Abstract
Purpose of Review
This review is geared to address the various forms of amyloidosis which have cardiac involvement and discuss the echocardiographic findings that may be present in patients with cardiac amyloidosis.
Recent Findings
The use of echocardiography has revolutionized the diagnosis of cardiac amyloidosis. The diagnosis is often first suspected due to characteristic echocardiographic findings. Although no single finding is pathognomonic, there are a constellation of features that point to the diagnosis including increased left and right ventricular wall thickness, thickened cardiac valves and atrial walls, and a pericardial effusion. Alterations in diastolic function with evidence of elevated filling pressures are common. The use of myocardial strain imaging has allowed for earlier detection of cardiac amyloidosis and has revealed a distinctive pattern, sometimes suggesting the diagnosis when more classic findings are subtle or absent. Multiple echocardiographic findings have been found to be prognostic in cardiac amyloid. Using current techniques, functional measures such as myocardial strain and stroke volume index are more powerful predictors of outcome than myocardial thickness. While other imaging modalities are complementary including cardiac magnetic resonance imaging and nuclear medicine scintigraphy techniques using bone tracers, echocardiography remains a mainstay in the diagnosis and follow-up of patients with cardiac amyloidosis.
Summary
Echocardiography is a critical tool in the evaluation of the patient with heart failure, as well as those with unexplained chest pain and arrhythmias. Key findings on an echocardiogram can help alert the clinician to the presence of cardiac amyloidosis. The degree of cardiac dysfunction in a patient with systemic amyloidosis correlates with prognosis. Recognition of subtle echocardiographic findings may allow for earlier and more aggressive treatment to improve overall mortality in patients with cardiac amyloidosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cardiac amyloidosis (CA) is a term applied to both inherited and acquired diseases of misfolded proteins which form amyloid fibrils that deposit within the heart. The term “amyloid” is Latin for “starch” as the misfolded protein was initially mistakenly thought to be a polysaccharide [1]. Over thirty proteins have now been identified as precursors to amyloid fibril deposition in multiple organs and tissues resulting in a wide variety of systemic manifestations. The presence or absence of cardiac involvement has been shown to be the prime determinant of survival in systemic amyloidosis, highlighting the importance of cardiac imaging and early diagnosis.
There are three major types of amyloidosis which commonly result in cardiac involvement. The nomenclature uses “A” for amyloid followed by the abbreviation of the precursor protein. Light chain amyloidosis (AL) is caused by a clonal plasma cell disorder of the bone marrow [2•]. Transthyretin amyloid (ATTR) results from an abnormal protein produced by the liver. ATTR can be hereditary, resulting from a DNA mutation (ATTRm) which produces an abnormal transthyretin protein, or it can result from deposition of non-mutated (“wild-type”) transthyretin (ATTRwt) [3]. The term “senile systemic amyloidosis” was previously used for ATTRwt, as this form of cardiac amyloidosis was initially thought to occur only in the elderly. However, ATTRwt is now the preferred term as it is recognized that patients may present with this condition as early as the fifth decade of life [4].
The infiltration of the amyloid fibrils within the myocardium results in thickened walls and abnormalities in left and right ventricular systolic and diastolic function. Diastolic abnormalities due to a restrictive cardiomyopathy are typically described in CA; however, systolic dysfunction may also occur, usually late in the disease [5]. The coronary arteries or cardiac valves may also manifest abnormalities due to amyloid deposition. This review will discuss in detail the various echocardiographic abnormalities that can be encountered in patients with cardiac amyloidosis. In addition, we will review some of the alternative imaging modalities which are part of the evaluation in a patient with suspected CA.
Background
Amyloidosis is the general term for the deposition of abnormal fibrils composed of specific precursor proteins. There are several forms of amyloidosis which result in a wide range of clinical manifestations.
