Abstract
Circumscapular pain is a frequent complaint in clinical practice. The dorsal scapular and long thoracic nerves course through the neck, where they may become entrapped between or within adjacent scalene muscles. Additionally, a high frequency of brachial plexus “piercing” variants have recently been documented, and it is unclear how they influence branching patterns distally along the brachial plexus. In the project reported here we strived to identify and quantify variations in dorsal scapular nerve and long thoracic nerve secondary to brachial plexus piercing variation. Ninety brachial plexuses from human cadavers (45 female/45 male) were evaluated to identify nerve branching patterns, specifically piercing versus non-piercing variants in the brachial plexus roots and nerves. Anatomical entrapment of the dorsal scapular nerve and long thoracic nerve was found in high frequencies (60.8% and 44.6%, respectively). Anomalous brachial plexus piercing variants were associated with higher frequencies of distal nerve branches also coursing through the scalene musculature, and there was a statistically significant correlation between brachial plexus and long thoracic nerve piercings (p = 0.027). Anatomical entrapment of nerves within scalene musculature is common and may be causative factors for idiopathic circumscapular pain, dorsalgia, and dysfunction of scapulohumeral rhythm. This study revealed a link between anatomical arrangement of the brachial plexus and occurrence of long thoracic nerve entrapment, which may lead to a series of cascading neurologic effects in which affected individuals may suffer from increased incidence of thoracic outlet syndrome and long thoracic nerve entrapment resulting in additional symptoms of interscapular pain and compromised shoulder mobility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The brachial plexus (BP) is a major nerve network that innervates the upper extremity and which in humans is formed by the merging of ventral rami from the C5–T1 nerves (Moore et al. 2014). These ventral rami are referred to as the roots of the BP, and they typically pass through the space between the anterior and middle scalene muscles, and then join to form the superior, middle, and inferior trunks. Each of these trunks diverges into an anterior and posterior division, and then reconverges to form three cords, namely, the lateral, posterior, and medial cords. As terminal branches split off from the plexus, they are named as individual peripheral nerves, most of which course distally to innervate the upper extremity.
Recent studies have identified anatomical variants in the BP, in which portions of the plexus pierce the scalene musculature (Harry et al. 1997; Sakamoto 2012; Leonhard et al. 2016, 2017). Impingement of the plexus at this location, especially in cases of hypertonicity of the scalene muscles, may result in neural symptomology. Specifically, these variants may predispose individuals to symptoms of neurogenic thoracic outlet syndrome (nTOS), including pain, paresthesia, and paresis of the upper extremity (Leonhard et al. 2017). Several peripheral nerves of the scapular, pectoral, and circumhumeral region branch off the BP proximally, suggesting that they too may be impinged by the scalene muscles in individuals in which the BP trunks pierce the scalenes.
Dorsal scapular nerve entrapment
Circumscapular and interscapular pain are common clinical complaints. While numerous etiologies exist for this pain, dorsal scapular nerve (DSN) entrapment is a recently recognized, common underlying cause (Sultan et al. 2013). After leaving the scalene region, the DSN typically travels inferiorly, deep to the BP and levator scapulae (e.g., Moore et al. 2014), coursing along the deep surfaces of the rhomboid major and minor where it pierces both of these muscles. Impingement of the DSN anywhere along its route could result in compromised shoulder function and/or radiating neuralgia through the back, upper extremity, and/or cervical region (Nguyen et al. 2016).
DSN entrapment results in impingement of the DSN nerve fibers, typically causing acute pain to originate along the vertebral border of the scapula, which may radiate distally to the lateral arm and forearm (Chen et al. 1995; Nakano 1978). Entrapment of the DSN is often accompanied by dysfunction of the shoulder girdle (Chen et al. 1995). If the condition persists, it may result in wasting of the shoulder musculature, and even winging of the scapula (Ravindran 2003). DSN entrapment is easily overlooked in differential diagnoses due to the relative vagueness of symptoms, myriad of common conditions with similar symptomology, and absence of sensory branches of the underlying nerve (Sultan et al. 2013).
