Abstract
The effects of fish size and nitrite level on metabolic rate and growth were investigated in the obligate air-breathing snakehead Channa striata, which is an important aquaculture species in Vietnam. Channa striata displayed respiratory size dependence, whereby the standard metabolic rate (SMR) and routine metabolic rate (RMR) decreased progressively in an exponential manner as fish size increased from 50 to 200 g. A mildly elevated nitrite level of 5% of the LC50 96 h (12 mg NO2−/L or safe concentration) induced significant increases in Channa striata SMR and RMR, which were almost double that of the control at the same size. At mild elevation, nitrite caused no significant effect on fish growth and survival during 3 months of rearing. However, both growth and survival rates of fish reared at severely elevated nitrite levels were significantly lower than those of the control; in particular, survival rates were under 50%. While changes in size reduced SMR and RMR, the percentage of air oxygen partitioning remained unchanged. Channa striata upregulation of SMR and RMR and air-breathing regulation were not significantly proven in this study. In summary, maintaining water environments at levels lower than 12 mg NO2−/L with ample oxygenation will not affect the growth and survival rate of snakeheads.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrite is produced from ammonia excreted by fishes via an anaerobic bacterial nitrification process and is then converted to nitrate (Lewis and Morris 1986; Jensen 2003). As a toxicant component, nitrite disrupts many physiological functions, including those of a respiratory or cardiovascular nature, as well as ion regulatory and endocrine excretory processes. Fish, as well as other aquatic animals, are considered to be at higher risk from their exposure to nitrite than terrestrial animals because nitrite can be actively passed through fish gills and accumulated in the fish body. Once nitrite is present in fish body fluid, nitrite combines with haemoglobin (oxidation of Fe2+ to Fe3+) to produce methaemoglobin (metHb), nitrate, and nitrosyl haemoglobin (nitrosylHb), thus reducing oxygen transport (Kosaka and Tyuma 1987; Jensen 2009; Jensen and Rohde 2010). The effects of nitrite on the respiratory process have been shown to be worse in species with a high metabolic rate (Perrone and Meade 1977), for instance, eliciting hyperventilation in carp and rainbow trout (Jensen et al. 1987; Aggergaard and Jensen 2001). Increased cardiac pumping is an appropriate response when blood oxygen-carrying capacity is reduced during nitrite exposure (Vleeming et al. 1997).
In water-breathing fish species, a large gill surface area supports respiratory gas exchange, ion, and pH regulation, and the excretion of nitrogenous waste (Evans et al. 2005). However, a large gill surface area also facilitates high nitrite absorption into the blood of the fish body. Most water-breathing fish have a low tolerance for nitrite, which consequently causes a reduction in hypoxic tolerance (Arillo et al. 1984) and an increase in plasma lactate concentrations (Jensen et al. 1987; Stormer et al. 1996). A lethal concentration (LC50 96 h) of below 0.5 mM has been found in sensitive water-breathing fish such as salmonids (Lewis and Morris 1986), and of several millimoles in tolerant water-breathing fish such as cyprinids. In air-breathing fish species, with the evolution of an air-breathing organ, a large gill surface area no longer seems necessary (Johansen 1968; Graham 1997), and therefore, the effects of nitrite seem to be diminished. However, studies are still limited as to these effects (Duncan et al. 1999; Boudreaux et al. 2007; Lefevre et al. 2011b, 2012a; Gam et al. 2017). Previous research has shown that air-breathing species can tolerate a relatively higher level of nitrite, with an LC50 96 h of 1.65 mM (~ 70 mg/L) in the facultative air-breather Pangasianodon hypophthalmus (Lefevre et al. 2011b), 4.9 mM (~ 230 mg/L) in the obligate air-breather Channa striata (Lefevre et al. 2012a), and 7.82 mM (~ 350 mg/L) in the facultative air-breather Chitala ornata (Gam et al. 2017). Lefevre et al. (2011b) showed that there is no evidence of air-breathing regulation in Pangasianodon hypophthalmus when measuring the metabolic rate in nitrite-exposed water. While it is possible that increased air breathing is the result of changes in arterial partial pressure of oxygen (PO2), it has also been argued that when oxygen need increases and mismatch with ventilation occurs, inducing increased air breathing, ion exchange, and toxicant uptake can be reduced. For instance, Hoplosternum littorale demonstrated increased hydrogen sulfide tolerance when exposed to both hydrogen sulfide and hypoxic conditions (Affonso and Rantin 2005), which matches that hypothesis.
