Abstract
In this study, we report the prevalence and aetiology of hyponatraemia after surgery for hip fracture. We conducted a retrospective analysis of 144 consecutive patients who underwent surgery after sustaining a hip fracture. Data were collected from medical case records, operative notes and electrolyte results. Univariate and logistic regression analysis was conducted in order to identify significant independent risk factors for the development of hyponatraemia. Mild hyponatraemia was relatively common affecting 19 % (28/144) of patients pre-operatively and 28 % (40/144) post-operatively. However, moderate/severe hyponatraemia (plasma sodium concentration <130 mmol/l) was uncommon, affecting 1 % (2/144) of patients at the time of admission and 6 % (9/144) of patients post-operatively. Univariate analysis identified: female gender, pre-operative hyponatraemia, hypotonic fluid administration and thiazide diuretic use as being associated with the development of post-operative hyponatraemia. Age had no statistically significant association. Logistic regression analysis identified female gender, pre-operative hyponatraemia and hypotonic fluid administration being significant, independent risk factors for the development of hyponatraemia. Age and thiazide diuretics both had positive risk associations; however, these were not statistically significant. Mild hyponatraemia is a common finding in hip fracture patients; however, more severe cases are relatively rare. Pre-operative hyponatraemia and hypotonic fluid administration were the only modifiable risk factors identified.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyponatraemia is one of the most common electrolyte disturbances to affect the elderly population affecting up to 12% of individuals over 75 years of age [1]. In hospitalised patients, the prevalence is even higher and is often iatrogenic in origin [1]. Severe hyponatraemia is associated with considerable morbidity and mortality; however, even mild hyponatraemia may negatively affect outcomes in patients with co-morbidities such as cardiac failure [2–4]. Hyponatraemia can be very difficult to diagnose clinically, particularly in the peri-operative period. Symptoms of hyponatraemia are non-specific and include nausea, vomiting, malaise muscle cramps and fatigue. Consequently, hyponatraemia may initially go unrecognised unless a high degree of clinical suspicion is maintained. More severe cases may result in confusion, seizures and permanent brain damage or death secondary to cerebral oedema.
The potential problem of hyponatraemia in orthopaedic patients was highlighted by a somewhat controversial article by Lane et al. [5]. The authors claimed that “too many orthopaedic surgeons seem unaware of the dangers of hyponatraemia or its characteristic neurological symptoms”. Whilst there is undoubtedly a degree of truth in this statement, it was however based on an anecdotal experience rather than scientific evidence from the medical literature. As such, this article provoked much debate regarding the issue of hyponatraemia in post-operative orthopaedic patients [6–10]. Each year, in the United Kingdom, approximately 80,000 patients undergo surgery after sustaining a hip fracture. There is however a relative paucity of evidence regarding the true prevalence and independent risk factors for the development of hyponatraemia in elderly trauma patients [11, 12]. Hip fracture patients represent a complex cohort to manage due to the requirement of multiple disciplines in their management and often significant past medical history. As such hip fractures are often described as a tracer condition, whereby the management of these patients represents the wider standard of trauma care.
Patients and methods
We performed a retrospective analysis of 144 consecutive patients who underwent surgery after sustaining a hip fracture. Medical case records were reviewed to identify patient demographics, drug prescription charts, operation notes and documentation of post-operative morbidity/mortality. Electrolyte results were also collected from the time of admission to discharge from the acute orthopaedic ward. Fluid administration charts were used to calculate the type and volume of intravenous fluids administered in the pre-operative, intra-operative and post-operative period. Normal (0.9 %) saline, packed red cells and Hartmann’s solution were classed as isotonic fluids and 5 % dextrose as hypotonic. Saline/dextrose and colloid solutions were not prescribed to any patient involved in the study. Hyponatraemia was subclassified into 3 categories based on the serum sodium concentration (mmol/l) as follows: mild (134.9–130.0), moderate (129.9–125.0) and severe (<125.0).
Statistical analysis was performed using SPSS versus 16.0 software (SPSS Inc, Chicago, Illinois). Univariate analysis was performed to determine the effects of five previously reported risk factors for the development of hyponatraemia including age, gender, thiazide diuretic use, pre-operative hyponatraemia and hypotonic fluid administration. As these factors may have confounding effects we subsequently used logistic regression models to investigate the independent effects of each variable.
