = 24) cardiac amyloidosis [33]. Echocardiograms were morphologically indistinguishable, with similar LV and RV wall thicknesses. Doppler indices of LV and RV function, LV volume, and EF were similar. Low-voltage electrocardiograms (< 0.5 mV) were more common in AL (67%) than in familial amyloidosis (25%; p < 0.05), and 1-year survival rates were 92% and 38%, respectively, with virtually all deaths being cardiac-related.
In addition, noninvasively derived LA kinetic energy, which is calculated from the formula ½ mV2, where m = the product of mean LA stroke volume (in cm3) and blood density (1.06 g/ml), and v = mean transmitral Doppler A-wave velocity (cm/s), is severely impaired in AL amyloidosis once ventricular infiltration is apparent on echocardiography [34].
RV dilatation appears to be associated with more severe involvement and poorer prognosis (median survival, 4 months) [35]. Of multiple clinical, echocardiographic, and Doppler features entered into a multifactorial model, RV dilation (LV/RV area ratio ≤ 2) remained the only independent predictor of survival. Doppler-derived LV diastolic filling variables are also important predictors of survival in cardiac amyloidosis [36], with patients with a transmitral early filling wave deceleration time ≤ 150 ms (restrictive pattern) showing poor cardiac outcomes.
Ng et al. compared the echocardiographic features and prognoses in patients with senile systemic amyloidosis (SSA) (n = 18) and AL amyloidosis (n = 18) [37]. SSA was characterized by amyloidosis limited to the heart. In contrast to the rapid progression of heart failure in AL amyloidosis, SSA was found to result in slowly progressive heart failure, despite the thicker LV wall and older age of patients.
Migrino et al. examined 42 biopsy-proven AL amyloidosis patients with echocardiography and followed them for 29 ± 16 months [38]. Among the two-dimensional/Doppler flow (mitral and LV outflow tract flow) and clinical parameters, only ejection time and alkaline phosphatase showed incremental values to classify heart failure and predict mortality. Rapezzi et al. conducted a longitudinal study of 233 patients with cardiac amyloidoses (AL, n = 157; ATTRv, n = 61; ATTRwt, n = 15) [39]. At diagnosis, the mean LV wall thickness was higher in ATTRwt than ATTRv and AL patients, and LVEF was moderately depressed in ATTRwt, but not in AL or ATTRv. Moreover, ATTRv displayed low voltage/mass ratio less frequently and was a strong favorable predictor of survival, whereas ATTRwt was found to predict freedom from major cardiac events.
Pinney et al. compared 102 ATTRwt with 36 isolated AL cases [40]. ATTRwt cases showed thicker LV walls, lower E/e′, and longer transmitral early filling wave deceleration time. Univariate analysis demonstrated that no echocardiographic parameters were associated with survival in ATTRwt.
Pulsed tissue Doppler imaging
Cardiac amyloidosis is characterized by an initial impairment of early cardiac relaxation, whereas congestive heart failure is associated with an impairment of peak systolic wall motion velocities, most prominently seen in the longitudinal axis [41]. Moreover, peak lateral and medial mitral annulus velocities and color m-mode tissue Doppler of the LV posterior wall (for measurements of mean myocardial velocities and myocardial velocity gradient) can differentiate cardiac amyloidosis from control patients with fair overall accuracy [42].
The longitudinal myocardial velocity gradients, indicating differences between basal and mid-myocardial velocities, using pulsed tissue Doppler imaging are reportedly significantly impaired in patients with congestive heart failure [43]. Conversely, single-point analysis of pulsed tissue Doppler imaging cannot distinguish between patients. Furthermore, the LV long-axis function was depressed in all (100%) cardiac amyloidosis patients compared to only 36% of idiopathic restrictive cardiomyopathy patients [44]. Even when compared to diastolic color Doppler myocardial imaging measurements, standard pulsed wave tissue Doppler imaging of the mitral annulus is the most accurate diastolic measure of early LV dysfunction in patients with AL amyloidosis [45].
Ultrasound myocardial tissue characterization
The prognostic value of cycle-dependent variation of myocardial integrated backscatter was compared with the other standard echocardiographic and Doppler flow indices in 208 consecutive biopsy-proven patients with primary cardiac amyloidosis [46]. Multivariate analysis showed that this parameter at the LV posterior wall was the only independent predictor of both cardiac and overall deaths. In this study, the Tei index [47, 48] did not correlate with mortality risk.