Exposure to low levels of nitrite can reduce the growth rates of some water-breathing species—for example, in Ictalurus punctatus, at less than 10 mg NO2−/L (20% reduction) (Colt et al. 1981), and in silver perch Bidyanus bidyanus and Atlantic cod Gadus morhua, at levels lower than 5 and 3.3 mg NO2−/L, respectively (Frances et al. 1998; Siikavuopio and Sæather 2006). In general, levels of 3.3 to less than 10 mg NO2−/L are thought to limit the growth of water-breathing fish. On the other hand, a growth study of air-breathing fish species has yet to be conducted. To address this lack of information, our study focuses on the snakehead Channa striata, an obligate air-breathing fish species that has been growing intensively in the Mekong delta. In particular, we investigate the effects of size and nitrite level on snakehead metabolic and growth rates.
Materials and methods
Animals and experimental conditions
Snakehead Channa striata were obtained from a local farm in Hau Giang province, Vietnam. Fish 50 g (54.4 ± 3.4 and 51.6 ± 0.9 g), 100 g (101.0 ± 2.5 and 101.1 ± 4.6 g), 150 g (151.0 ± 1.1 and 150.0 ± 0.4 g), and 200 g (200.0 ± 4.0 and 202.0 ± 2.8 g) in size were held in separate 2 m2 tanks for use in measuring oxygen consumption of control and nitrite treatments using a respirometer, whereas fish sized 12.0 ± 0.4 g were selected to be raised in the growth experiment. The water quality in the respirometry experiment was regulated and maintained by a recirculation unit (water parameters: NO2− < 0.5 mg/L, NO3− < 90 mg/L, NH3 < 0.02 mg/L, dissolved oxygen > 95% saturation, CO2 < 0.5 mg/L, pH ~ 7.6 and temperature of 27 °C). Sodium (Na+) and chloride (Cl−) ion concentrations in water were low, varying from 0.26 to 0.30 mmol/L and 0.28 to 0.32 mmol/L, respectively. Fish were fed commercial floating pellets containing 43% protein (Stella S3, Nutreco Company, Ho Chi Minh City, Vietnam). Fish were randomly caught and moved to a holding tank in the respirometry laboratory, fasted for 48 h, and maintained at a temperature of 27 °C before being measured in the respirometer. Bimodal intermittent-flow respirometry (Lefevre et al. 2011a, 2014, 2016; Tuong et al. 2018) was used to measure snakehead metabolic rates.
All experiments were carried out in accordance with national guidelines on the protection and welfare of experimental animals in Vietnam (Law of Animal Health 2015).