Results
A total of 155 consecutive patients who underwent operative treatment of a hip fracture were initially identified. Due to incomplete data being available, 11/155 (8 %) patients were excluded. The mean age of the remaining cohort of 144 patients was 80 years (range 32–102 years, SD ± 11.3 years). Females accounted for 75 % of those in the study. Operative procedures included dynamic hip screw 70/144 (49 %), hemi-arthroplasty 69/144 (48 %), intra-medullary nail 3/144 (2 %) and total hip arthroplasty 2/144 (1 %).
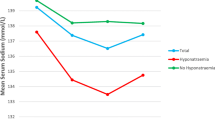
Hyponatraemia was identified in 21 % (30/144) of patients at the time of admission. However, although hyponatraemia was relatively common, 28/30 (93 %) cases were mild and only 2/30 (7 %) moderate. As such, 19 % (28/144) of patients were diagnosed with mild hyponatraemia and 1 % (2/144) moderate hyponatraemia on admission. No severe cases were identified. Correction of hyponatraemia was initiated in all patients on admission, but achieved pre-operatively in only ten of these 30 cases (33 %). In the patients who had hyponatraemia at the time of surgery, anaesthetic review was preformed pre-operatively, and the risks of proceeding in the presence of hyponatraemia balanced against the risks of delaying surgery. Consequently, at the time of surgery, 124/144 (86 %) patients had a normal serum sodium concentration, and 20/144 (14 %) had on-going hyponatraemia (two moderate cases and 18 mild).
Post-operatively, the number of patients identified as having hyponatraemia increased from 20/144 (14 %) to 49/144 (34 %) patients. Of the 20 patients who underwent surgery with a low serum sodium concentration, all remained hyponatraemic post-operatively up to 72 h. Of the 18 cases of uncorrected mild hyponatraemia, 11/18 remained unchanged, 6/18 developed moderate and 1/18 developed severe hyponatraemia. The two remaining patients with uncorrected moderate hyponatraemia both improved to mild within 72 h of surgery.
In the remaining 124/144 patients who underwent surgery with a normal serum sodium concentration, 29/49 (59 %) cases of post-operative hyponatraemia developed. As such, 23 % (29/124) of patients with a normal serum sodium concentration at the time of surgery developed hyponatraemia post-operatively. Again, the majority of these new cases were mild (27/29, 93 %) and 2/29 (7 %) moderate. Of this cohort of new onset cases of hyponatraemia, all were corrected prior to discharge from the acute orthopaedic unit.
At the time of transfer/discharge from the acute orthopaedic ward, 7/141 (5 %) surviving patients had a serum sodium concentration less than 135 mmol/l; all of whom had uncorrected mild hyponatraemia at the time of surgery. Of this cohort, 5/7 cases had mild, 1/7 moderate and 1/7 severe hyponatraemia. No permanent neurological impairment was recorded as a consequence of hyponatraemia.
Three deaths occurred in the peri-operative period, two of which involved patients with no history of hyponatraemia. The remaining death occurred in a patient who developed severe congestive cardiac failure post-operatively with a plasma sodium concentration of 127 mmol/l. The hyponatraemia was secondary to the cardiac failure and not the cause of death.
Risk factors for the development of post-operative hyponatraemia
Pre-operative hyponatraemia
One hundred percent (20/20) of patients who had hyponatraemia at the time of surgery continued to have a low plasma sodium concentration at 72 h post-operatively compared to 23 % (29/124) of those who had a normal plasma sodium concentration. Although mild hyponatraemia was relatively common, only 2 % (2/124) of patients who underwent surgery with a normal plasma sodium concentration developed moderate hyponatraemia post-operatively (no severe cases recorded) compared to 35 % (7/20) in the cohort who were known to have hyponatraemia at the time of surgery. Univariate analysis identified a significant association between pre-operative hyponatraemia and the risk of post-operative hyponatraemia (chi2 = 18.0, odds ratio = 5.72 (95 % CI 2.40–14.0, p < 0.001)).
Dextrose administration
The mean total volume of all intravenous fluids administered to each patient in the peri-operative period (from admission to 72 h post-op) was 6436 mls (range 2000–14500 mls, SD ± 2390 mls). The mean volume of 5 % dextrose administered was 1150 mls (range 0–4000 mls, SD ± 930 mls). Five percent dextrose accounted for a total of 18 % of pre-operative, 0 % of intra-operative and 23 % of post-operative fluids administered.