Despite the recent recognition of the role of DSN entrapment in shoulder pain and dysfunction, the frequency of anatomical DSN impingement in the general population is unknown. In addition, it is unclear whether recently discovered anomalies in the BP may affect the course of the DSN and its predisposition to impingement. Individuals with piercing variant BP patterns report higher incidences of thoracic outlet syndrome symptoms (Leonhard et al. 2017), and it has been suggested that impingement of the DSN may also contribute to symptoms of nTOS (Chen et al. 1995; Boezaart et al. 2010; Saporito 2013). Additionally, it is important for clinicians to have an accurate understanding of the important neurological relationships in the scalene region for any surgical procedures or nerve blocks, which are common treatment modalities for both nTOS and DSN entrapment (e.g., Roos 1982; Povlsen et al. 2014).
Long thoracic nerve compression
The long thoracic nerve (LTN) shares a classic anatomical relationship with the BP, in which the former courses through the cervicoaxillary canal posterior to the trunks of the BP. In individuals with BP piercing variants discussed above, it is unclear how the LTN would be impacted. Injury to or compromise of the LTN has been documented to cause debilitating paralysis or paresthesia of the serratus anterior muscle (Wiater and Flatow 1999). As a primary stabilizer of the scapula, serratus anterior is crucial in maintaining the normal position of the scapula against the thoracic wall and in sustaining proper scapulohumeral function (Inman et al. 1944). Patients with lesions or other impingements of the LTN often present with pain, paresthesia, and weakness of the serratus anterior. In severe cases, scapular winging, medial translation or rotation of the scapula, or projection of the medial scapular border may co-occur (Connor et al. 1997; Wiater and Flatow 1999). Chronic weakness of the serratus anterior may result in substantial shoulder pain, particularly in individuals who experience heavy demands on their shoulders (Kaupilla and Vastamaki 1996).
It has been documented that LTN dysfunction may occur in the absence of reported trauma or other apparent pathology (Foo and Swann 1983; Vukov et al. 1996). In particular, Vukov et al. (1996) describe a case of idiopathic dysfunction of the LTN resulting in paralysis of the serratus anterior. The appearance of LTN dysfunction in the absence of any reported trauma suggests that anatomical variation may predispose certain individuals to LTN palsy, including entrapment of the LTN within the scalene musculature. As with the DSN, there is limited published information on the variability of the course of the LTN through the neck and upper thorax and its relationships to other neurovascular structures in the area. Information on the various courses and frequencies is crucial for comprehensive patient care and safe and proper surgical procedures in this region.
Neuropathies of the DSN and LTN
The lack of knowledge on the variation and frequency of DSN and LTN pathways could complicate medical treatments, differential diagnoses, and surgical interventions in the scapular and scalene regions. It has been noted that nerve injuries occur in 1–8% of shoulder surgeries and that three-dimensional (3D) information on nerve anatomy is crucial for the safe placement of portals and trocar direction (Boardman and Cofield 1999). However, while numerous studies have assessed the 3D relationships of several nerves relevant to shoulder surgery, these studies have focused primarily on the suprascapular nerve (Bigliani et al. 1990; Warner et al. 1992), musculocutaneous nerve (Flatow et al. 1989), and axillary nerve (Bryan et al. 1986; Duval et al. 1993; Duparc et al. 1997; Eakin et al. 1998). Little research has focused on the role of anatomical variation in the DSN and LTN in undiagnosed shoulder pain, and the potential of variation for iatrogenic causes of nerve damage when they deviate from the expected course through the neck.
In the study reported here, we evaluated the course of the DSN and LTN with relation to the scalene musculature in order to assess the frequency of anatomical entrapment of these nerves and to assess whether BP branching variants are correlated with DSN and/or LTN piercing patterns. In particular, we quantified how DSN and LTN anatomical entrapment co-varied with BP anatomy, with the goal of determining whether individuals with BP piercing variants were more likely to also possess DSN and/or LTN entrapment.
