Abstract
Claes et al. recently documented and described the anterolateral ligament (ALL) of the knee, demonstrating its existence in 97% of their samples. Here, we further examined the anatomy of this ligament, documented its morphological variation, and assessed the feasibility of its dissection in preserved cadaveric specimens. To achieve this, we dissected 53 preserved cadaveric knees and documented their morphological variation in the anterolateral ligament. The originally described dissection technique for identifying and following the ALL requires flexion of the knee, a state which is often not possible in stiff, preserved cadavers. Here, we describe and confirm the feasibility of an alternate dissection technique in which the quadriceps femoris tendon is incised, for use on specimens in which flexion of the undissected knee is not possible. We also identify a novel technique for assessing whether the anterolateral ligament is absent from a specimen or has simply been obliterated or overlooked, using the lateral inferior genicular vasculature. These dissection techniques have great potential for the dissection of preserved cadavers used in gross anatomy laboratories, and we discuss the applications of such an approach in student-led dissections. Our dissections also uncovered noticeable variation in the anterolateral ligament course and position. Most notably, it often inserts significantly more laterally than the classical presentation (30.2%), or originates more proximally with superficial fibers extending superiorly and laterally over the distal femur (7.5%).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The discovery of the anterolateral ligament (ALL) can be traced back as far as 1879 by the French surgeon, Dr. Paul Segond, who first highlighted a “pearly, resistant, fibrous band” at the anterolateral aspect of the human knee (Segond 1879); however, this ligament has only recently been formally recognized and described in the anatomical literature (Claes et al. 2013). Since its formal description, a series of papers have attempted to document the presence and variation of the ALL in cadaveric specimens (e.g., Helito et al. 2013; Dodds et al. 2014; Caterine et al. 2015; Kittl et al. 2016; Kosy et al. 2016; Macchi et al. 2016). The ALL was initially described as originating from the lateral femoral epicondyle, typically sharing connecting fibers with the lateral collateral ligament, and coursing anterolaterally towards the proximal tibia to attach midway between Gerdy’s tubercle and the tip of the fibular head (Claes et al. 2013; Fig. 1). However, subsequent studies have identified a greater range of variation in the attachment points of this ligament (Caterine et al. 2015; Daggett et al. 2016; Kosy et al. 2016). Two recent studies described variation in the origin of the ALL, indicating that in as many as 44–77% of individuals it may originate proximally or posteroproximally to the lateral epicondyle (Caterine et al. 2015; Daggett et al. 2016). Additionally, several studies have now demonstrated that there is also greater variation in the insertion of the ALL than originally described (Vincent et al. 2012; Helito et al. 2014; Caterine et al. 2015), although the precise location of its insertion point has been quantified in various ways that are often not directly comparable. Along its path, there are firm attachments to the lateral meniscus (Claes et al. 2013; Caterine et al. 2015), which envelop the inferior lateral genicular artery and vein. Macchi et al. recently showed that the ALL inserts on the middle third of the lateral meniscus in 46% of cases, and on the inferior third in 54% (Macchi et al. 2016). This meniscal attachment often helps to isolate the clearly distinguishable ligament from the joint capsule. The insertion point of the ALL on the tibia is also clearly distinct from the iliotibial band (ITB). The knee joint consists of three primary layers of tissue (described in detail by Miller et al. 2012), numbered sequentially from superficial to deep. Layer I consists of the iliotibial (IT) band and the biceps femoris tendon and its connecting fascia. Layer II consists primarily of the patellar retinaculum. Layer III includes the joint capsule and the lateral collateral ligament, superficial to the joint capsule (Miller et al. 2012; Moore et al. 2013; Thompson, 2009), and the anterolateral ligament (Claes et al. 2013). In 2000, LaPrade et al. described the mid-third lateral capsular ligament as a thickening of the lateral capsule of the knee, attaching to the femur in the region of the lateral epicondyle, with capsular attachments to the lateral meniscus and insertion onto the tibia posterior to Gerdy’s tubercle and anterior to the popliteal hiatus (LaPrade et al. 2000). As the proximal origins of the LCL and ALL are so intimately associated, we support the designation of both structures under the term “lateral collateral ligament complex” (LCLC).