We subsequently analysed the effects of volume of dextrose administration and risk of post-operative hyponatraemia. An initial analysis using volume four categories (with the first being 0 mls and the second being 1–999 mls) showed no difference in risk of developing post-operative hyponatraemia between the first two categories. As such, three categories were subsequently used to improve the power of the analysis: 0–999 mls, 1000–1999 mls and 2000 mls or greater (as a total administered over the peri-operative period). Univariate analysis identified a significant association (chi2 = 9.94, p < 0.001) between volume of dextrose administered and the proportion of patients who developed post-operative hyponatraemia.
Age
The incidence of hyponatraemia in patients over 80 years of age was 58 % (29/50) versus 24 % (4/17) for those under 70 years of age. In order to more accurately assess the association between age and the risk of post-operative hyponatraemia we divided age into 10-year cohorts. Univariate analysis did not identify any significant relationship between age and post-operative hyponatraemia (chi2 = 4.9, p = 0.45). A similar analysis had similar findings for the risk of pre-operative hyponatraemia.
Thiazide diuretic administration
At the time of admission, 15/144 (10 %) patients were prescribed thiazide diuretics. Of this cohort, 6/15 (40 %) were diagnosed with hyponatraemia on admission and 9/15 (60 %) post-operatively (all cases mild). Univariate analysis identified a significant relationship between thiazide diuretic use and the development of post-operative hyponatraemia (odds ratio = 3.3 (95 % CI 1.1–10.0, p = 0.03)).
Gender
The incidence of post-operative hyponatraemia was significantly different between genders. Seventy-one percent (44/62) of females developed post-operative hyponatraemia versus 15 % (5/3) of males (chi2 = 10.0, odds ratio = 3.2 (95 % CI 1.4–7.4, p = 0.001)).
Such univariate analyses are complicated by the fact that each of the variables analysed may confound the effects of the others. To control for such effects a hierarchical logistic regression model was constructed (with post-operative hyponatraemia as the dependent variable). In the first block, four potential confounding variables were entered, such as gender, age, thiazide diuretic use and pre-operative hyponatraemia. The −2Log Likelihood value fell by 29.72 showing that these four variables were significantly related to post-operative hyponatraemia (chi2 = 29.7, p < 0.001). In the second block, volume of dextrose was added as a three level categorical variable with those with under 1 L of dextrose as the baseline. This significantly reduced the −2Log Likelihood value by a further 9.44 (chi2 (2) = 9.44, significant at p < 0.05). Hence, percentage of dextrose was a significant predictor of post-operative hyponatraemia even after allowing the effects of the other confounding variables.
Table 1 lists the regression coefficients for the final model. Of the five predictor variables, three of them are significant, such as gender, pre-operative hyponatraemia and volume of dextrose administered. Increasing age and prescription of thiazide diuretics had coefficients that were in line with existing literature and increased the odds of post-operative hyponatraemia, however, were not statistically significant predictors.
Discussion
Despite the fact that each year, in the United Kingdom, approximately 80,000 patients undergo surgery after sustaining a hip fracture; very few studies directly address the important issue of hyponatraemia in this patient group [11, 12]. Hyponatraemia warrants investigation and assessment as mortality risk increases with even mild derangement and is associated with increased length of hospital stay [13]. Admission hyponatraemia has been associated with increased in hospital death in hip fracture patients with an odds ratio of 3.64 [14].
Post-operative hyponatraemia has also been reviewed. Tambe et al. [12] found a hyponatraemia incidence of 2.8 %; however, this study included all trauma patients admitted. However, 78 % of the patients that developed hyponatraemia had sustained hip fractures, reiterating that hyponatraemia is a particular concern in this patient group. Hyponatraemia is not a problem specific to trauma admissions. Rates of up to 40 % have been identified in patients undergoing elective arthroplasty surgery [15].
However, this is the first study of elderly orthopaedic patients to use logistic regression models to investigate the independent effects of several risk factors for the development of hyponatraemia in the peri-operative period. In an earlier study of the prevalence hyponatraemia in hip fracture patients, McPherson et al. [11] reported an incidence of pre-operative and post-operative hyponatraemia of 2.8 % (defined as a plasma sodium concentration less than 130 mmol/l). This compares to 1 % and 6 %, respectively, in this series.