Experimental protocols of the effect of fish size and nitrite levels on Channa striata metabolic rate
Experimental protocol
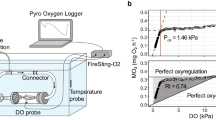
In this experiment, the aquatic consumption, standard metabolic rate (SMR), and pharyngeal critical pressure (Pcrit) were measured using intermittent-closed respirometry, and four sizes of obligate air-breathing fish were chosen, i.e. 50 g, 100 g, 150 g, and 200 g, in combination without (control) and with 5% LC50 96 h (12 mg NO2−/L) of snakehead. The respirometer, which was submerged in water in a large tank (~ 30 L), consisted of a chamber containing the fish and a closed loop where water passed a galvanic oxygen electrode that continuously measured the oxygen partial pressure of the water (PO2w) within the respirometer. In addition, an open loop could be activated to flush the respirometer with fresh water from the tank containing the system. The opening and closure of the flush pump and the logging of the PO2w measurements were automated by a software system (Respirometer 2.0) developed at the Zoophysiology section at Aarhus University, Denmark. Every fish was fasted for 48 h and placed in the respirometer chamber for measuring for 20 h; MO2 measurement lasted 15 min, where the respirometer was closed for 3 min, so the decline in PO2w could be used to calculate MO2, followed by 12 min of flush to renew oxygen levels. This setup provided independent measures of MO2 at 15-min intervals. MO2 for all points was calculated as MO2 = {[ΔPO2/Δt] ⋅βO2⋅Vsys}/Mb, where ΔPO2/Δt is the rate at which PO2w declined during the closed period, βO2 is the solubility of oxygen in water at 27 °C (1.617 μmol mmHg/L), Vsys is the volume of water in the system corrected for the volume of the fish (density assumed to be 1 kg/L), and Mb is body mass. The background bacterial oxygen uptake was also measured after 2 h. The system was then cleaned with chlorinated water and tap water, and other fish were continuously measured for 20 h. The nitrite was converted into NaNO2 and was added until reaching the desired treatment concentration, and water samples were collected to measure concentration levels once at the beginning until the end of the experiment, because the period of the experiment was only 20 h, and the nitrite concentration in the system was ~ 12 mg/L. The background oxygen consumption of bacteria was subtracted, and the metabolic rate was presented as routine metabolic rate (RMR), standard metabolic rate (SMR), and percentage of oxygen consumption in the air. The partitioning of oxygen consumption as the RMR and SMR of each fish was the combined water and air oxygen uptake and was calculated by using R script (Chabot et al. 2016; Lefevre et al. 2011a, 2012b). The air phase to measure aerial oxygen uptake (MO2a) was used by the same intermittent-closed respirometer. PO2 of the air phase was measured using a mini optic O2 sensor (Fibox 3, PreSens Precision Sensing GmbH, Regensburg, Germany) and data were collected by accompanying software (OxyView PST3, V6.02, PreSens Precision Sensing GmbH). The system consisted of a Plexiglass chamber submerged in water, with a small air-breathing chamber with plastic on top. The air inlet and air outlet were sealed with solenoid valves (Type 6013, Bürkert Fluid Control Systems, Ingelfingen, Germany) connected to the computer-controlled relays. An air pump, connected to the inlet, blew fresh air into the chamber when the valves were opened, and this flushing of the air phase was only performed during the intervals where water flushing was turned off (i.e., during measurement of MO2w). This prevented changes in the water level and thus changes in the air space volume. The air chamber was flushed for 1 min every hour, after which the outlet solenoid remained open for a further 2 min to prevent changes in air pressure during the closure of the inlet solenoid valve. During aquatic hypoxia, PO2w was lowered by bubbling the water phase with N2 gas. The necessary flow of N2 gas was adjusted manually until the required steady-state PO2w was reached (Lefevre et al. 2011a).