Strain Doppler imaging
Longitudinal LV myocardial function assessed by strain and strain rate tissue Doppler echocardiography can detect the early impairments in systolic longitudinal LV function in AL amyloidosis patients upon fractional shortening, but before it is detectable by tissue velocity imaging [49, 50] (Fig. 4). This sensitive method can detect differences in LV function between ATTRv and AL amyloidosis, which cannot be distinguished by standard echocardiographic parameters, despite the severity of congestive heart failure and cardiac mortality being much lower in ATTRv [51]. In fact, this method can detect impaired LV systolic function in AL amyloidosis patients with no evidence of cardiac involvement on standard two-dimensional and Doppler echocardiography [52].
In one study, 249 AL amyloidosis patients were prospectively studied to identify independent predictors of survival, compare clinical data, hematologic and cardiac biomarkers, and standard echocardiographic and Doppler myocardial imaging measures [53]. Multivariate analysis identified NYHA class III or IV, presence of pleural effusion, brain natriuretic peptide level > 493 pg/mL, ejection time < 273 ms, and peak longitudinal systolic basal anteroseptal strain ≥ − 7.5%, as independent predictors of poor outcomes. The mean basal LS of LV was reported to be a powerful predictor of clinical outcome and was superior to standard two-dimensional echocardiographic, Doppler flow measurements, and simple tissue velocity indices [54]. In addition, RV function assessed by Doppler myocardial imaging can also identify the early impairment of cardiac function and stratify mortality risk in AL amyloidosis patients [55].
Speckle tracking echocardiography
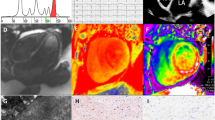
Modesto et al. compared two-dimensional and tissue Doppler-derived strain echocardiographic measurements in amyloid cardiomyopathy patients and age-matched healthy volunteers [56]. They found that two-dimensional strain echocardiography values correlated closely with tissue Doppler-derived strain echocardiography values (r = 0.94 and 0.96 for strain rate and strain, respectively).
Speckle tracking echocardiographic (STE) measurement of myocardial strain and strain rate can discriminate cardiac amyloidosis from the other causes of cardiac hypertrophy [57] (Fig. 5). Cardiac amyloid profoundly alters all strain parameters (longitudinal, circumferential, and radial strain) compared to other causes. In one study, endocardial and epicardial longitudinal and circumferential strain and radial strain were found to be significantly lower in hypertrophic cardiomyopathy and ATTR cardiac amyloidosis patients compared to controls [58]. Furthermore, epicardial circumferential strain was significantly lower in ATTR cardiac amyloidosis than hypertrophic cardiomyopathy. The peak systolic radial strain and strain rate have been reported to be indicative of LV segments with cardiac amyloidosis involvement, as confirmed by cardiac magnetic resonance imaging in a patient with secondary amyloidosis [59]. A systolic septal longitudinal base-to-apex strain gradient (septal apical/basal longitudinal systolic ratio > 2.1), combined with a shortened diastolic deceleration time of early filling (< 200 ms), aids in differentiating cardiac amyloidosis from the other causes of concentric LV hypertrophy [60]. Using parametric polar maps of regional LS, regional variations in strain are easily recognizable, and represent an accurate and reproducible means of differentiating hypertrophic cardiomyopathy or cardiac amyloidosis from hypertensive heart disease [61]. Positron emission tomography and cardiovascular magnetic resonance studies indicate that segmental differences in the distribution of total amyloid mass, rather than the proportion of amyloid deposits, account for marked regional differences in LS in cardiac amyloidosis [62]. The echocardiographic imaging parameters, mean tissue Doppler-derived and two-dimensional global LS of LV, cardiac serological biomarkers, and comprehensive clinical characteristics were prospectively assessed in 206 consecutive patients with biopsy-proven systemic AL amyloidosis [63]. In the multivariate analysis, only diastolic dysfunction and two-dimensional global LS remained independent predictors of survival. Barros-Gomes et al. demonstrated that 2D-STE predicted outcomes and provided incremental prognostic information over the current prognostic staging system, especially in patients without cardiac amyloidosis [64]. Liu et al. assessed LV longitudinal, circumferential, and radial systolic strains by speckle tracking imaging in 44 biopsy-proven systemic AL amyloidosis patients with LV hypertrophy and 30 normal controls [65]. The EF was preserved, while the longitudinal systolic strain was significantly reduced in both compensated and decompensated AL amyloidosis patients. The longitudinal systolic strains were similar in the apical segments and significantly reduced in the basal segments in both groups, and multivariate analysis showed that NYHA class and mid-septum systolic LS were independent predictors of survival.