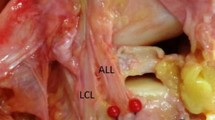
Classical anatomy of the anterolateral ligament and its relationships to surrounding anatomical structures. ALL anterolateral ligament, FH fibular head, GT Gerdy’s tubercle, ITB iliotibial band, LCL lateral collateral ligament, LFE lateral femoral epicondyle, LM lateral meniscus, MM medial meniscus, PT popliteus tendon
Claes et al. documented the presence of the ALL in 97% of the cadavers they surveyed, suggesting that this ligament, despite its history of vague descriptions and lack of official recognition, is present in the vast majority of individuals (Claes et al. 2013). Subsequent studies have also verified the presence of the ALL in the majority of nonpathological adult knees (Helito et al. 2014; Dodds et al. 2014; Caterine et al. 2015; Kosy et al. 2016). Interestingly, the ALL is not present in many young children (Shea et al. 2016), suggesting that the development of this ligament may be initiated by the biomechanical pressures experienced by the knee joint later in life.
Despite the fact that the anterolateral ligament has a documented frequency of greater than 95% in healthy adult knees (e.g., Vincent et al. 2012; Claes et al. 2013; Helito et al. 2014; Van der Watt et al. 2015; Kosy et al. 2016), it is lacking from standard dissection manuals used in gross anatomy classes, and is generally not part of the knee joint dissections in these settings. Additionally, there is currently no standard protocol for isolating the ALL in embalmed specimens, or definitively assessing its presence. The goal of the current research was to refine dissection techniques appropriate for embalmed cadavers, which may be stiff and relatively unpliable, such as those typically found in gross anatomy laboratory courses, as well as to contribute to the developing understanding of morphological variation in size and attachment points of the ALL.
Materials and methods
This investigation assessed 53 embalmed human cadaveric knees, which included 22 paired and 9 unpaired samples, from the gross anatomy teaching laboratories at Midwestern University. Cadavers were obtained for teaching purposes from the National Body Donation Program (St. Louis, MO, USA). Specimens were preserved with 22 l of an embalming fluid composed of 3% formaldehyde, 4% phenol, 31% glycerin, and 62% water. They were embalmed through the internal jugular vein, and stored at room temperature for a minimum of one month prior to dissection. Twenty-six of the samples were male, while 27 were female knees. The average age of death was 67 years (range 54–88 years). The presence, course, and morphological characteristics of the anterolateral ligament (ALL) were investigated. Cadavers with gross deformities (i.e., fracture) or a torn anterior cruciate ligament were excluded from the study.
In the initial dissection protocol, we attempted to follow the procedure described by Claes et al. (2013). However, this original dissection procedure was modified early on in our study, because the lack of mobility in the extremities of our preserved cadavers made it quite difficult to successfully identify the ALL.
In addition to an assessment of qualitative and quantitative variation in this newly described ligament, we also sought to assess the feasibility of its dissection in cadaveric anatomy courses using preserved specimens. The majority of dissection-based cadaveric studies of the ALL have employed fresh (Vincent et al. 2012) or fresh-frozen (e.g., Dodds et al. 2014; Caterine et al. 2015; Kennedy et al. 2015; Rahnemai-Azar et al. 2016; Imbert et al. 2016; Kosy et al. 2016; Roessler et al. 2016) cadavers. Even in the few studies using embalmed cadavers, the dissection steps were not described in great detail (e.g., Daggett et al. 2016; Runer et al. 2016), presumably simply because that was not the focus of the study. Thus, the feasibility and most effective method for dissecting this structure in embalmed cadavers has not been assessed. We determined that in the preserved cadaveric specimens used in gross anatomy laboratories at Midwestern University, the impliability of the preserved tissue rendered flexion of an undissected knee extremely difficult. Thus, we utilized an alternate dissection approach in which the quadriceps femoris tendon was incised transversely. This additional cut permitted full mobility at the knee joint, even in our stiff preserved cadavers, and allowed the knee to be flexed and rotated, making the LCL easily identifiable. The LCL could then be cleaned and separated from the clearly distinguishable fibers of the ALL coursing medially. Placing the knee in a flexed position also helped to identify the ALL as a thickened, fibrous band, distinctly separate from the joint capsule, since the capsule did not become taut in this internally rotated position. The comprehensive set of steps for this alternate dissection approach, which we suggest should be employed in gross anatomy laboratory classes, are described and illustrated in detail below.
A step-by-step protocol for dissecting the anterolateral ligament (ALL) in cadaveric specimens
-
1.