Experimental protocols of the effect of nitrite levels on growth performance of Channa striata
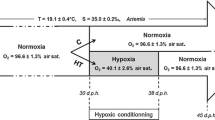
Forty fish were stocked in a 500 L composite tank for evaluating growth performance of the control and three levels of nitrite—12 mg/L (5% LC50 96 h), 185 mg/L (LC10 96 h), and 202 mg/L (LC20 96 h)—for 90 days; each treatment was replicated three times. Thirty percent of the tank water was changed every 3 days to maintain optimal environmental conditions (oxygen concentration was 5–5.6 mg/L, pH 7.4–7.6, temperature 27–28 °C, NO3− < 90 mg/L, NH3 < 0.02 mg/L and CO2 < 0.5 mg/L). Nitrite was added to the water until reaching the desired concentrations before stocking fish to the tanks. The nitrite concentrations were measured every 2 days and when necessary. Nitrite was added during the experiment to maintain the concentration as a desirable treatment. Forty fish were sampled from each tank for weighing body mass (day 0, 15, 30, 60, and 90) and calculating weight gain (WG), daily weight gain (DWG), specific growth rate (SGR). Fish were fed to saturation with commercial floating pellets containing 43% protein (Stella S3, Nutreco Company, Ho Chi Minh City, Vietnam). Feed conversion ratio (FCR) was calculated from food intake which was recorded every day until the end of the experiment. Survival rates (SR) were calculated from the final number of fish. The growth and SR data were determined as below:
Where W0: initial weight of fish; Wt: fish weight at time point t; t: culturing time.
Feed conversion ratio: FCR = feed intake/fish weight gained.
Note: Feed intake = feed given − uneaten feed.
Weight gain = (final weight of fish − initial weight of fish).
Statistical analysis
All figures were produced in SigmaPlot 12.5. Data were analysed using SPSS 12.0 software. Results were tested by two-way analysis of variance (ANOVA) to evaluate the effects of size and nitrite level on RMR, SMR, and percent air uptake, followed by least significant difference (LSD) post hoc comparison to test significant differences among treatments for each factor. One-way ANOVA, followed by the Duncan post hoc test, was used to compare growth parameters (weight gain, daily weight gain, specific growth rate), survival rate, and food conversion ratio between treatment levels of nitrite, with a p value of less than 5% (p < 0.05). All data were shown as the standard error of the mean (SEM).
Results
Effect of fish size and nitrite on metabolic rate of Channa striata
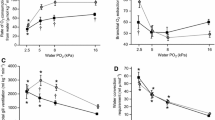
Snakeheads became calmer and less active as they became bigger (Fig. 1). The routine metabolic rate (RMR) in the treatment without nitrite decreased significantly with fish size, from 194 ± 27.1 mgO2 /kg/h for 50-g fish size to 56.5 ± 0.86 mgO2/kg/h for 200-g fish size (Table 1). These results show that the metabolic rate undergoes exponential regression within the body mass (regressive equation expressed in Fig. 2). Similarly, the standard metabolic rate (SMR) also decreased regressively and significantly with an increase in fish size, from 134 ± 23.2 mgO2/kg/h to 31.8 ± 5.9 mgO2 /kg/h for 50- to 200-g fish, respectively (Table 1). However, the increase in body mass did not cause significant changes in the percentage of air partitioning uptake, which varied from roughly 40.6% to 62.0% (p = 0.199) (Table 1). In the nitrite treatment, fish became more active than in the control (Fig. 3). Nitrite levels caused a significant increase in the RMR, from 1.3- to 2.1-fold, and in the SMR, from 1.5- to 3.0-fold, for fish weighing from 50 to 200 g, respectively (Table 1). Increases in RMR and SMR of nitrite compared to the control paralleled and regressively decreased with the increase in air partitioning uptake in the nitrite treatment, varying from 50.3% to 69.1%; however, it is not a significant difference compared to the control treatment (p = 0.083).
Effect of nitrite levels on growth performance of Channa striata
Significantly higher survival of Channa striata was observed in the control and 12 mg NO2−/L treatment than in 185 and 202 mg NO2−/L treatment (Fig. 4). The survival rate with the higher nitrite treatment decreased almost 50% compared to the control (Fig. 4). After 90 days of growth, fish weight gain was 3.3-fold compared to the initial weight at the control level and 1.8–2.0 at the 185 and 202 mg NO2−/L treatments (with the highest found in the control, 50.7 ± 9.8 g, and the lowest in 202 mg/L, 35.6 ± 8.48 g). There was significantly higher final weight, DWG, and SGR in the fish at the control and low nitrite levels compared to those in high nitrite concentrations (Figs. 4 and 5). The control and low nitrite concentrations had low FCR values (1.2 and 1.4, respectively), while the treatments of 185 and 202 mg/L showed the highest FCR values of 1.8 (Fig. 6). Generally, weight gain, DWG, and SGR at the control and low nitrite concentrations were similar, whereas the higher nitrite concentration led to a significantly higher level of food consumption.