Quarta et al. analyzed 172 patients with cardiac amyloidosis (80 AL, 36 ATTRv, and 56 ATTRwt) using standard echocardiography and two-dimensional speckle-tracking imaging-derived LV longitudinal, radial, and circumferential strains [66]. Compared with ATTRv and AL amyloidosis, ATTRwt was characterized by greater LV wall thickness and lower EF, and the LS of LV was more depressed in both ATTRwt and AL amyloidosis. TTR-related causes were favorable predictors of survival, whereas LS and advanced NYHA class were negative predictors.
Minamisawa et al. compared cardiac function in patients with ATTRwt and ATTRv using standard and speckle tracking echocardiography [67]. Twenty-one patients with biopsy-proven ATTRwt were compared with 21 age-matched patients with ATTRv. LV wall thickness, LVEF, LV basal circumferential/radial strain, and mid-radial strain were significantly lower in ATTRwt patients compared with ATTRv. There were no other significant differences between the two groups. In the receiver-operating characteristics curve analysis, LVEF and LV basal mean radial strain were the best parameters for distinguishing between the two groups. Siepen et al. analyzed clinical predictors of mortality in 191 patients with histologically proven ATTRwt. Basal and midventricular, but not apical LS correlated with disease severity. Multivariable analysis revealed mitral annular plane systolic excursion and NT-pro BNP as independent predictors of mortality in the whole cohort and midventricular strain in the subgroup of patients in sinus rhythm [68].
Several studies have focused on RV function. Among RV fractional shortening, tricuspid annular plane systolic excursion (TAPSE), tissue Doppler systolic velocity, and global RV-LS, NYHA class, and low TAPSE independently predicted major adverse cardiac events in multivariate analysis [69]. Free-wall RV longitudinal strain (FWRVLS) discriminates AL amyloidosis from patients with increased wall thickness due to other etiologies [70].
Left atrial function has been a focus in cardiac amyloidosis. In ATTR cardiomyopathy with increased LV wall thickness, LA myocardial function is abnormal, irrespective of atrial cavity size. Reduced LA myocardial strain rate during atrial systole, irrespective of cavity volume, E/e′, and LV deformation, is also a strong predictor of atrial arrhythmic events [71]. In contrast to LV measures, there is a strong linear relationship between volumetric and longitudinal deformation indices of the LA irrespective of cardiomyopathy (dilated, hypertrophic, and AL amyloidosis) etiology [72]. LA function was severely impaired and highly correlated with LV deformation in cardiac amyloidosis. Differences in LA function between amyloid subtypes suggest that amyloid etiology plays a role in the pathophysiology of cardiac dysfunction in cardiac amyloidosis [73]. Three-dimensional speckle tracking echocardiography (3D-STE) provides new insight into the mechanism of LV dysfunction in cardiac amyloidosis. 3D-STE reveals regional and global biventricular dysfunction (LV basal LS, LV peak basal rotation, and RV basal free-wall LS) in cardiac amyloidosis. Assessment of RV ventricular dysfunction has an additive value in differentiating cardiac amyloidosis from the other causes of myocardial wall thickening [74].
Functional LA parameters are progressively altered in AL patients according to the Mayo Clinic staging system. A decrease in 3D-peak atrial LS is associated with worse outcomes, independent of LA volume [75].
Transthyretin cardiac amyloidosis in patients with aortic stenosis
Transthyretin cardiac amyloidosis (ATTR-CA) has been reported in patients with aortic stenosis. Among 151 patients with severe symptomatic aortic stenosis undergoing transcatheter aortic valve replacement, 16% (n = 24) screened positive for ATTR-CA with 99mTc-PYP scintigraphy. ATTR-CA is associated with a severe AS phenotype of low-flow low-gradient with mildly reduced EF. Average tissue Doppler mitral annular S′ < 6 cm/s may be a sensitive measure to prompt a confirmatory 99 mTc-PYP scan and subsequent testing for ATTR-CA [76]. A total of 146 patients with severe aortic stenosis requiring surgical valve replacement underwent cardiovascular magnetic resonance and intraoperative biopsies. 99m Tc DPD scintigraphy identified 6/146 patients as ATTR-CA patients. Occult cardiac ATTRwt was associated with poor outcomes [77].