Materials and methods
Ninety BPs from 47 adult cadavers (24 male/23 female) from Anatomy teaching laboratories were examined to determine the rates of variation in the course, branching patterns, and anatomical relationships of the BP, DSN, and LTN. The body donors ranged in age from 51 to 97 years old, with a mean age of 74.7 years. Following medical student dissections of the neck and shoulder, a bilateral evaluation was completed for each cadaveric specimen. Each evaluation started with reflection of the sternocleidomastoid muscle and the identification of the phrenic nerve along the superficial surface of the anterior scalene muscle. Once the inferior and lateral borders of the anterior scalene were defined, the positions of the trunks and cords of the BP in relation to the scalene muscles were determined and recorded. Any BP variants were recorded, especially piercing variations, in which the superior trunk courses through the anterior scalene.
Next, the DSN was identified and traced both proximally and distally to reveal the entirety of its course. In particular, the relationship of the DSN to the scalene muscles was noted, including whether the nerve pierced the anterior, middle, or posterior scalene. Alternatively, if the DSN was found to course between two adjacent muscle bellies, it was noted which muscles surrounded it. This information is relevant because the space between the middle and posterior scalenes is quite tight in comparison to the relatively spacious gap between the anterior and middle scalenes, commonly referred to as the “interscalene gap.” Finally, the latissimus dorsi muscle was reflected to reveal the serratus anterior muscle and identify the LTN, which was traced proximally to determine the entirety of its course. As with the DSN, the relationship of the LTN to each of the scalene muscles was noted. If the ventral rami pierced the muscle in any way, it was recorded as a piercing variant. Any unique circumstances were noted and described. Each specimen was evaluated by both authors to confirm the assessment and then photodocumented for future confirmation. If the BP, DSN, or LTN were damaged on either side from the student dissections, those data were excluded from consideration.
Chi-squared analyses were conducted using the SPSS version 25 software package (IBM Corp., Armonk, NY, USA) to assess whether DSN or LTN piercing patterns were more common in individuals with BP piercing variations than in individuals with “classic” BP anatomy. Chi-squared analyses were also utilized to compare patterns between the sexes and sides of the body (left vs. right).
Results
Since the cadavers in this study were first dissected by medical students, some of the nerves investigated were inadvertently severed and therefore were not able to be included in the sample. Of the 47 cadavers examined in this study, on a few sides, the majority of relevant muscles and nerves had been severed or removed completely or partially and, therefore, data from those sides were not included in the sample. Consequently, the total number of usable cadaveric BP sides was 90. All results reported in the following sections are reported as numbers of sides.
Brachial plexus
In the evaluation of BP branching and relationships, 35 sides were found to exhibit classic BP anatomy (38.9%), while 54 sides (60.0%) possessed a piercing variant in which portions of the plexus pierced a scalene muscle, and one side (1.1%) displayed a C5 root coursing superficial to the anterior scalene muscle (Table 1). Chi-squared analyses revealed no significant differences in BP branching patterns between males and females or sides of the body.
In many of the piercing variant sides (N = 54), there were additional variations, such as C5 and/or C6 roots located superficial to the anterior scalene or trunks located between the middle and posterior scalenes instead of between the anterior and middle scalenes. The majority of the piercing variants (N = 41) were caused by the C5 and C6 roots or the entire superior trunk piercing the anterior scalene, including one male side where both the superior and middle trunks pierced the anterior scalene muscle. Among the sides piercing the anterior scalene (N = 53), in eight sides (4 male/4 female) only the C5 root pierced the anterior scalene muscle, while in three sides (2 male/1 female) only C6 pierced the anterior scalene muscle while the C5 ramus emerged anterior to the muscle.
In one side (male), the inferior trunk pierced the anterior scalene, while the superior and middle trunks classically emerged between the anterior and middle scalenes. In one side (male), the C5 and C6 roots pierced the middle scalene muscle, and the middle trunk emerged between the middle and posterior scalene muscles, while the inferior trunk maintained the classic pattern between the anterior and middle scalenes. These highly atypical conformations are unique, and to our knowledge, have not previously been documented.
Dorsal scapular nerve
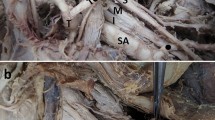
For the DSN, data were available on 74 sides (36 male/38 female), summarized in Table 2. In 16 sides (21.6%), the DSN emerged between the middle and posterior scalene muscles, while 60.8% demonstrated one of the piercing patterns (Fig. 1). In the majority of the piercing DSN sides, the nerve pierced the middle scalene muscle (68.9%), while 11.1% pierced the anterior scalene muscle and 20.0% pierced the posterior scalene muscle. The chi-squared tests revealed no statistical differences in the frequencies of pathways between sexes or sides of the body.