Discussion
Channa striata is an active obligate air-breathing fish whose metabolic rate is quite high. The SMR of 50 g Channa striata in the present study was 134 ± 23.2 mgO2/kg/h, which compared well to 106 ± 3.20 mgO2/kg/h of 65.2-g Channa striata from an earlier study of Lefevre et al. (2012b), and 107 ± 6 mgO2/kg/h of 50 g Pangasianodon hypophthalmus (Lefevre et al. 2011a) and almost double that of Chitala ornata (Tuong et al. 2018). There was also a favourable comparison of the RMR of 194 ± 27.2 mgO2/kg/h and 169.6 ± 7.2 mgO2/kg/h of the present study and from Lefevre et al. (2012b), respectively. The percentage of air oxygen uptake was not significantly different among the four size groups; a similar result of 67% was reported for Channa striata by Lefevre et al. (2012b). Fish at bigger sizes became calmer and inactive compared to small sizes (Fig. 1). Channa striata showed a decrease in metabolic rate with an increase in body weight. Both SMR and RMR decreased significantly with exponential regression curves, corroborating an inverse relationship principal between body mass and metabolic rate (Schmidt-Nielsen 1997). Similar results of decreased metabolic rates have been found in walleye (Cai and Sumerfelt 1992; Yager and Summerfelt 1993), yellow perch Perca flavescens (Enders et al. 2006), sockeye salmon Oncorhynchus nerka (Brett 1965), black carp Mylopharyngodon piceus (Lv et al. 2018) and grass carp Ctenopharyngodon idellus (Zhang et al. 2014). Fish sizes from 50 to 100 g had the greatest decrease in RMR and SMR; however, fish from 100 to 200 g had a slight decrease (Fig. 1 and Table 1).
The low concentration of nitrite treatment (12 mg NO2−/L or safe concentration) in this metabolic rate study was chosen as 5% LC50 96 h of nitrite on snakehead, based on the LC50 96 h (4.9 mM, ~ 230 mg NO2−/L) of this species reported earlier (Lefevre et al. 2012a). The safe concentration of nitrite for this fish was calculated based on the application factor (AF), which has been calculated as 5% LC50 96 h (IJC 1977; CCREM 1991; Boyd 2014; Lingaraja and Venugopalan 1978). The safe concentration has been widely used in many studies to evaluate the effects of the pollutant in aquatic environments on aquatic animals (Asifa et al. 2016; Nwani et al. 2013a, 2013b, 2010; Magesh and Kumaraguru 2006; Somdare et al. 2015; Tiwari et al. 2011; Saoud et al. 2014). The effect of nitrite was significant on both SMR and RMR (Fig. 3, Table 1), which increased almost two-fold compared to all sizes of the control. Air oxygen uptake contributed higher percentages than in the control; however, as the level of nitrite concentration used in this study may be quite low, the increase in air oxygen partitioning was not significantly different. Snakehead exposure to nitrite led to the combination of nitrite with erythrocyte haemoglobin, producing metHb (Kosaka and Tyuma 1987; Jensen and Rohde 2010), which reduces blood oxygen transport or oxygen carrying capacity (Jensen 2007). There is, however, an inverse result found in snakeheads compared to the facultative air-breathing fish Pangasianodon hypophthalmus, which decreased their SMR and RMR and displayed unchanged air partitioning (Lefevre et al. 2011a). It is possible that striped catfish mainly use the ion regulation mechanism instead of the ventilation, metabolic, and air-breathing regulation mechanism. Snakeheads are able not only to tolerate higher concentrations of nitrite and maintain low internal nitrite levels due to their better detoxification ability than Pangasianodon hypophthalmus, but they also have the ability to increase their metabolic rate, and air breathing can reduce the level of nitrite entering their gills (Lefevre et al. 2011a,b; Lefevre et al. 2012a). It has also been reported that there is an increase in gasping behavior and hyperventilation when fish are short of oxygen due to nitrite exposure (Jensen et al. 1987; Aggergaard and Jensen 2001).