Conclusion
Recent advances in echocardiography allow the noninvasive measurement of cardiac function, and novel echocardiographic parameters may represent prognosticators, especially in AL amyloidosis. LV longitudinal function and the cyclic variation of myocardial integrated backscatter may be the best predictors of adverse outcomes [46, 53, 54, 62]. Newer echocardiographic techniques, such as fully automated echocardiogram interpretation, are expected to add further useful information for assessing cardiac function and prognosis in cardiac amyloidosis patients [78].
References
Falk RH, Comenzo RL, Skinner M. The systemic amyloidoses. N Engl J Med. 1997;337:898–909.
Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003;349:583–96.
Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation. 2012;126:1286–300.
Koike H, Misu K, Ikeda S, et al. Type I (transthyretin Met30) familial amyloid polyneuropathy in Japan. Early- vs late-onset form. Arch Neurol. 2002;59:1771–6.
Koike H, Sobue G. Late-onset familial amyloid polyneuropathy in Japan. Amyloid. 2012;19:55–7.
Chew C, Ziady GM, Raphael MJ, et al. The functional defect in amyloid heart disease. The “stiff heart” syndrome. Am J Cardiol. 1975;36:438–44.
Borer JS, Henry WL, Epstein SE. Echocardiographic observations in patients with systemic infiltrative disease involving the heart. Am J Cardiol. 1977;39:184–8.
Child JS, Krivokapich J, Abbasi AS. Increased right ventricular thickness on echocardiography in amyloid infiltrative cardiomyopathy. Am J Cardiol. 1979;44:1391–5.
Child JS, Krivokapich J, Perloff JK. Effect of left ventricular size on mitral E point to ventricular septal separation in assessment of cardiac performance. Am Heart J. 1981;101:797–805.
St John Sutton MG, Reichek N, Kastor JA, et al. Computerized M-mode echocardiographic analysis of left ventricular dysfunction in cardiac amyloid. Circulation. 1982;66:790–9.
Siqueira-Filho AG, Cunha CL, Tajik AJ, et al. M-mode and two-dimensional echocardiographic features in cardiac amyloidosis. Circulation. 1981;63:188–96.
Mohty D, Pradel S, Magne J, et al. Prevalence and prognostic impact of left-sided valve thickening in systemic light-chain amyloidosis. Clin Res Cardiol. 2017;106:331–40.
Carroll JD, Gaasch WH, McAdam KP. Amyloid cardiomyopathy: characterization by distinctive voltage/mass relation. Am J Cardiol. 1982;49:9–13.
Simons M, Isner JM. Assessment of relative sensitivities of noninvasive tests for cardiac amyloidosis in documented cardiac amyloidosis. Am J Cardiol. 1992;69:425–7.
Hamer JP, Janssen S, van Rijswijk MH, et al. Amyloid cardiomyopathy in systemic non-hereditary amyloidosis. Clinical, echocardiographic and electrocardiographic findings in 30 patients with AA and 24 patients with AL amyloidosis. Eur Heart J. 1992;13:623–7.
Rahman JE, Helou EF, Gelzer-Bell R, et al. Noninvasive diagnosis of biopsy-proven cardiac amyloidosis. J Am Coll Cardiol. 2004;43:410–5.
Bhandari AK, Nanda NC. Myocardial texture characterization by two-dimensional echocardiography. Am J Cardiol. 1983;51:817–25.
Nicolosi GL, Pavan D, Lestuzzi C, et al. Prospective identification of patients with amyloid heart disease by two-dimensional echocardiography. Circulation. 1984;70:432–7.
Falk RH, Plehn JF, Deering T, et al. Sensitivity and specificity of the echocardiographic features of cardiac amyloidosis. Am J Cardiol. 1987;59:418–22.
Falk RH, Rubinow A, Cohen AS. Cardiac arrhythmias in systemic amyloidosis: correlation with echocardiographic abnormalities. J Am Coll Cardiol. 1984;1:107–13.
Cueto-Garcia L, Reeder GS, Kyle RA, et al. Echocardiographic findings in systemic amyloidosis: spectrum of cardiac involvement and relation to survival. J Am Coll Cardiol. 1985;6:737–43.