If removal of the skin and subcutaneous fat over the knee joint has not been completed during a previous lower limb lab, then now is the time to complete that task. If the skin of the lower limb is still intact, and you wish to isolate only the anterolateral ligament, then start by creating a large rectangular flap on the anterolateral aspect of the flexed knee, extending approximately from 10 cm proximal to 5 cm distal to the patella (Fig. 2a). Remove skin and any subcutaneous fat.
Fig. 2a–b Initial steps for the alternate dissection approach for the anterolateral ligament (ALL) employed here. This technique is appropriate for preserved cadavers in which the knee joint is initially stiff and relatively immobile, because it opens up the knee to permit greater rotation. Please refer to text for details. a Skin cuts to reveal region to be dissected; b muscular incisions through the quadriceps femoris and iliotibial band. ITB iliotibial tibial band, QuadFem quadriceps femoris tendon
-
2.
Make an incision with a scalpel that transects the quadriceps tendon approximately 5 cm proximal to the patella. Continue this cut medially and laterally to include any quadriceps femoris muscle fibers that are limiting flexion of the knee joint (Fig. 2b).
-
3.
Hang the knee off the side of the table (place a towel on the floor underneath to collect any fluids that may drip), and use force to flex the knee to a full 90° of flexion. If there are any remaining quadriceps muscle fibers limiting this full range of motion, incise them with the scalpel.
-
4.
Cut the iliotibial band (ITB) using a scalpel approximately 6 cm proximal to the lateral epicondyle of the femur (Fig. 2b).
-
5.
Begin to reflect the ITB distally, CAREFULLY using scissors to free any deep fibers that are limiting the reflection. There should be deep fibers connecting to the lateral intermuscular septum in the anterior thigh, as well as distal fibers connecting to the lateral patellar retinaculum.
-
6.
Once the ITB has been reflected all the way to its distal attachment (Gerdy’s tubercle on the tibia), use a scalpel to separate and completely remove it.
-
7.
Next, isolate the lateral collateral ligament (LCL) of the knee, which runs from the lateral epicondyle to the fibular head. To achieve this, apply a varus force on the knee joint. If the limb is hanging off the table, this can be accomplished by pushing the ankle in towards the table (Fig. 3). Using the fingers of your other hand, you should be able to feel the LCL become taut. Repeat this motion with the ankle to demonstrate how the ligament is relaxed in a neutral position and becomes taut in a varus position.
-
8.
With the LCL in a taut position, use a reverse-scissoring technique to open up the lamina of the joint capsule to reveal the ligament at its midpoint (Fig. 4). Continue the cut distally/inferiorly to fully expose the ligament. Next, extend the cut proximally/superiorly to expose the origin of the LCL.
Fig. 4 Procedure for identifying and cleaning the lateral collateral ligament (LCL) and anterolateral ligament (ALL) in the alternate dissection approach. In this step, the LCL is exposed along its entire course, the lamina of the joint capsule is opened, and the ALL is identified and cleaned. Please see text for further details. ALL anterolateral ligament, FH fibular head, LCL lateral collateral ligament, JC joint capsule
-
9.
Lastly, find the anterolateral ligament (ALL). It should still be enveloped by the lamina of the joint capsule (Fig. 4).
-
10.
Internally rotate the foot, which will also internally rotate the tibia (Fig. 5). This causes the ALL to become taut. Use the fingers of your other hand to palpate the taut ALL, just as you did with the LCL. Again, repeat the motion to appreciate that the ALL is relaxed in a neutral position and becomes taut when the tibia is internally rotated.
-
11.
With the ALL taut, use the reverse scissoring technique to separate the joint capsule from the ligament at its borders (Fig. 4). Continue with the scissors proximally and distally to completely free the ligament from the joint capsule.
-
12.
You should notice that the ALL will have connecting fibers to the LCL at its origin, and may even have superficial fibers extending more proximally. You can confirm your finding of the ALL by examining its deep fibers connecting to the lateral meniscus, and also finding the lateral inferior genicular blood vessels enveloped by this connection (Fig. 7).
-
13.
The ALL originates at the lateral femoral epicondyle, and runs in an oblique course, attaching to the anterolateral tibia approximately midway between Gerdy’s tubercle and the fibular head, typically slightly proximal to these two points.