Respiration is an important phenomenon of fish life, and oxygen consumption is related to metabolism. Change in respiration has been used as an indicator of stress in pollutant-exposed aquatic animals (Subrahmanyam 2004). Studies on oxygen consumption are the main tool to give an index of energy expenditure mechanisms for environmental conditions (Franklin et al. 2010; Logaswamy and Remia 2009). The increase in oxygen consumption in fish affected by nitrite has rarely been documented. Lingaraja and Venugopalan (1978) found that the oxygen consumption of Therapon jarbua increased twofold when exposed to an environment containing LC25 96 h of DDT, dimethoate, and carbaryl. The oxygen consumption of Labeo rohita doubled after exposure to a sublethal concentration (0.57 μg/L) of cypermethrin at 10 and 15 days (Marigoudar et al. 2009). Similarly, increased oxygen consumption but decreased growth was reported in trout exposed to a sublethal concentration of permethrin (Haya 1989). Lokhande (2017) reported increased oxygen consumption in freshwater Rasbora daniconus from 24 to 48 h at sublethal and lethal concentrations of dimethoate. These results coincide with those of the present study, wherein the oxygen consumption of the snakehead exposed to 12 mg/L nitrite increased in fish of all sizes compared to that of the control; the RMR and SMR increased while the air uptake increased in the largest fish (Figs. 1, 2, and 3 and 2). In contrast to the present study, some studies have found a decrease in oxygen consumption in fish exposed to toxicants. A significant decrease in both aquatic and total oxygen consumption was found in Channa gachua exposed to pesticides (Rahman and Sadhu 2009). As reported by Patil and David (2008), Labeo rohita exposed to malathion at a lethal concentration demonstrated an increase in oxygen consumption, while it was decreased on day 15. Moreover, total oxygen consumption of air-breathing fish Heteropneustes fossilis exposed to bayrusil was found to decrease due to a decrease in aquatic oxygen consumption and an increase in air oxygen consumption (Lakshmi and Alam 2009). Catla catla showed a decrease in oxygen consumption in both sublethal concentrations (1/10th) and (1/20th) of LC50 96 of profenofos (Maharajan et al. 2013). In summary, the alternation of oxygen consumption of the fish exposed to the toxicant environment depends on the species, time of exposure, and toxicants.
Oxygen consumption is related to metabolism; however, the metabolism in fish is divided into different parts for ventilation, digestion, reproduction, and growth. Fish exposed to toxic environments display an elevated basal metabolic rate due to the increased energy requirements for physiological stress response, detoxification, and tissue recovery (Kumaraguru and Beamish 1983; Bradbury et al. 1987). As reported by Voslárová et al. (2008), the growth of Danio rerio was not affected in nitrite concentrations lower than 73 mg/L, while suppressed growth was observed in high concentrations of 130 mg/L, although the LC50 96 h of this species was 242 mg/L. Prolongation of sublethal nitrite exposure resulted mainly in decreased growth (Kroupová et al. 2018). Food intake of common carp exposed to quinalphos at sublethal and lethal concentrations decreased after 48 h while oxygen consumption increased because the fish used energy for tissue defense and toxicant excretion (Multappa et al. 2014). It appears that the effects on growth are dependent on species, concentrations, and toxicants. The growth of snakeheads in this study was unaffected in the safe concentration of 12 mg/L of nitrite exposure.