Immunoglobulin light chain (AL) amyloidosis is the result of a plasma cell dyscrasia of the bone marrow resulting in the over-production of immunoglobulin light chain fragments. These monoclonal proteins can be measured using protein electrophoresis with immunofixation, and the circulating free light chains can be measured in the serum. AL amyloidosis often presents as multi-organ disease with fibrils depositing in the kidneys causing nephrotic syndrome, the liver resulting in hepatomegaly, and the heart causing heart failure [5]. However, isolated or predominant cardiac AL may occur. Peripheral and autonomic neuropathies are common in AL. Macroglossia and periorbital purpura are infrequent manifestations but are almost pathognomonic for AL amyloidosis [6]. Significant cardiac involvement occurs in up to 50% of patients with AL amyloidosis and is the main determinant of outcome [5, 7]. Although AL can occur in association with multiple myeloma, it is increasingly recognized that cardiac amyloid in elderly patients with myeloma may be due to ATTRwt.
Transthyretin amyloid (ATTR) may be hereditary where an identified DNA mutation results in an unstable transthyretin protein produced by the liver (ATTRm). There is a spectrum of disease in ATTR, from a purely neuropathic phenotype with peripheral and/or autonomic neuropathy to a predominant cardiac involvement or a mixed phenotype [8]. The presence and extent of cardiac involvement depends partly on the specific mutation in ATTRm, but significant heterogeneity is present, even within members of the same family.
Wild-type ATTR (ATTRwt) occurs without an identified genetic mutation and historically had been referred to as “senile” cardiac amyloidosis. Although initially it was reported that amyloid deposition in the hearts of elderly individuals is of little clinical relevance [9], ATTRwt is now increasingly recognized as an important cause of heart failure with advancing age [10]. One series demonstrated that ATTRwt was present in 13% of patients over the age of 60 with heart failure and preserved ejection fraction and left ventricular wall thickness greater than 12 mm [11•]. Although small deposits of amyloid may be found throughout the body, the clinical manifestations of ATTRwt are primarily cardiac with associated carpal tunnel syndrome, often 6–10 years before cardiac symptoms, spinal ligament involvement causing spinal stenosis, and bladder involvement [12•]. Atrial arrhythmias and conduction system disease in ATTRwt may precede the onset of heart failure by several years.
Other forms of cardiac amyloid are much less common than AL and ATTR. Chronic inflammatory conditions may be associated with secondary amyloidosis in which an acute-phase reactant, serum amyloid A, is the responsible protein. However, this condition rarely produces cardiac dysfunction.
Determining the type of cardiac amyloidosis has historically relied on tissue biopsy (fat aspirate, bone marrow, or endomyocardial biopsy). However, the use of nuclear scintigraphy using bone tracers, such as technetium pyrophosphate (PYP), allows for diagnosis of ATTR in the setting of typical echocardiographic and/or cardiac MRI findings in the absence of a plasma cell dyscrasia [13]. When a monoclonal protein is present, tissue biopsy with amyloid typing is required. The most accurate method of tissue typing is laser micro-dissection laser mass spectrometry [14]. Accurate typing of CA is critical for the determination of treatment options and prognosis. Incorrect amyloid typing has life-threatening consequences.
Clinical Presentation
Typically, the consideration of cardiac amyloidosis is made when a patient presents with signs and symptoms of heart failure without an identifiable cause such as ischemic heart disease, hypertension, or significant valvular heart disease. Patients may present with dyspnea on exertion, lower extremity edema, hepatomegaly, abdominal distension due to ascites, orthopnea, and paroxysmal nocturnal dyspnea. These symptoms are suggestive of biventricular dysfunction. Patients who present with heart failure with preserved ejection fraction should be evaluated for the possibility of CA. Unfortunately, many patients are diagnosed late in the course of the disease, often after having undergone multiple cardiac imaging studies over several years before the diagnosis was established [12•]. Thus, it is crucial to recognize early manifestations and subtle echocardiographic findings.
Other common cardiac symptoms include presyncope or syncope. This can be secondary to the autonomic neuropathy that occurs alongside CA which results in orthostatic hypotension. However, syncope may also be a reflection of poor cardiac output, particularly if it occurs during exertion [15].