Kristen AV, Perz JB, Schonland SO, et al. Non-invasive predictors of survival in cardiac amyloidosis. Eur J Heart Fail. 2007;9:617–24.
Dubrey SW, Bilazarian S, LaValley M, et al. Signal-averaged electrocardiography in patients with AL (primary) amyloidosis. Am Heart J. 1997;134:994–1001.
Eriksson P, Backman C, Bjerle P, et al. Non-invasive assessment of the presence and severity of cardiac amyloidosis A study in familial amyloidosis with polyneuropathy by cross sectional echocardiography and technetium-99 m pyrophosphate scintigraphy. Br Heart J. 1984;52:321–6.
Fournier C, Grimon G, Rinaldi JP, et al. Usefulness of technetium-99 m pyrophosphate myocardial scintigraphy in amyloid polyneuropathy and correlation with echocardiography. Am J Cardiol. 1993;72:854–7.
Sperry BW, Vranian MN, Tower-Rader A, et al. Regional variation in technetium pyrophosphate uptake in transthyretin cardiac amyloidosis and impact on mortality. JACC Cardiovasc Imaging. 2018;11:234–42.
Dubrey S, Pollak A, Skinner M, et al. Atrial thrombi occurring during sinus rhythm in cardiac amyloidosis: evidence for atrial electromechanical dissociation. Br Heart J. 1995;74:541–4.
Feng D, Edwards WD, Oh JK, et al. Intracardiac thrombosis and embolism in patients with cardiac amyloidosis. Circulation. 2007;116:2420–6.
Feng D, Syed IS, Martinez M, et al. Intracardiac thrombosis and anticoagulation therapy in cardiac amyloidosis. Circulation. 2009;119:2490–7.
Klein AL, Hatle LK, Burstow DJ, et al. Doppler characterization of left ventricular diastolic function in cardiac amyloidosis. J Am Coll Cardiol. 1989;13:1017–26.
Klein AL, Hatle LK, Burstow DJ, et al. Comprehensive Doppler assessment of right ventricular diastolic function in cardiac amyloidosis. J Am Coll Cardiol. 1990;15:99–108.
Klein AL, Hatle LK, Taliercio CL, et al. Serial Doppler echocardiographic follow-up of left ventricular diastolic function in cardiac amyloidosis. J Am Coll Cardiol. 1990;16:1135–41.
Dubrey SW, Cha K, Skinner M, et al. Familial and primary (AL) cardiac amyloidosis: echocardiographically similar diseases with distinctly different clinical outcomes. Heart. 1997;78:74–82.
Murphy L, Falk RH. Left atrial kinetic energy in AL amyloidosis: can it detect early dysfunction? Am J Cardiol. 2000;86:244–6.
Patel AR, Dubrey SW, Mendes LA, et al. Right ventricular dilation in primary amyloidosis: an independent predictor of survival. Am J Cardiol. 1997;80:486–92.
Klein AL, Hatle LK, Taliercio CP, et al. Prognostic significance of Doppler measures of diastolic function in cardiac amyloidosis. A Doppler echocardiography study. Circulation. 1991;83:808–16.
Ng B, Connors LH, Davidoff R, et al. Senile systemic amyloidosis presenting with heart failure: a comparison with light chain-associated amyloidosis. Arch Intern Med. 2005;165:1425–9.
Migrino RQ, Mareedu RK, Eastwood D, et al. Left ventricular ejection time on echocardiography predicts long-term mortality in light chain amyloidosis. J Am Soc Echocardiogr. 2009;22:1396–402.
Rapezzi C, Merlini G, Quarta CC, et al. Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation. 2009;120:1203–12.
Pinney JH, Whelan CJ, Petrie A, et al. Senile systemic amyloidosis: clinical features at presentation and outcome. J Am Heart Assoc. 2013;2:e000098. https://doi.org/10.1161/JAHA.113.000098 (accessed 20 Dec 2014).
Koyama J, Ray-Sequin PA, Davidoff R, et al. Usefulness of pulsed tissue Doppler imaging for evaluating systolic and diastolic left ventricular function in patients with AL (primary) amyloidosis. Am J Cardiol. 2002;89:1067–71.
Palka P, Lange A, Donnelly JE, et al. Doppler tissue echocardiographic features of cardiac amyloidosis. J Am Soc Echocardiogr. 2002;15:1353–60.