In this study, high nitrite concentrations (185 and 202 mg/L) led to a significant decrease in WG, DWG, and SGR compared to rates at the control and safe nitrite concentration (12 mg/L). The body mass of the snakehead increased from 12 g to almost 50 g at the control and low nitrite level (12 mg NO2−/L), and to only 35 g at high levels of nitrite (185 and 202 mg NO2−/L). While elevated nitrite environments have not been investigated with regard to many air-breathing fish, they have been shown to have little to no effect on the growth of many air-breathing fishes. High nitrite concentration (1 mM ~ 46 mg/L) in the water combined with high temperature did not affect the growth rate in terms of WG, DWG, and SGR of the air-breathing clown knife fish (Chitala ornata); the highest growth rate of DWG was found in high temperatures (30 and 33 ºC) combined with high nitrite concentrations (Huong et al. 2020). For instance, exposing Ictalurus punctatus to sublethal levels of nitrite (< 10 mg NO2−/L) led to a growth reduction of 20% (Colt et al. 1981). Similarly, nitrite exposure caused decreased growth in silver perch Bidyanus bidianus (Mitchell 1838) at levels exceeding 5 mg NO2−/L (Frances et al. 1998). There are other sensitive species whose growth has been shown to be limited at low levels of 3.3 mg NO2−/L, such as Gadus morhua and Oncorhynchus mykiss (Siikavuopio and Sæather 2006; Kroupova et al. 2008). There are differences in tolerance to nitrite in water-breathing compared to air-breathing fish. Air-breathing fish can avoid absorption of nitrite by using their air-breathing organ to obtain oxygen from the air. The oxygen consumption of the snakehead increased at a low nitrite concentration (12 mg/L) in this study, and decreased at 13.8 mg/L in striped catfish (Lefevre et al. 2011b). This reveals that the fish have to increase their metabolism for adaptation to the environment and consumption of more energy, so the growth rate of the fish decreases in high nitrite concentration. The FCR values in this study also explained the fish intake of much food, but the growth performance of snakehead fish was low in high nitrite concentration compared to control or low nitrite concentration treatment. The FCR increase in the high nitrite concentrations reveals an increase in energy demand to support physiological changes that accompany nitrite exposure, ion-regulatory, respiratory, and cardiovascular change (Grosell and Jensen, 2000; Jensen 2003). Although the information on the effects of nitrite on air breathing is limited, the nitrite concentration for inducing growth reduction is mostly around 3–10 mg/L (Tomasso 1994), and the level of 12 mg NO2−/L is still considered safe for Channa striata. The survival rates (42%) of snakehead in the two highest levels of nitrite were lower than that of the control (~ 80%). In this study, mortality occurred in the initial culture stage in high nitrite concentrations, subsequently decreasing over the experimental period of 90 days.
In summary, our results showed that a mildly elevated nitrite level of 5% of LC50 96 h (12 mg NO2−/L, safe concentration) induced significant increases in Channa striata SMR and RMR but had no significant effect on the growth or survival rate. At the higher nitrite levels, these parameters were significantly lower than those of the control, with a survival rate of less than 50%. An increase in size reduced SMR and RMR, while the percentage of air oxygen partitioning remained unchanged. Therefore, maintaining the water environment at levels lower than 12 mg NO2−/L, with ample oxygenation, will not affect the growth or survival rate of snakeheads.