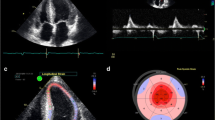
Atrial fibrillation is seen in up to 10–20% of patients with AL and 40–60% of ATTRwt at diagnosis [4, 5, 16, 17]. Patients with both AL and ATTR have an increased risk of left atrial thrombus formation and thromboembolism, even in sinus rhythm [18]. Once atrial fibrillation or flutter is detected, life-long anticoagulation is recommended, even for those with low CHADs-Vasc scores. Left atrial appendage thrombus may persist despite anticoagulation and TEE is recommended prior to elective cardioversion (Fig. 1). Unfortunately, stroke due to thromboembolism may be the initial presentation of CA [19].
Other symptoms which can occur in patients with CA include angina (due to small vessel disease typically), weight loss, and fatigue.
Non-cardiac findings can be a clue to the presence of amyloidosis. Carpal tunnel syndrome typically predates the diagnosis of CA and can be seen in AL or ATTR, although it is more common with ATTR [12•]. Identification of periorbital purpura (raccoon eyes) due to small vessel disease and vascular fragility and macroglossia, both rare, are almost pathognomonic of AL amyloid.
Echocardiographic Abnormalities
The entire cardiac structure can be affected by the deposition of the amyloid protein. In addition, elevated levels of circulating free light chains cause myocardial dysfunction and may result in clinical manifestations out of proportion to the degree of wall thickening [20]. Echocardiography is performed routinely when the diagnosis of systemic amyloidosis is made officially due to another organ’s clinical involvement. Certain echocardiographic features are highly suggestive of the infiltrative cardiomyopathy, although none are pathognomonic. However, findings on a transthoracic echocardiogram (TTE) may be the first clue about the presence of CA, particularly in the proper clinical context. Echocardiography is also utilized during treatment to assess for improvement in cardiac parameters or to determine progression of disease. Findings on echocardiography are related to overall survival in CA, particularly AL amyloid [21].
Ventricles
Increased left ventricular (LV) wall thickness is one of the most characteristic echocardiographic abnormalities. This increase in wall thickness may be concentric or asymmetric (Fig. 2). Increases in septal thickness can mimic findings of hypertrophic cardiomyopathy, and dynamic left ventricular outflow obstruction and systolic anterior motion may occur [22]. Concentric wall thickness can be mistaken for hypertensive heart disease. However, low voltage on an electrocardiogram (ECG) especially in association with increased wall thickness should raise suspicion of CA. Although low voltage criteria suggest cardiac amyloid, the absence does not exclude the diagnosis. In AL, approximately 45% of patients meet low-voltage criteria, but this is present in only ~ 25% of patients with ATTRwt [4, 12•, 23]. Thus, normal voltage or even left ventricular hypertrophy pattern on an ECG does not exclude the possibility of CA. Patients with ATTRwt often have thicker walls than AL patients. In fact, AL patients may have wall thickness within the normal range [24] (Fig. 3). Right ventricular (RV) wall thickness is often increased in CA (Fig. 4) and is a clue to the diagnosis, especially in those with less impressive left ventricular wall thickening. In addition, other causes of left ventricular wall thickening, such as hypertrophic cardiomyopathy, rarely result in increased right ventricular wall thickness. However, if there is an unusual pattern of amyloid deposition, such as endomyocardially, classic findings of CA may be missing on echocardiography [25].
Diastolic function is frequently impaired in patients with CA. This progresses to a restrictive pattern of diastolic dysfunction with advancing disease (Fig. 5). Early studies demonstrated that increased mitral E wave velocities with short deceleration times were associated with poor prognosis in AL [26] although these factors are less important with the advent of myocardial strain imaging. Abnormalities in the mitral inflow Doppler pattern are accompanied by severely reduced tissue Doppler velocities of the mitral annulus and are consistent with high left ventricular filling pressures. AL patients who have less LV wall thickness may still exhibit significant diastolic dysfunction [8].
LV cavity dilatation is not commonly seen in CA [8], and LV ejection fraction (EF) is usually preserved early in the disease process. However, a minority of patients with AL may present with reduced ejection fraction and normal wall thickness [24]. Systolic function is likely impaired in most patients with CA as is demonstrated by abnormalities of strain imaging (discussed below). As the disease progresses, systolic function deteriorates as manifested by worsening strain and a decline in the LVEF.