The clinical presentation of hyponatraemia depends upon both the severity and the rate of fall in the plasma sodium concentration. Reduced plasma sodium concentration creates an osmotic gradient between the extracellular and intracellular fluid in brain. This leads to movement of water molecules into cells causing cerebral oedema and raised intracranial pressure. Patients with mild hyponatraemia (plasma sodium 130–135 mmol/l) are often asymptomatic. When the plasma sodium concentration falls to 125–130 mmol/l, the patient may report nausea and malaise [16]. Headache, lethargy and disorientation may follow as the sodium concentration falls further. With severe and rapidly evolving hyponatraemia the patient may develop acute cerebral oedema resulting in seizures, coma, respiratory arrest, permanent brain damage and ultimately death [16]. In patients who develop chronic hyponatraemia over a long period of time, the brain is able to adapt and the patient may be entirely asymptomatic even in the presence of quite severe hyponatraemia [16].
Although there are many causes of hyponatraemia, the aetiology in the post-operative hip fracture patient is generally consistent, although it is usually multi-factorial (Table 2). Females account for up to 80 % of patients admitted to hospital with a hip fracture and are more likely to develop post-operative hyponatraemia as a consequence of their relatively smaller total body fluid volume [5]. Many patients who sustain a hip fracture (in this series 10 %) have long term prescriptions for thiazide diuretics to control hypertension, which may cause chronic mild/moderate hyponatraemia [16]. The use of spinal anaesthesia may result in excessive intravenous fluid administration to counteract the effects of sympathetic block [10].
When a patient sustains a significant trauma and/or undergoes surgery, electrolyte homeostasis is often altered. Mediated by vasopressin (anti-diuretic hormone), the rennin angiotensin system and cathecholamines, water is retained, thus effectively diluting serum sodium concentrations. One should however remember that although commonly cited as a cause of hyponatraemia in post-surgical patients, the syndrome of inappropriate diuretic hormone (SIADH) release is a diagnosis of exclusion in the euvolaemic patient, and other causes of hyponatraemia must always be considered and excluded.
Five percent dextrose solution is termed a hypotonic fluid as the dextrose is the solution that is quickly metabolised in the blood leaving free water which is hypotonic [16, 17]. Although 5 % dextrose is an important source of free water for maintenance, it should be used with great caution as excessive amounts may cause dangerous hyponatraemia, especially in the elderly. Similarly, 5 % dextrose is not appropriate for resuscitation or replacement therapy except in conditions of significant free water deficit such as diabetes insipidus [16, 17]. Several studies have linked hypotonic infusions with increased morbidity and mortality in post-operative patients [1–4]. This is a particular problem in elderly trauma patients who are already at significant risk of developing hyponatraemia for the reasons listed previously. The principle patient group who require administration of 5 % dextrose is the diabetic patient who is prescribed an insulin/dextrose/potassium infusion whilst fasting for surgery. However, only 3/144 (3 %) patients required glycaemic control to be managed in our study cohort. However, in our study, there were relatively large amounts of dextrose given to patients, and further education of junior staff on the appropriate use of 5 % dextrose is clearly imperative.
The British Consensus Guidelines on Intravenous Fluid Therapy for Adult Surgical Patients (GIFTASUP 2008) makes the following evidence based recommendations on fluid replacement therapy [17]. No intravenous infusion should be continued routinely and intravenous infusions discontinued as soon as possible. Prescribers of intravenous fluids must take care to assess the patient’s sodium, chloride, potassium and water requirements, and prescription should not be made without such knowledge. No intravenous fluid should be regarded as “intrinsically safe” [16, 17].
Whilst detailed descriptions of the causes and treatment of hyponatraemia are beyond the scope of this article, the authors would refer the reader to a comprehensive article by Reynolds et al. which covers this topic in great detail [16]. All hip fracture patients should have their serum electrolytes checked on admission, on the first post-operative day and regularly thereafter. If a patient is found to be hyponatraemic, an assessment should be made of their fluid status to guide further investigation and management (Fig. 1). If a patient is unable to tolerate oral fluids, then the electrolytes should be monitored daily whilst receiving intravenous fluids. A patient who displays any of the non-specific symptoms or signs associated with hyponatraemia should have their serum electrolytes checked as a matter of urgency. If hyponatraemia develops then management should be discussed at an early stage with a clinical biochemist or endocrinologist. It is important that patients who are acutely confused are investigated thoroughly, so that potentially reversible causes such as hyponatraemia can be identified and treated. Although the confusional state associated with more severe hyponatraemia may be reversible, it can result in permanent brain damage or even death. Table 2 outlines the common causes of hyponatraemia.