Koyama J, Davidoff R, Falk RH. Longitudinal myocardial velocity gradient derived from pulsed Doppler tissue imaging in AL amyloidosis: a sensitive indicator of systolic and diastolic dysfunction. J Am Soc Echocardiogr. 2004;17:36–44.
Perugini E, Rappezi C, Reggiani LB, et al. Comparison of ventricular long-axis function in patients with cardiac amyloidosis versus idiopathic restrictive cardiomyopathy. Am J Cardiol. 2005;95:146–9.
Al-Zahrani GB, Bellavia D, Pellikka PA, et al. Doppler myocardial imaging compared to standard two-dimensional and Doppler echocardiography for assessment of diastolic function in patients with systemic amyloidosis. J Am Soc Echocardiogr. 2009;22:290–8.
Koyama J, Ray-Sequin PA, Falk RH. Prognostic significance of ultrasound myocardial tissue characterization in patients with cardiac amyloidosis. Circulation. 2002;106:556–61.
Tei C. New non-invasive index for combined systolic and diastolic ventricular function. J Cardiol. 1995;26:135–6.
Tei C, Dujardin KS, Hodge DO, et al. Doppler index combining systolic and diastolic myocardial performance: clinical value in cardiac amyloidosis. J Am Coll Cardiol. 1996;28:658–64.
Koyama J, Ray-Sequin PA, Falk RH. Longitudinal myocardial function assessed by tissue velocity, strain, and strain rate tissue Doppler echocardiography in patients with AL (primary) cardiac amyloidosis. Circulation. 2003;107:2446–52.
Bellavia D, Abraham TP, Pellikka PA, et al. Detection of left ventricular systolic dysfunction in cardiac amyloidosis with strain rate echocardiography. J Am Soc Echocardiogr. 2007;20:1194–202.
Ogiwara F, Koyama J, Ikeda S, et al. Comparison of the strain Doppler echocardiographic features of familial amyloid polyneuropathy (FAP) and light-chain amyloidosis. Am J Cardiol. 2005;95:538–40.
Bellavia D, Pellikka PA, Abraham TP, et al. Evidence of impaired left ventricular systolic function by Doppler myocardial imaging in patients with systemic amyloidosis and no evidence of cardiac involvement by standard two-dimensional and Doppler echocardiography. Am J Cardiol. 2008;101:1039–45.
Bellavia D, Pellikka PA, Al-Zahrani GB, et al. Independent predictors of survival in primary systemic (Al) amyloidosis, including cardiac biomarkers and left ventricular strain imaging: an observational cohort study. J Am Soc Echocardiogr. 2010;23:643–52.
Koyama J, Falk RH. Prognostic significance of strain Doppler imaging in light-chain amyloidosis. JACC Cardiovasc Imaging. 2010;3:333–42.
Bellavia D, Pellikka PA, Dispenzieri A, et al. Comparison of right ventricular longitudinal strain imaging, tricuspid annular plane systolic excursion, and cardiac biomarkers for early diagnosis of cardiac involvement and risk stratification in primary systematic (AL) amyloidosis: a 5-year cohort study. Eur Heart J Cardiovasc Imaging. 2012;8:680–9.
Modesto KM, Cauduro S, Dispenzieri A, et al. Two-dimensional acoustic pattern derived strain parameters closely correlate with one-dimensional tissue Doppler derived strain measurements. Eur J Echocardiogr. 2006;7:315–21.
Sun JP, Stewart WJ, Yang XS, et al. Differentiation of hypertrophic cardiomyopathy and cardiac amyloidosis from other causes of ventricular wall thickening by two-dimensional strain imaging echocardiography. Am J Cardiol. 2009;103:411–5.
Di Bella G, Minutoli F, Pingitore A, et al. Endocardial and epicardial deformations in cardiac amyloidosis and hypertrophic cardiomyopathy. Circ J. 2011;75:1200–8.
Kusunose K, Yamada H, Iwase T, et al. Images in cardiovascular medicine. Cardiac magnetic resonance imaging and 2-dimensional speckle tracking echocardiography in secondary cardiac amyloidosis. Circ J. 2010;74:1494–6.
Liu D, Hu K, Niemann M, et al. Effect of combined systolic and diastolic functional parameter assessment for differentiation of cardiac amyloidosis from other causes of concentric left ventricular hypertrophy. Circ Cardiovasc Imaging. 2013;6:1066–72.