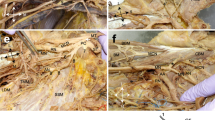
Variations in the pathway of the dorsal scapular nerve (DSN). a DSN branches directly off superior trunk or C5, avoiding the scalene musculature, b DSN pierces scalene musculature, c DSN courses between middle and posterior scalenes. AS Anterior scalene, IT inferior trunk, MS middle scalene, MT middle trunk, PS posterior scalene, ST superior trunk
Extensive variation was observed in the course and branching positions of the DSN. In addition to the aforementioned piercing pathways, the DSN also varied in the position from which it diverged from the rest of the plexus. In many sides, it diverged posteriorly, as has been previously described. However, in two sides, the DSN branched off the superior trunk approximately 2 cm distal to the lateral border of anterior scalene. In three sides, it emerged off a shared trunk with the LTN. In one case, the DSN branched directly off the middle trunk, and in two cases, it was a direct branch from C4.
Long thoracic nerve
For the LTN, data were available from 83 cadaveric sides (38 male/45 female). Forty-six sides (55.4%) demonstrated a non-piercing LTN configuration, while 44.6% displayed a version of piercing morphology (Table 3; Fig. 2). Of the 37 LTNs that pierced a muscle, 32 (86.5%) pierced the middle scalene, while only one side (male) pierced the anterior scalene (2.7%), and four sides (male) pierced the posterior scalene (10.8%). Interestingly, the female sides had nearly equal distribution between the classic LTN anatomy and the LTN piercing the middle scalene muscle. Only two female sides possessed an LTN emerging between the middle and posterior scalene muscles, and when combined with the male sides, this pattern composes only 8.4% of the total sample. Of the LTN piercing sample, five LTNs were found to have a partial piercing, if one or more of their contributing spinal nerve rami traveled through the scalenes, but not the complete nerve trunk. Chi-squared tests found no statistical differences between sexes or sides of the body.
Variation was noted in the pathway of the LTN and its relationships with other neurovascular structures in the cervical region. In addition to the piercing variations described above, there were also a few anomalous pathways worth mentioning: two sides were observed in which the LTN and DSN shared a common trunk; on one side the LTN branched directly off the inferior trunk; on another the LTN branched directly off the superior trunk; and on one side, the LTN received contributions from the superior and middle trunks, rather than direct branches from C5–7. In another side, the LTN exhibited classic anatomy proximally, but was found to give off a branch to pectoralis minor as it traveled distally. In this side, the medial pectoral nerve branched off the middle trunk and coursed to pectoralis minor, as usual. However, the LTN also branched posteriorly off the middle trunk and coursed inferolaterally over the superficial surface of pectoralis minor. As it passed over the lateral border of pectoralis minor, it gave off a small branch to the muscle.
Covariations
While variation is not an unexpected phenomenon in many instances of human anatomy, we wanted to further investigate the relationship of the BP, DSN, and LTN. We hypothesized that proximal BP variants may lead to cascading distal effects in the courses of the peripheral nerves downstream.
Covariation of BP and DSN
To evaluate the covariation among courses of the BP and DSN, we combined the variables explored using the singular nerve approach above. We found that only 12.3% of sides had the entirely non-piercing anatomical pattern in which the BP trunks emerged between the anterior and middle scalene muscles and DSN was non-piercing (Table 4; Fig. 3). In a plurality of sides (38.4%), both the BP and DSNwere classified as piercing variants (Table 4).
In assessing the association between DSN and BP patterns, no clear pattern emerged (Table 4). The largest number of sides displayed both BP and DSN piercing patterns (38.4%). However, the number of non-piercing DSNs associated with a piercing BP was also substantial (27.4%). In fact, there were more sides with piercing DSNs in association with a classic BP (21.9%) than there were non-piercing DSNs with a classic BP (12.3%). The chi-squared test revealed no significant differences in the occurrence of piercing DSNs between sides with piercing versus non-piercing BPs (χ2 = 0.2204, p = 0.639).