Conclusion
Mild hyponatraemia is a common diagnosis in hip fracture patients both pre and post-operatively. Whilst the majority of cases in this series were mild, only 6 % of patients developed moderate/severe hyponatraemia post-operatively. Female gender, pre-operative hyponatraemia and hypotonic fluid administration are all important and independent risk factors for the development of hyponatraemia. Hypotonic intravenous fluids should be avoided unless specifically clinically indicated in hip fracture patients. The orthopaedic surgeon must be aware that hyponatraemia is a common and potentially life threatening condition which must be considered in any confused or unwell patient.
References
Bagshaw SM, Townsend DR, McDermid RC (2009) Disorders of sodium and water balance in hospitalized patients. Can J Anaesth 56(2):151–167
Anderson RJ (1986) Hospital acquired hyponatraemia. Kidney Int 29:1237–1247
Arieff AI (1986) Hyponatraemia, convulsions, respiratory arrest, and permanent brain damage after elective surgery in healthy women. N Engl J Med 314:1529–1535
Klein L, O’Connor CM, Leimberger JD, Gattis-Stough W, Pina IL, Felker GM et al (2005) Lower serum sodium is associated with increased short-term mortality in hospitalized patients with worsening heart failure: results from the outcomes of a prospective trial of intravenous milrinone for exacerbations of chronic heart failure (OPTIME-CHF) study. Circulation 111:2454–2460
Lane N, Allen K (1999) Hyponatraemia after orthopaedic surgery. BMJ 318(7195):1363–1364
Harrington P (1999) Hyponatraemia after orthopaedic surgery. General journals must not alienate particular specialties. BMJ 319(7208):514
Hoffbrand B (1999) Hyponatraemia after orthopaedic surgery. Hypotonic solutions should be used infrequently. BMJ 319(7208):515–516
Marino A, Krikler S, Blakemore M (1999) Hyponatraemia after orthopaedic surgery. Rigorous audit and introduction of guidelines decreased hospital's figures. BMJ 319(7208):515
Mojiminiyi OA (1999) Hyponatraemia after orthopaedic surgery. Laboratory must play a part in patients' management. BMJ 319(7208):515
Severn AM, Dodds C (1999) Hyponatraemia after orthopaedic surgery. Failsafe system is needed. BMJ 319(7208):514
McPherson E, Dunsmuir RA (2002) Hyponatraemia in hip fracture patients. Scott Med J 47(5):115–116
Tambe AA, Hill R, Livesley PJ (2003) Post-operative hyponatraemia in orthopaedic injury. Injury 34(4):253–255
Wald R, Jaber BL, Price LL, Upahyay A, Madias NE (2010) Impact of hospital associated hyponatraemia on selected outcomes. Arch Intern Med 170(3):294–302
Hagino T, Ochiai S, Watanabe Y, Senga S, Saito M, Takayama Y, Wako M, Ando T, Sata E, Haro H (2013) Hyponatraemia at admission is associated with in hospital death in patients with hip fracture. Arch Orthop Trauma Surg 133:507–511
Sah A (2014) Hyponatraemia after primary hip and knee arthroplasty: incidence and associated risk factors. Am J Orthop 43(4):E69–E73
Reynolds RM, Padfield PL, Seckl JR (2006) Disorders of sodium balance. BMJ 332(7543):702–705
Powell-Tuck J, Gosling P, Lobo DN, Allison SP, Carlson GL, Gore M, et al (2008) British Consensus Guidelines on Intravenous Fluid Therapy for Adult Surgical Patients—GIFTASUP. Available from: http://www.ics.ac.uk/downloads/2008112340_GIFTASUP%20FINAL_31-10-08.pdf (Accessed 04th Oct 20011)
Wakil A, Ng JM, Atkin SL (2011) Investigating hyponatraemia. BMJ 342:d1118
Ethical statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grant, S.J., Winter, A., McGlynn, J. et al. Aetiology of hyponatraemia after hip fracture. Eur Orthop Traumatol 6, 163–168 (2015). https://doi.org/10.1007/s12570-015-0303-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12570-015-0303-5