Cardiac Valves
Nonspecific thickening of all of the cardiac valves is seen with CA. Most often, the aortic and mitral valves are involved. The degree of thickening is out of proportion to the patient’s age. Significant valve pathology is not typically seen. However, recently, ATTRwt has been recognized in a high percentage (16%) of patients who have undergone transcatheter aortic valve procedures (TAVR) [27•]. As both ATTRwt and severe aortic stenosis (AS) can occur in an elderly population, the significance of these findings remains unclear. In a patient with AS, the presence of ATTR cardiac amyloidosis, by causing a restrictive LV filling pattern, may be the etiology for a low flow-low gradient state [27•]. In addition, the concomitant presence of an infiltrative cardiomyopathy may adversely affect outcomes in patients undergoing TAVR.
Other Echocardiographic Findings
A pericardial effusion is a common finding in patients with CA (Fig. 4). These effusions tend to be small and rarely cause cardiac tamponade. Atrial enlargement is also common. Left atrial enlargement can be due to the elevated left atrial pressure from diastolic dysfunction but may also be secondary to direct deposition of fibrils within the atrial walls. Similarly, the atrial septum can be thickened, which may be best appreciated on a subcostal view (Fig. 6).
Pulmonary hypertension may be identified on TTE and is typically due to left heart diastolic dysfunction, although pulmonary amyloid infiltration may contribute in some patients [28]. A dilated inferior vena cava is common as it is a reflection of high central venous pressure. A pleural effusion or ascites may additionally be seen during an echocardiogram.
Hemodynamic assessment reveals a low stroke volume and cardiac output in patients with clinically significant CA. The independent prognostic value of indexed stroke volume in AL has recently been reported [29]. Over 700 AL patients who underwent echocardiography were studied, and stroke volume was found to be as predictive of survival as myocardial strain measurements and was independent of LVEF, blood pressure, and cardiac biomarkers [29]. Stoke volume calculation by Doppler is part of routine echocardiographic studies and can be obtained in most patients. Serial echocardiography is often performed in patients with known or suspected CA, and tracking stroke volume/cardiac index can be helpful to follow symptoms and prognosis in CA.
Myocardial Strain Imaging
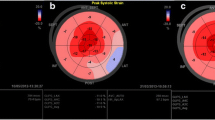
The constellation of findings such as thick myocardial walls with a restrictive cardiomyopathy, pericardial effusion, thickened valves, and enlarged atria are highly suggestive of CA. If these findings are seen in the right clinical context, then they can more definitely be labeled as “consistent with cardiac amyloidosis.” However, it is when there are less obvious echocardiographic features that the clinician remains unsure as to the presence of CA. Assessing myocardial deformation using strain imaging has evolved as a tool to identify early stages of any myocardial disease, prior to a drop in LVEF [30]. Strain imaging has been used to identify earlier cardiac involvement in amyloidosis [31]. Abnormal global longitudinal peak systolic strain (GLS) can predict outcomes [32] and provides incremental prognostic value to patients with amyloidosis and may be even more useful in those without documented CA [33•]. A characteristic pattern is seen in all patients with CA (AL or ATTR) where there is sparing of the longitudinal strain at the apex of the LV (Fig. 7) [34]. It is unclear if this pattern is unique to CA but as more pathologic conditions are assessed with GLS, we will be able to characterize various diseases with their patterns. These pattern differences may help guide the workup for patients who present with thick walls on echocardiography in the setting of clinical heart failure.
Other Imaging Modalities for the Diagnosis of Cardiac Amyloidosis
Cardiac magnetic resonance imaging (CMR) is often utilized during the work up for suspected CA. Similar to echocardiography, abnormalities detected by CMR are sometimes the first suggestion of CA in patients not specifically referred for this diagnosis. Late gadolinium enhancement (LGE) has been shown to identify the presence of CA and be prognostically important [35]. The pattern of late gadolinium seen includes diffuse subendocardial LGE as well as localized patterns that correspond with the extent and distribution of amyloid [36, 37]. Altered gadolinium kinetics in patients with CA results in abnormal “nulling” of the myocardium in which it is difficult to distinguish the myocardium from the blood pool [38]. Non-contrast T1 mapping by detecting myocardial edema and fibrosis may have a strong diagnostic yield for AL amyloid and have improved accuracy over LGE [39]. Myocardial extracellular volume (ECV) measurements have shown promise for the detection of CA and may be more reproducible, thus possibly being a parameter to follow treatment response [40].