Phelan D, Thavendieanathan P, Popovic Z, et al. Application of parametric display of two-dimensional speckle-tracking longitudinal strain to improve the etiologic diagnosis of mild to moderate left ventricular hypertrophy. J Am Soc Echocardiogr. 2014;27:888–95.
Bravo PE, Fujikura K, Kijewski MF, et al. Relative apical sparing of myocardial longitudinal strain is explained by regional differences in total amyloid mass rather than the proportion of amyloid deposits. JACC Cardiovasc Imaging. 2018. https://doi.org/10.1016/j.jcmg.2018.06.016.
Buss SJ, Emami M, Mereles D, et al. Longitudinal left ventricular function for prediction of survival in systemic light-chain amyloidosis: incremental value compared with clinical biochemical markers. J Am Coll Cardiol. 2012;60:1067–76.
Barros-Gomes S, Williams B, Nhola LF, et al. Prognosis of light chain amyloidosis with preserved LVEF: added value of 2D speckle-tracking echocardiography to the current prognostic staging system. JACC Cardiovasc Imaging. 2017;10:398–407.
Liu D, Hu K, Niemann M, et al. Impact of regional left ventricular function on outcome for patients with AL amyloidosis. PLoS One. 2013;8:e56923.
Quarta CC, Solomon SD, Uraizee I, et al. Left ventricular structure and function in transthyretin-related versus light-chain cardiac amyloidosis. Circulation. 2014;129:1840–9.
Minamisawa M, Koyama J, Sekijima Y, et al. Comparison of the standard and speckle tracking echocardiographic features of wild-type and mutated transthyretin cardiac amyloidoses. Eur Heart J Cardiovasc Imaging. 2016;17:402–10.
Siepen FAD, Bauer R, Voss A, et al. Predictors of survival stratification in patients with wild-type cardiac amyloidosis. Clin Res Cardiol. 2018;107:158–69.
Bodez D, Ternacle J, Guellich A, et al. Prognostic value of right ventricular systolic function in cardiac amyloidosis. Amyloid. 2016;23:158–67.
Uzan C, Lairez O, Raud-Raynier P, et al. Right ventricular longitudinal strain: a tool for diagnosis and prognosis in light-chain amyloidosis. Amyloid. 2018;25:18–25.
Henein MY, Suhr OB, Arvidsson S, et al. Reduced left atrial myocardial deformation irrespective of cavity size: a potential cause for atrial arrhythmia in hereditary transthyretin amyloidosis. Amyloid. 2018;25:46–53.
Kobayashi Y, Moneghetti KJ, Boralkar K, et al. Challenging the complementarity of different metrics of left atrial function: insight from a cardiomyopathy-based study. Eur Heart J Cardiovasc Imaging. 2017;18:1153–62.
Nochioka K, Quarta CC, Claggett B, et al. Left atrial structure and function in cardiac amyloidosis. Eur Heart J Cardiovasc Imaging. 2017;18:1128–37.
Vitarelli A, Lai S, Petrucci MT, et al. Biventricular assessment of light-chain amyloidosis using 3D speckle tracking echocardiography: differentiation from other forms of myocardial hypertrophy. Int J Cardiol. 2018;271:371–7.
Mohty D, Petitalot V, Magne J, et al. Left atrial function in patients with light chain amyloidosis: a transthoracic 3D speckle tracking imaging study. J Cardiol. 2018;71:419–27.
Castano A, Narotsky DL, Hamid N, et al. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J. 2017;38:2879–87.
Treibel TA, Fontana M, Gilbertson JA, et al. Occult transthyretin cardiac amyloid in severe calcific aortic stenosis: Prevalence and prognosis in patients undergoing surgical aortic valve replacement. Circ Cardiovasc Imaging. 2016. https://doi.org/10.1161/circimaging.116.005066.
Zhang J, Gajjala S, Agrawal P, et al. Fully automated echocardiogram interpretation in clinical practice. Circulation. 2018;138:1623–35.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Jun Koyama, Masatoshi Minamisawa, Yoshiki Sekijima, Koichiro Kuwahara, Tsutomu Katsuyama, and Kazutoshi Maruyama declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Koyama, J., Minamisawa, M., Sekijima, Y. et al. Role of echocardiography in assessing cardiac amyloidoses: a systematic review. J Echocardiogr 17, 64–75 (2019). https://doi.org/10.1007/s12574-019-00420-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12574-019-00420-5