Quantitative analyses
Each cadaver was photographed to allow qualitative analysis of the ALL and its attachments to the femur and tibia, as well as its anatomical relationships with surrounding structures. Mitutoyo Hillson–Fitzgerald digital calipers (Hillson et al. 2005) with an accuracy of 0.01 mm were used to measure the length of the ALL in full extension and at 90° of flexion. The thickness of the ligament at the joint line between the femur and tibia was also recorded. During measurements, care was taken to ensure that the foot remained in a neutral position. Further image analysis was performed using the Image J software on photos of the specimens. This digital measurement confirmed the distance between the ALL insertion and both the fibular head and Gerdy’s tubercle.
A series of statistical analyses were conducted to assess the relationships among the various quantitative variables. Paired samples t tests were conducted to determine whether the ALL significantly increased in length during flexion as opposed to extension. A regression analysis was performed to determine whether a significant correlation existed between the width of the ALL at the joint line and its length at flexion and/or extension. All statistical analyses were conducted using SPSS v.19 (IBM Corp.).
Results
Dissection
We determined that this novel technique for deep knee joint dissection effectively revealed all relevant deep knee structures and facilitated identification of the ALL in preserved cadavers. In general, we found that the ALL was readily distinguishable from the joint capsule using this technique, particularly when tension was applied by internally rotating the tibia. This protocol also decreased the amount of time required for each knee dissection, from over an hour to less than 30 min. In two specimens that did not demonstrate a distinct ALL, the assessment that the ALL was absent was reinforced by the unique superficial presence of the lateral inferior genicular vessels upon dissection. In both cases, after identification of the LCL, teasing away the nearby tissue revealed those vessels in a much more superficial location than was typically expected. These vessels typically course at a deeper plane, protected between the deep ALL fibers and the lateral meniscus. However, in these two knees, since the ALL was not present, they were visible in a much more superficial position.
Qualitative ALL results
In 51 of the 53 dissected knees (96.2%), a distinct ligamentous structure was identified coursing anterolaterally from the femur to the tibia (Table 1; Fig. 6). Of the two knees that lacked a clear ALL, each belonged to an individual who possessed a definite ALL on the contralateral knee. One of the knees without an ALL belonged to a female, while the other was male. Both cadavers had a fully intact ACL. There were no other remarkable observations about these specimens.
Anterolateral ligament of the knee, demonstrated in a deep dissection of a cadaveric specimen. a Lateral view; b anterior view. ALL anterolateral ligament, FH fibular head, GT Gerdy’s tubercle, LCL lateral collateral ligament, LFC lateral femoral condyle, LFE lateral femoral epicondyle, LM lateral meniscus
In the majority of cases, the origin of the ALL fell on the prominence of the lateral femoral epicondyle, situated immediately anterior to the origin of the lateral collateral ligament (LCL). In every sample, there was some degree of connecting fibers between the ALL and LCL at this origination point. The ligament ran obliquely across the joint line to insert on the anterolateral side of the proximal tibia. In all cases, there was a clear deep attachment between the ALL and lateral meniscus. In fact, this was usually the primary determining factor for proper identification of the ligament. In samples that permitted the cleanest dissection along with the most pliable tissue, fine dissection of this attachment highlighted the lateral inferior genicular artery and vein running in a small furrow between the deep fibers of the ALL and the lateral meniscus (Fig. 7). Distally, the ALL inserted on the proximal tibia, with some of its deep fibers forming a thick capsular insertional fold on the joint capsule (Claes et al. 2013). In a small majority of the specimens, the location of the insertion was precisely as Claes et al. initially described it: almost exactly halfway between Gerdy’s tubercle and the tip of the fibular head, situated just proximal to an imaginary line connecting these two bony features.
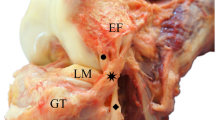
Image showing the relationship between the inferior lateral genicular blood vessels, the anterolateral ligament (ALL), and lateral meniscus of the knee. a Lateral view; b anterolateral view. The inferior lateral genicular artery and vein run in a furrow between the lateral meniscus and the deep fibers of the ALL. Due to this predictable relationship, the presence of these vessels can be used to confirm the absence of the ALL in specimens that appear to be lacking it. ALL anterolateral ligament, ILGA inferolateral genicular artery, ITB iliotibial band, LCL lateral collateral ligament, LFC lateral femoral condyle, LFE lateral femoral epicondyle, LTC lateral tibial condyle, LM lateral meniscus
However, several examples of variation were observed in the path of the ALL (Table 2) compared to the original description by Claes et al. (2013). In a large number of samples (n = 16; 30.2%), the ALL did not insert at the midpoint between Gerdy’s tubercle and the fibular head, but instead inserted substantially more laterally, closer to the fibular head (Fig. 8). In four cases (7.5%), there were clear superficial fibers of the ALL that continued proximally and laterally over the distal femur (Fig. 9), which elongated the ALL in the proximal direction. In one specimen (1.9%), the ALL divided off near the midpoint of the LCL, coursing in a much more oblique orientation towards its insertion on the tibia.