Covariation of BP and LTN
A plurality of the sides (32.9%) presented with instances in which both the BP and LTN displayed a piercing pattern (Table 5, Fig. 4). In only 10 sides (12.2%) did the LTN pierce the scalenes in the absence of a piercing BP. The Chi-squared test revealed statistically significant higher occurrence of piercing LTNs in sides with piercing compared to non-piercing BPs (χ2 = 4.8975, p = 0.027).
Discussion
Patient complaints of circumscapular pain and/or shoulder dysfunction may be indicative of DSN or LTN entrapment and compression. Given the high frequency of anatomical entrapment of both the DSN and LTN and the importance of both nerves in maintaining normal scapular position and scapulohumeral function, we suggest that these anatomical relationships may be an underlying cause of some cases of idiopathic shoulder pain. It is also crucial for clinicians to be aware of the range of variability in the positions of these nerves as it relates to surgical and other medical interventions. Procedures such as nerve blocks, scalenectomy, or removal of the first rib, all common treatments for thoracic outlet syndrome (e.g., Roos 1982; Povlsen et al. 2014), could place a variantly positioned DSN or LTN at greater risk of iatrogenic damage. As such, it is recommended that ultrasonography be employed to accurately assess the positions and pathways of the DSN and LTN for both patients with unidentified shoulder pain and those with planned surgical interventions in the scalene region.
The incidence of anatomical DSN and LTN entrapment revealed here is far higher than that based on clinical reports. The reasons for this may be two-fold. First, not all patients with anatomical entrapment will present with symptoms. Instead, this relationship may indicate only a predisposition to develop DSN or LTN entrapment symptoms. In particular, occupations requiring frequent long-term elevation of the upper extremity may predispose individuals to present symptoms (Akgun et al. 2008). Second, DSN and LTN entrapment may be clinically underdiagnosed. As a primarily motor nerve, the DSN does not have a sensory territory. Thus, interscapular pain will arise in association with DSN entrapment only in certain conditions: (1) If the syndrome has progressed to the point in which scapular winging occurs, the cutaneous branches of the thoracic posterior rami adjacent to the scapula may become stretched out, referring pain to the interscapular region; (2) If the nerve is impinged by taut bands within the scalene or rhomboid musculature, nerve trunk pain may be induced, referring pain into the neck (Sultan et al. 2013). Thus, as pain is not always concomitant with DSN entrapment, the somatic dysfunction may go largely unnoticed.
The LTN is composed of branches of multiple anterior rami (typically C5–C7) that converge to form a single nerve trunk (Moore et al. 2014). It has been noted that in some individuals, branches of anterior rami pierce the middle scalene muscle before joining together to form the trunk of the LTN (e.g., Yazar et al. 2009; Nasu et al. 2012). This anatomical arrangement leaves open the possibility of partial LTN compression, in which some of the contributing rami course through the scalene musculature even though the main trunk of the LTN does not. Individuals with this pattern were scored as LTN piercing variants, because at least part of the nerve was considered to be compressed in these cases. However, it is predicted that these individuals would likely experience reduced severity of symptoms compared to full LTN compression, with pain, paresthesia, and/or paresis along only the affected dermatomes and myotomes.
While the DSN consists primarily of motor fibers, entrapment of the nerve may still result in interscapular pain from several associated complications, as noted by Sultan et al. (2013). First, paresis (muscle weakness) of the rhomboids may place additional physical burden on other muscles of the pectoral girdle and back, causing muscle fatigue or strain. Second, myofascial pain of the rhomboids may co-occur with entrapment (Sultan et al. 2013). Third, stretch or compression of the nocioceptors within the DSN sheath induce increased sensitization of the nerve fibers, resulting in nerve trunk pain (Sultan et al. 2013). Finally, resulting muscular deformities, such as scapular winging, may result in additional stretching of the cutaneous nerve fibers, exacerbating the previously described condition.
While in this study we primarily defined anatomical entrapment as a nerve coursing through a muscle belly, as mentioned above, not all individuals with this condition will present with symptoms. In addition, the middle and posterior scalene muscles were noted to adhere very closely together in some individuals, to the point that the border between them was virtually imperceptible. In these individuals, any nerve passing between the middle and posterior scalenes could also become impinged between the two muscle bellies. We found that the DSN, in particular, travels this route approximately 20% of the time, which could result in additional opportunities for nerve compression.