Nuclear scintigraphy using phosphate-based isotopes (99m Technetium pyrophosphate (PYP)) has been identified as being helpful in the diagnosis of ATTR [41, 42]. However, uptake can occur in AL amyloid and PYP alone cannot be used for diagnosis in the presence of a monoclonal protein (abnormal serum/urine free light chain assay or monoclonal protein detected by immunofixation). In addition, nuclear scintigraphy does not differentiate wild-type from mutant-type ATTR and DNA sequencing is recommended to determine if a mutation is present [13].
Conclusion
Cardiac involvement with amyloidosis is variable and advanced disease is associated with a poor prognosis. Investigating for the presence of cardiac amyloidosis is frequently undertaken to better characterize prognosis as well as to determine treatment approaches. Echocardiography is one of the first imaging tools utilized during a work up for suspected amyloidosis. In fact, echocardiography may be the first indicator of the disease when clinical signs and symptoms do not establish an etiology for heart failure. Characteristic findings on transthoracic echocardiography can be highly suggestive of cardiac amyloidosis; however, echocardiography alone is not sufficient for the diagnosis. Recognition of subtle imaging findings may help identify CA at a time when patients are more likely to benefit from therapy The varying types of amyloid protein can impact the degree of cardiac abnormalities seen on echocardiography, but echocardiography is not reliable for determining amyloid type. Advances in echocardiographic technique, with the use of myocardial speckle strain imagining, have enhanced the ability to establish an earlier diagnosis of cardiac amyloid. CMR and nuclear scintigraphy are complimentary to echocardiography in suggesting the diagnosis, and in the case of scintigraphy, determining amyloid type. A multimodality/multidisciplinary approach is critical in the evaluation of patients with suspected CA and will hopefully promote earlier diagnosis.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Maleszewski JJ. Cardiac amyloidosis: pathology, nomenclature, and typing. Cardiovasc Pathol. 2015;24(6):343–50.
• Grogan M, Dispenzieri A. Natural history and therapy of AL cardiac amyloidosis. Heart Fail Rev. 2015;20(2):155–62 Comprehensive review of light chain cardiac amyloidosis with focus on clinical and imaging findings as well as treatment options.
Gertz MA, Benson MD, Dyck PJ, Grogan M, Coelho T, Cruz M, et al. Diagnosis, prognosis, and therapy of transthyretin amyloidosis. J Am Coll Cardiol. 2015;66(21):2451–66.
Grogan M, Scott CG, Kyle RA, Zeldenrust SR, Gertz MA, Lin G, et al. Natural history of wild-type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J Am Coll Cardiol. 2016;68(10):1014–20.
Falk RH. Diagnosis and management of the cardiac amyloidoses. Circulation. 2005;112(13):2047–60.
Grogan M, Dispenzieri A, Gertz MA. Light-chain cardiac amyloidosis: strategies to promote early diagnosis and cardiac response. Heart. 2017;103(14):1065–72.
Dubrey SW, Cha K, Anderson J, Chamarthi B, Reisinger J, Skinner M, et al. The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJM. 1998;91(2):141–57.
Rapezzi C, Merlini G, Quarta CC, Riva L, Longhi S, Leone O, et al. Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation. 2009;120(13):1203–12.
Cornwell GG III, Westermark P. Senile amyloidosis: a protean manifestation of the aging process. J Clin Pathol. 1980;33(12):1146–52.
Mohammed SF, Mirzoyev SA, Edwards WD, Dogan A, Grogan DR, Dunlay SM, et al. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Heart Failure. 2014;2(2):113–22.
• Gonzalez-Lopez E, Gallego-Delgado M, Guzzo-Merello G, de Haro-Del Moral FJ, Cobo-Marcos M, Robles C, et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36(38):2585–94 Discussion about the role of ATTRwt amyloidosis as an etiology for the diagnosis of heart failure with preserved ejection fraction (itself a complex clinical dilemma).