The more lateral insertion of the anterolateral ligament (ALL) in some specimens (n = 16; 30.2%). In these variants, the ALL inserted closer to the fibular head than to Gerdy’s tubercle, rather than midway between these features as in most specimens. ALL anterolateral ligament, BiFem biceps femoris, FH fibular head, GT Gerdy’s tubercle, ITB iliotibial band, LCL lateral collateral ligament, LFE lateral femoral epicondyle
The more proximal origin of the anterolateral ligament (ALL) in some specimens (n = 4; 7.5%). In these variants, ALL fibers originated proximally and laterally, superior to the normal origin on the lateral femoral epicondyle, resulting in an elongated ALL. ALL anterolateral ligament, BiFem biceps femoris, FH fibular head, GT Gerdy’s tubercle, LCL lateral collateral ligament, LFE lateral femoral epicondyle
Quantitative ALL results
Values for length of the ALL during flexion and extension and ALL width at the joint line for all individual specimens are presented in Table 1. The mean length of the ALL was 38.39 ± 4.30 mm (range 28.97–48.41 mm) at extension and 42.95 ± 4.35 mm (range 32.97–50.88 mm) at flexion (90°) (Table 3). The mean width of ALL at the joint line was 4.26 ± 0.84 mm (range 2.35–6.52 mm).
Quantitative analyses supported our interpretation that the ALL inserted significantly more laterally in a subset of the specimens. The ratio of the insertion point relative to the fibular head versus Gerdy’s tubercle was found to be an average of 0.488 in the normally inserting specimens, and 0.428 in the laterally inserting specimens (Table 4). An analysis of variance (ANOVA) revealed highly significant differences in the position of the insertion relative to the fibular head and Gerdy’s tubercle (F = 26.95, p < 0.001). In addition, a regression analysis indicated different spatial relationships between the ALL insertion relative to these bony landmarks between the normal and laterally inserting specimens (Fig. 10). In the normally inserting specimens, the correlation coefficient was R 2 = 0.557, while in the laterally inserting specimens, R 2 = 0.390.
A paired samples t test revealed highly significant differences between the length of the ALL at extension compared to its length at 90° flexion (t = −11.93, p < 0.001), indicating that the increase in length of the ALL during flexion of the knee is significant. Interestingly, a regression analysis revealed no significant correlations between the width of the ALL and either its length at flexion (r = 0.055, p = 0.703) or length at extension (r = 0.018, p = 0.899), suggesting that the width and length of the ALL do not covary in predictable ways.
Discussion
Our new dissection technique was found to effectively facilitate the identification of the ALL and other relevant knee structures in embalmed cadavers. In addition, it greatly decreased the amount of time required for each knee dissection, reducing our dissection time from over an hour to less than 30 min. In addition, this technique provides a definitive method of determining whether an apparently absent ALL is the result of a dissection error or misidentification or is truly absent in a specimen—by observing the position of the lateral inferior genicular blood vessels. The only limitation to this approach is that it requires the incision of the quadriceps femoris tendon. Thus, we suggest that student-led dissections, such as those in gross anatomy courses, attempt this technique, but instructors could consider performing this procedure unilaterally, so as to maintain the quadriceps femoris tendon on one side of the body. Alternatively, this procedure could also be performed during a final “joints of the lower extremity” laboratory session, after all of the musculature has been thoroughly studied. Given the potential clinical significance of this ligament and its role in knee stability, it is important for gross anatomy students, especially those entering medical fields, to be aware of its presence and have the opportunity to explore it during their cadaveric dissection experiences.