The results of this study revealed for the first time a significant association between the pathway of the LTN through the neck and the branching pattern of the BP. We found that sides in which portions of the plexus traveled through the scalene musculature were significantly more likely to also possess a piercing variant of the LTN. These individuals are therefore at higher risk of cascading symptomology. In addition to the increased risk of thoracic outlet syndrome caused by BP compression (Leonhard et al. 2017), they may also exhibit a previously unrecognized predisposition for LTN neuralgia. These compounding symptoms may further obscure the underlying condition and render differential diagnosis especially challenging. A follow-up study using ultrasonography in living subjects will compare patients with interscapular pain to those without to assess whether those with complaints of scapular pain display a higher frequency of anatomical DSN and/or LTN entrapment than the general population.
In summary, anatomical entrapment of the DSN and LTN both within and between the scalene musculature is common, and may be causative factors for undiagnosed presentations of circumscapular pain, dorsalgia, and dysfunction of scapulohumeral rhythm. Additionally, this study revealed for the first time a link between the anatomical arrangement of the BP and the occurrence of LTN entrapment. The link between these conditions may lead to a series of cascading neurologic effects in which individuals with BPs that pierce the scalene muscles and consequently prone to thoracic outlet syndrome may also suffer from an increased incidence of LTN entrapment, resulting in additional symptoms of interscapular pain and compromised shoulder mobility. Providers evaluating patients with generalized shoulder pain or dysfunction are recommended to evaluate the pathways of the DSN and LTN ultrasonographically as part of their diagnostic modalities.
References
Akgun K, Aktas I, Terzi Y (2008) Winged scapula caused by a dorsal scapular nerve lesion. Arch Phys Med Rehabil 89:2017–2020
Bigliani LU, Daisey RM, McCann PD, April EW (1990) An anatomical study of the suprascapular nerve. Arthroscopy 6:301–305
Boardman ND, Cofield RH (1999) Neurologic complications of shoulder surgery. Clin Orthop Relat Res 368:44–53
Boezaart AP, Haller A, Laduzenski S, Koyyalamudi VB, Inhatsenka B, Wright T (2010) Neurogenic thoracic outlet syndrome: a case report and review of the literature. Int J Shoulder Surg 4:27–35
Bryan WJ, Schauder K, Tullos HS (1986) The axillary nerve and its relationship to common sports medicine shoulder procedures. Am J Sports Med 14:113–116
Chen D, Gu Y, Lao J, Chen L (1995) Dorsal scapular nerve compression: Atypical thoracic outlet syndrome. Chin Med J 108:582–585
Connor PM, Yamaguchi K, Manifold SG, Pollock RG, Flatow EL, Bigliani LU (1997) Split pectoralis major transfer for serratus anterior palsy. Clin Orthop Relat Res 341:134–142
Duparc F, Bocquet G, Simonet J, Freger P (1997) Anatomical basis of the variable aspects of injuries of the axillary nerve (excluding the terminal branches in the deltoid muscle). Surg Radiol Anat 19:127–132
Duval MJ, Parker AW, Drez D, Hinton MA (1993) The anterior humeral circumflex vessels and the axillary nerve: an anatomic study. Orthop Rev 22:1023–1026
Eakin CL, Dvimak P, Miller CM, Hawkins RJ (1998) The relationship of the axillary nerve to arthroscopically placed capsulolabral sutures: an anatomic study. Am J Sports Med 26:505–509
Flatow EL, Bigliani LU, April EW (1989) An anatomic study of the musculocutaneous nerve and its relationship to the coracoid process. Clin Orthop Relat Res 244:166–171
Foo CL, Swann M (1983) Isolated paralysis of the serratus anterior: a report of 20 cases. J Bone Joint Surg Br 65:552–556
Harry WG, Bennett JD, Guha SC (1997) Scalene muscles and the brachial plexus: anatomical variations and their clinical significance. Clin Anat 10:250–252