• Maurer MS, Elliott P, Comenzo R, Semigran M, Rapezzi C. Addressing common questions encountered in the diagnosis and management of cardiac amyloidosis. Circulation. 2017;135(14):1357–77 Review of the approach to diagnosis cardiac amyloidosis particularly as it related to various presentations.
Gillmore JD, Maurer MS, Falk RH, Merlini G, Damy T, Dispenzieri A, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133(24):2404–12.
Vrana JA, Gamez JD, Madden BJ, Theis JD, Bergen HR III, Dogan A. Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood. 2009;114(24):4957–9.
Chamarthi B, Dubrey SW, Cha K, Skinner M, Falk RH. Features and prognosis of exertional syncope in light-chain associated AL cardiac amyloidosis. Am J Cardiol. 1997;80(9):1242–5.
Pinney JH, Whelan CJ, Petrie A, Dungu J, Banypersad SM, Sattianayagam P, et al. Senile systemic amyloidosis: clinical features at presentation and outcome. J Am Heart Assoc. 2013;2(2):e000098.
Connors LH, Sam F, Skinner M, Salinaro F, Sun F, Ruberg FL, et al. Heart failure resulting from age-related cardiac amyloid disease associated with wild-type transthyretin: a prospective, observational cohort study. Circulation. 2016;133(3):282–90.
Feng D, Edwards WD, Oh JK, Chandrasekaran K, Grogan M, Martinez MW, et al. Intracardiac thrombosis and embolism in patients with cardiac amyloidosis. Circulation. 2007;116(21):2420–6.
Zubkov AY, Rabinstein AA, Dispenzieri A, Wijdicks EF. Primary systemic amyloidosis with ischemic stroke as a presenting complication. Neurology. 2007;69(11):1136–41.
Falk RH, Alexander KM, Liao R, Dorbala S. AL (light-chain) cardiac amyloidosis: A Review of Diagnosis and Therapy. J Am Coll Cardiol. 2016;68(12):1323–41.
Siddiqi OK, Sanchorawala V, Ruberg FL. Echocardiography and survival in light chain cardiac amyloidosis: back to basics. Circ Cardiovasc Imaging. 2018;11(5):e007826.
Mookadam F, Haley JH, Olson LJ, Cikes M, Mookadam M. Dynamic left ventricular outflow tract obstruction in senile cardiac amyloidosis. Eur J Echocardiogr. 2006;7(6):465–8.
Murtagh B, Hammill SC, Gertz MA, Kyle RA, Tajik AJ, Grogan M. Electrocardiographic findings in primary systemic amyloidosis and biopsy-proven cardiac involvement. Am J Cardiol. 2005;95(4):535–7.
Suresh R, Grogan M, Maleszewski JJ, Pellikka PA, Hanna M, Dispenzieri A, et al. Advanced cardiac amyloidosis associated with normal interventricular septal thickness: an uncommon presentation of infiltrative cardiomyopathy. J Am Soc Echocardiogr. 2014;27(4):440–7.
Fealey ME, Edwards WD, Buadi FK, Syed IS, Grogan M. Echocardiographic features of cardiac amyloidosis presenting as endomyocardial disease in a 54-year-old male. J Cardiol. 2009;54(1):162–6.
Klein AL, Hatle LK, Taliercio CP, Oh JK, Kyle RA, Gertz MA, et al. Prognostic significance of Doppler measures of diastolic function in cardiac amyloidosis. A Doppler echocardiography study. Circulation. 1991;83(3):808–16.
• Castano A, Narotsky DL, Hamid N, Khalique OK, Morgenstern R, DeLuca A, et al. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J. 2017;38(38):2879–87 Paper illustrating two complex conditions which may occur in the elderly patient: severe aortic stenosis and cardiac amyloidosis. How the presence of cardiac amyloidosis may result in low flow, low gradient aortic stenosis.
Cirulis MM, Emerson LL, Bull DA, Hatton N, Nativi-Nicolai J, Hildebrandt GC, et al. Pulmonary arterial hypertension in primary amyloidosis. Pulm Circ. 2016;6(2):244–8.