The main finding of the morphological portion of this study reinforces the conclusions, initially of Claes et al. (2013), but more recently of many other researchers (e.g., Helito et al. 2013; Dodds et al. 2014; Caterine et al. 2015; Kittl et al. 2016; Kosy et al. 2016; Macchi et al. 2016), that the anterolateral ligament (ALL) can be identified as a distinct ligamentous structure at the anterolateral aspect of the human knee in almost all adult specimens. Mobilization of the knee joint during dissection helped further support the hypothesis that the ALL functions to help limit internal rotation of the tibia, as the ligament becoming taut in this position was one of the key mechanisms that allowed for its accurate identification. Our observed values for the dimensions of the ALL are similar to those recorded in previous studies (e.g., Claes et al. 2013; Helito et al. 2013; Stijak et al. 2016; Caterine et al. 2015; Kosy et al. 2016). The mean ALL length in our sample, 38.4 mm in extension and 43.0 mm in flexion, is similar to that initially described by Claes et al. (2013) of 38.5 mm in extension and 41.5 mm in flexion. Subsequent studies have typically reported a single length, rather than separate values at flexion and extension, but our mean value generally coincides with theirs quite well: 37.3 mm (Helito et al. 2013), 40.1 mm (Kosy et al. 2016), 40.3 mm (Caterine et al. 2015), and 41 mm (Stijak et al. 2016). Only Dodds et al. (2014) reported a substantially longer ligament of 59 mm, although this disparity may result from a difference in definition of the ALL, as they define the insertion as a blending with the joint capsule rather than a distinct osseous attachment. We recorded a mean width of 4.3 mm, which is comparable to the 4 mm mean width obtained by Stijak et al. (2016), but narrower than several other previous studies: 8.2 mm (Vincent et al. 2012 ), 8.3 mm (Claes et al. 2013), 7.4 mm (Helito et al. 2014), and 8.9 mm (Caterine et al. 2015).
The origin of this ligament, as described by Claes et al. (2013), occurs on the lateral femoral epicondyle. The ligament then runs obliquely across the joint line and attaches on the proximal tibia, often at the midpoint between Gerdy’s tubercle and the fibular head, situated proximal to an imaginary line connecting these two structures. Despite the distinctness of the ALL, it demonstrates an intimate relationship with the LCL and lateral meniscus, sending connecting fibers to both structures. Claes et al. went so far as to suggest the term “lateral collateral ligament complex” to refer to the LCL+ALL, a term that is supported by our findings here (Claes et al. 2013). While we confirmed the presence of the ALL in 96% of our cadavers, we also found variation in the course and attachment pattern compared to that originally described by Claes et al. (2013). Most notably, in our samples there was more variation in the insertion point than originally described (Claes et al. 2013). In several individuals, distinct fibers of the ALL continued proximolaterally onto the distal femur (Fig. 9). This finding is similar to recent studies that described the ALL as originating proximally or posteroproximally to the lateral epicondyle in the majority of specimens (Helito et al. 2013; Caterine et al. 2015; Kosy et al. 2016). In 30% of our specimens, the insertion point was also situated more laterally than initially described by Claes et al. (2013), closer to the fibular head (Table 4; Fig. 8). We can hypothesize that mechanically, this variation would not provide as much rotational stability for the tibia as the classical presentation in which the insertion falls more medially. While they did not subdivide their specimens into two insertion groups as we have here (i.e., normal and lateral insertions), Helito et al. (2013) described the ALL as inserting 38 ± 11% of the way from the fibular head to Gerdy’s tubercle, suggesting that the insertion point in their sample ranged by greater than 20% of the distance between these two landmarks. Two additional studies also included several specimens with more lateral ALL insertions (Claes et al. 2013; Kosy et al. 2016); however, both studies reported and focused on average values, so neither discussed these laterally inserting variants in detail.
Clinical implications
In 1879, the surgeon Paul Segond described the existence of an anterolateral capsular thickening in the human knee that can cause a small avulsion fracture at the level of the anterolateral tibia when exposed to excessive internal (medial) rotation (Segond 1879). More specifically, the mechanism of injury is believed to be varus and internal rotation causing traction on the fragment by the ITB and the ALL (Hess et al. 1994; Davis and Post 1997), with a flexion component also possibly playing a role (Hess et al. 1994), which may result in anterolateral rotatory instability (De Maeseneer et al. 2015). This fracture has become known as Segond fracture, and is considered pathognomonic of ACL rupture (Segond 1879), with Segond fractures co-occuring in 9–12% of ACL injuries (Stallenberg et al. 1993; Hess et al. 1994; De Maeseneer et al. 2015).