Inman VT, Saunders JB, Abbott LC (1944) Observations on the function of the shoulder joint. J Bone Joint Surg 26:1–30
Kaupilla LI, Vastamaki M (1996) Iatrogenic serratus anterior paralysis: long-term outcome in 26 patients. Chest 109:31–34
Leonhard V, Landreth R, Caldwell G, Smith HF (2016) Anatomical variations in the brachial plexus roots: implications for neurogenic thoracic outlet syndrome. Ann Anat 206:21–26
Leonhard V, Caldwell C, Goh M, Reeder S, Smith HF (2017) Ultrasonographic diagnosis of thoracic outlet syndrome secondary to brachial plexus piercing variation. Diagnostics 7:40
Moore KL, Dalley AF, Agur AMR (2014) Clinically oriented anatomy, 7th edn. Lippincott Williams & Wilkins, Baltimore
Nakano KK (1978) The entrapment neuropathies. Muscle Nerve 1:264–279
Nasu H, Yamaguchi K, Nimura A, Akita K (2012) An anatomic study of structure and innervation of the serratus anterior muscle. Surg Radiol Anat 34:921–928
Nguyen VH, Liu H, Rosales A, Reeves R (2016) A cadaveric investigation of the dorsal scapular nerve. Anat Res Int 2016:4106981. https://doi.org/10.1155/2016/4106981
Povlsen B, Hansson T, Povlsen SD (2014) Treatment for thoracic outlet syndrome. Cochrane Database Syst Rev 11:CD007218
Ravindran M (2003) Two cases of suprascapular neuropathy in a family. Br J Sports Med 37:539–541
Roos DB (1982) The place for scalenectomy and first-rib resection in thoracic outlet syndrome. Surgery 92:1077–1085
Sakamoto Y (2012) Spatial relationships between the morphologies and innervations of the scalene and anterior vertebral muscles. Ann Anat 194:381–388
Saporito A (2013) Dorsal scapular nerve injury: a complication of ultrasound-guided interscalene block. Br J Anaesth 3:883–885
Sultan HE, Gihan A, El-Tantawi Y (2013) Role of dorsal scapular nerve entrapment in unilateral interscapular pain. Arch Phys Med Rehabil 94:1118–1125
Vukov V, Ukropina D, Bumbasirevic M, Pecotic G, Zdravkovic M, Ille M (1996) Isolated serratus anterior paralysis: a simple surgical procedure to reestablish scapulo-humeral dynamics. J Orthop Trauma 10:341–347
Warner JJP, Krushell RJ, Masquelet A, Gerber C (1992) Anatomy and relationships of the suprascapular nerve: anatomical constraints to mobilization of the supraspinatus and infraspinatus muscles in the management of massive rotator-cuff tears. J Bone Joint Surg Am 74:36–45
Wiater JM, Flatow EL (1999) Long thoracic nerve injury. Clin Orthop Relat Res 368:17–27
Yazar F, Kilic C, Acar HI, Candir N, Comert A (2009) The long thoracic nerve: its origin, branches, and relationship to the middle scalene muscle. Clin Anat 22:476–480
Acknowledgements
Funding for this study was provided by faculty intramural research funds to HFS. The authors would like to thank student doctors Aleksandra Dunin-Borkowska, Kelsey Eaton, and Wade Wright for assistance with dissections. We thank Brent Adrian for consultation regarding figures, and Ashley Bergeron and the Anatomy laboratory staff for their accommodation in the anatomy laboratories. Finally, we are immensely grateful to the generous body donors whose cadavers formed the basis of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no potential conflicts of interest to disclose.
Ethical standard statement
Cadavers utilized in this study were obtained from Midwestern University Body Donation Program in Glendale, AZ, USA and the National Body Donor Program in St. Louis, MO, USA. The dissection of cadaveric specimens was performed according to The Common Rule regulations established in the Code of Federal Regulations (USA). The Institutional Review Board at Midwestern University indicated that IRB approval was not required for this project.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Williams, A.A., Smith, H.F. Anatomical entrapment of the dorsal scapular and long thoracic nerves, secondary to brachial plexus piercing variation. Anat Sci Int 95, 67–75 (2020). https://doi.org/10.1007/s12565-019-00495-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12565-019-00495-1