Milani P, Dispenzieri A, Scott CG, Gertz MA, Perlini S, Mussinelli R, et al. Independent prognostic value of stroke volume index in patients with immunoglobulin light chain amyloidosis. Circ Cardiovasc Imaging. 2018;11(5):e006588.
Bellavia D, Abraham TP, Pellikka PA, Al-Zahrani GB, Dispenzieri A, Oh JK, et al. Detection of left ventricular systolic dysfunction in cardiac amyloidosis with strain rate echocardiography. J Am Soc Echocardiogr. 2007;20(10):1194–202.
Porciani MC, Lilli A, Perfetto F, Cappelli F, Massimiliano Rao C, Del Pace S, et al. Tissue Doppler and strain imaging: a new tool for early detection of cardiac amyloidosis. Amyloid. 2009;16(2):63–70.
Koyama J, Falk RH. Prognostic significance of strain Doppler imaging in light-chain amyloidosis. JACC Cardiovasc Imaging. 2010;3(4):333–42.
• Barros-Gomes S, Williams B, Nhola LF, Grogan M, Maalouf JF, Dispenzieri A, et al. Prognosis of light chain amyloidosis with preserved LVEF: added value of 2D speckle-tracking echocardiography to the current prognostic staging system. JACC Cardiovasc Imaging. 2017;10(4):398–407 Discussion about the utility of strain imaging, along with other markers of cardiac amyloidosis, for prognostic determination.
Quarta CC, Solomon SD, Uraizee I, Kruger J, Longhi S, Ferlito M, et al. Left ventricular structure and function in transthyretin-related versus light-chain cardiac amyloidosis. Circulation. 2014;129(18):1840–9.
Boynton SJ, Geske JB, Dispenzieri A, Syed IS, Hanson TJ, Grogan M, et al. LGE provides incremental prognostic information over serum biomarkers in AL cardiac amyloidosis. JACC Cardiovasc Imaging. 2016;9(6):680–6.
Maceira AM, Joshi J, Prasad SK, Moon JC, Perugini E, Harding I, et al. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2005;111(2):186–93.
Syed IS, Glockner JF, Feng D, Araoz PA, Martinez MW, Edwards WD, et al. Role of cardiac magnetic resonance imaging in the detection of cardiac amyloidosis. JACC Cardiovasc Imaging. 2010;3(2):155–64.
White JA, Kim HW, Shah D, Fine N, Kim KY, Wendell DC, et al. CMR imaging with rapid visual T1 assessment predicts mortality in patients suspected of cardiac amyloidosis. JACC Cardiovasc Imaging. 2014;7(2):143–56.
Karamitsos TD, Piechnik SK, Banypersad SM, Fontana M, Ntusi NB, Ferreira VM, et al. Noncontrast T1 mapping for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging. 2013;6(4):488–97.
Banypersad SM, Sado DM, Flett AS, Gibbs SD, Pinney JH, Maestrini V, et al. Quantification of myocardial extracellular volume fraction in systemic AL amyloidosis: an equilibrium contrast cardiovascular magnetic resonance study. Circ Cardiovasc Imaging. 2013;6(1):34–9.
Rapezzi C, Guidalotti P, Salvi F, Riva L, Perugini E. Usefulness of 99mTc-DPD scintigraphy in cardiac amyloidosis. J Am Coll Cardiol. 2008;51(15):1509–10 author reply 10.
Bokhari S, Castano A, Pozniakoff T, Deslisle S, Latif F, Maurer MS. (99m)Tc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ Cardiovasc Imaging. 2013;6(2):195–201.
Author information
Authors and Affiliations
Ethics declarations
Conflict of Interest
Both authors declare no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of Topical Collection on Echocardiography
Rights and permissions
About this article
Cite this article
Mankad, R., Grogan, M. Diagnosis of Cardiac Amyloidosis: Clinical and Echocardiographic Features. Curr Cardiovasc Imaging Rep 11, 32 (2018). https://doi.org/10.1007/s12410-018-9472-2
Published:
DOI: https://doi.org/10.1007/s12410-018-9472-2