In patients with a rupture of the ACL and/or LCL, a more laterally inserting ALL could increase its vulnerability to rupture or avulsion at the insertion, which could put these individuals at a greater risk for a concomitant Segond fracture. Individuals with a more lateral insertion could be predisposed to rupturing the ALL. If such a patient incurred a dual ACL-ALL rupture, then he or she would be a candidate for dual reconstruction. In a case like this, the reconstructed ALL could be inserted onto the tibia in a classically oriented position, at the midway point between Gerdy’s tubercle and the fibular head. This should provide even greater rotational stability post-surgery than prior to injury.
Conclusions
Here, we describe a new dissection technique for effectively isolating the ALL in embalmed cadavers. We also describe a definitive technique for determining whether an apparently absent ALL was actually absent in life, or was simply misidentified or destroyed in dissection, using the inferior lateral genicular vessels. It is our hope that this technique will be widely applicable in gross anatomy teaching laboratories and cadaveric research studies. Additionally, we assessed the morphological variation in the anterolateral ligament in a large sample of cadaveric knees, and observed variation in its morphology. Similar to previous studies, we confirmed the presence of an ALL in the vast majority of our sample (96%), and we confirmed previously described anatomical variations in the origin and insertion of this ligament compared to its originally described morphology (Claes et al. 2013). The significantly more lateral insertion position observed in 30% of our sample has potential clinical implications, including decreased rotational stability of the knee and predisposition to Segond fracture. We hope this study further elaborates on the anatomy of the anterolateral ligament (ALL) and its potential variations in appearance and morphology.
References
Caterine S, Litchfield R, Johnson M, Chronik B, Getgood A (2015) A cadaveric study of the anterolateral ligament: re-introducing the lateral capsular ligament. Knee Surg Sports Traumatol Arthrosc 23:3186–3195
Claes S, Vereecke E, Maes M, Victor J, Verdonk P, Bellemans J (2013) Anatomy of the anterolateral ligament of the knee. J Anat 223:321–328
Daggett M, Ockuly AC, Cullen M, Busch K, Lutz C, Imbert P, Sonnery-Cottet B (2016) Femoral origin of the anterolateral ligament: an anatomic analysis. Arthroscopy 32(5):835–841
Davis DS, Post WR (1997) Segond fracture: lateral capsular ligament avulsion. J Orthop Sports Phys Ther 25(2):103–106
De Maeseneer M, Boulet C, Willekens I et al (2015) Segond fracture: involvement of the iliotibial band, anterolateral ligament, and anterior arm of the biceps femoris in knee trauma. Skeletal Radiol 44(3):413–421
Dodds AL, Halewood C, Gupte CM, Williams A, Amis AA (2014) The anterolateral ligament: anatomy, length changes and association with the Segond fracture. Bone Jt J 96:325–331
Helito CP, Demange MK, Bonadio MB, Tirico LE, Gobbi RG, Pecora JR, Camanho GL (2013) Anatomy and histology of the knee anterolateral ligament. Orthop J Sports Med 1(7):2325967113513546
Helito CP, Demange MK, Bonadio MB, Tirico LEP, Gobbi RG, Pecora JR, Camanho GL (2014) Radiographic landmarks for locating the femoral origin and tibial insertion of the knee anterolateral ligament. Am J Sports Med 42:2356
Hess T, Rupp S, Hopf T, Gleitz M, Liebler J (1994) Lateral tibial avulsion fractures and disruptions to the anterior cruciate ligament: a clinical study of their incidence and correlation. Clin Orthop 303:193–197
Hillson S, Fitzgerald C, Flinn H (2005) Alternative dental measurements: proposals and relationships with other measurements. Am J Phys Anthropol 126:413–426
Imbert P, Lutz C, Daggett M, Niglis L, Freychet B, Dalmay F, Sonnery-Cottet B (2016) Isometric characteristics of the anterolateral ligament of the knee: a cadaveric navigation study. Arthroscopy 32(10):2017–2024
Kennedy MI, Claes S, Fuso FA, Williams BT, Goldsmith MT, Turnbull TL, Wijdicks CA, LaPrade RF (2015) The anterolateral ligament: an anatomic, radiographic, and biomechanical analysis. Am J Sports Med 43(7):1606–1615
Kittl C, El-Daou H, Athwal KK, Gupte CM, Weiler A, Williams A, Amis AA (2016) The role of the anterolateral structures and the ACL in controlling laxity of the intact and ACL-deficient knee. Am J Sports Med 44(2):345–354
Kosy JD, Sonui A, Venkatesh R, Mandalia VI (2016) The anterolateral ligament of the knee: unwrapping the enigma. Anatomical study and comparison to previous reports. J Orthopaed Traumatol 17(4):303–308
LaPrade RF, Gilbert TJ, Bollom TS, Wentorf F, Chaljub G (2000) The magnetic resonance imaging appearance of individual structures of the posterolateral knee: a prospective study of normal knees and knees with surgically verified grade III injuries. Am J Sports Med 28(2):191–199
Macchi V, Porzionato A, Morra A et al (2016) The anterolateral ligament of the knee: a radiologic and histotopographic study. Surg Radiol Anat 38(3):341–348
Miller MD, Thompson SR, Hart J (2012) Review of orthopaedics, 6th edn. Elsevier Health Sciences, Philadelphia
Moore KL, Dalley AF, Agur AM (2013) Clinically oriented anatomy, 7th edn. Lippincott Williams and Wilkins, Philadelphia
Rahnemai-Azar AA, Miller RM, Guenther D, Fu FH, Lesniak BP, Musahl V, Debski RE (2016) Structural properties of the anterolateral capsule and iliotibial band of the knee. Am J Sports Med 44(4):892–897
Roessler PP, Schüttler KF, Heyse TJ, Wirtz DC, Efe T (2016) The anterolateral ligament (ALL) and its role in rotational extra-articular stability of the knee joint: a review of anatomy and surgical concepts. Arch Orthop Trauma Surg 136:305–313
Runer A, Birkmaier S, Pamminger M, Reider S, Herbst E, Kunzel KH, Brenner E, Fink C (2016) The anterolateral ligament of the knee: a dissection study. Knee 23(1):8–12
Segond P (1879) Recherches cliniques et experimentales sur lesepanchements sanguins du genou par entorse. Progrès Médical (Paris). http://www.patrimoine.edilivre.com/. Accessed 7 May 2015
Shea KG, Polousky JD, Jacobs JC Jr, Yen YM, Ganley TJ (2016) The anterolateral ligament of the knee: an inconsistent finding in pediatric cadaveric specimens. J Pediatr Orthop 36(5):e51–e54
Stallenberg B, Gevenois PA, Sintzoff SA Jr, Matos C, Andrianne Y, Struyven J (1993) Fracture of the posterior aspect of the lateral tibial plateau: radiographic sign of anterior cruciate ligament tear. Radiology 187(3):821–825
Stijak L, Bumbaširević M, Radonjić V, Kadija M, Puškaš L, Milovanović D, Filipović B (2016) Anatomic description of the anterolateral ligament of the knee. Knee Surg Sports Traumatol Arthrosc 24:2083–2088
Thompson JC (2009) Netter’s concise orthopaedic anatomy, 1st edn. Saunders Elsevier Health Sciences, Philadelphia
Van der Watt L, Khan M, Rothrauff BB, Ayeni OR, Musahl V, Getgood A, Peterson D (2015) The structure and function of the anterolateral ligament of the knee: a systematic review. Arthroscopy 31:569–582
Vincent JP, Magnussen RA, Gezmez F, Uguen A, Jacobi M, Weppe F, Al-Saati MF, Lustig S, Demey G, Servien E, Neyret P (2012) The anterolateral ligament of the human knee: an anatomic and histologic study. Knee Surg Sports Traumatol Arthrosc 20:147–152
Acknowledgements
Funding for this study came from Midwestern University faculty start-up funds (HFS). The authors wish to thank the generous individuals who donated their bodies to medical education. Figures were created by Brent Adrian.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no potential conflicts of interest to disclose.
Ethical standard statement
Cadavers utilized in this study were obtained from the National Body Donor Program in St. Louis, MO, USA. The dissection of cadaveric specimens was performed according to The Common Rule regulations established in the Code of Federal Regulations (USA). The Institutional Review Board at Midwestern University indicated that IRB approval was not required for this project.
Rights and permissions
About this article
Cite this article
Parker, M., Smith, H.F. Anatomical variation in the anterolateral ligament of the knee and a new dissection technique for embalmed cadaveric specimens. Anat Sci Int 93, 177–187 (2018). https://doi.org/10.1007/s12565-016-0386-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12565-016-0386-2