References
Affonso EG, Rantin FT (2005) Respiratory responses of the air-breathing fish Hoplosternum littorale to hypoxia and hydrogen sulfide. Comp Biochem Phys C Toxicol Pharmacol 141:275–280. https://doi.org/10.1016/j.cca.2005.07.003
Aggergaard S, Jensen FB (2001) Cardiovascular changes and physiological response during nitrite exposure in rainbow trout. J Fish Biol 59:13–27. https://doi.org/10.1111/j.1095-8649.2001.tb02335.x
Arillo A, Gaino E, Margiocco C, Mensi P, Schenone G (1984) Biochemical and ultrastructural effects of nitrite in rainbow trout: liver hypoxia as the root of the acute toxicity mechanism. Environ Res 34:135–154. https://doi.org/10.1016/0013-9351(84)90083-5
Asifa KP, Viya PV, Chitra KC (2016) Assessment of median lethal concentration (LC50-96h) and behavioral modification of nonylphenol in the cichlid fish, Etroplus maculatus (Bloch, 1795). Int J Adv Lif Sci 9(2):190–195
Boudreaux PJ, Ferrara AM, Fontenot QC (2007) Chloride inhibition of nitrite uptake for non-teleost Actinopterygiian fishes. Comp Biochem Physiol Part A Mol Integr Physiol 147:420–423. https://doi.org/10.1016/j.cbpa.2007.01.016
Boyd CE (2014) Nitrite toxicity affected by species susceptibility, environmental conditions. Glob Aquac Advocate 17:34–36
Bradbury SP, McKim JM, Coats JR (1987) Physiological response of rainbow trout (Salmo gairdneri) to acute fenvalerate intoxication. Pestic Biochem Physiol 27:275–288
Brett J (1965) The relation of size to rate of oxygen consumption and sustained swimming speed of sockeye salmon Oncorhynchus nerka. J Fish Res Board Can 22:1491–1501. https://doi.org/10.1139/f65-128
Cai Y, Summerfelt RC (1992) Effects of temperature and size on oxygen consumption and ammonia excretion by walleye. Aquaculture 104:127–138. https://doi.org/10.1016/0044-8486(92)90143-9
CCREM (1991) Canadian Water Quality Guideline. Canadian Council of Resource and Environmental Ministry, Inland Water Directorate. Environment Canada, Ottawa
Chabot D, Steffensen JF, Farrell A (2016) The determination of standard metabolic rate in fishes. J Fish Biol 88:81–121. https://doi.org/10.1111/jfb.12845
Colt J, Ludwig R, Tchobanoglous G, Cech JJ Jr (1981) The effects of nitrite on the short-term growth and survival of channel catfish, Ictalurus punctatus. Aquaculture 24:111–122. https://doi.org/10.1016/0044-8486(81)90048-X
Duncan WP, de Nazare Paula-Silva M, Almeida-Val VMF (1999) Effects of nitrite on hematology and metabolic parameters of an Amazonian catfish Hoplosternum littorale (Callychthyidae). In: International Congress on the Biology of Fish: Special Adaptations of Tropical Fish (Eds. Nelson J, MacKinlay D.), pp. 29–35. Towson University, Baltimore, MD, United State, July 27–30, 1998. https://repositorio.inpa.gov.br/handle/1/29515
Enders E, Boisclair D, Boily P, Magnan P (2006) Effect of body mass and water temperature on the standard metabolic rate of juvenile yellow perch, Perca flavescens (Mitchill). Environ Biol Fishes 76:399–407. https://doi.org/10.1007/s10641-006-9045-0
Evans DH, Piermarini PM, Choe KP (2005) The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev 85:97–177. https://doi.org/10.1152/physrev.00050.2003
Frances J, Allan GL, Nowak BF (1998) The effects of nitrite on the short-term growth of silver perch Bidyanus bidyanus. Aquaculture 163:63–72. https://doi.org/10.1016/S0044-8486(98)00219-1
Franklin RK, Loo HS, Osumanu HA (2010) Incorporation of bentazone with Exserohilum rostratum for controlling Cyperus iria. Am J Agri Biol Sci 5:210–214. https://doi.org/10.3844/ajabssp.2010.210.214
Gam LTH, Jensen FB, Damsgaard C, Huong DTT, Phuong NT, Bayley M (2017) Extreme nitrite tolerance in the clown knifefish Chitala ornata is linked to up-regulation of methaemoglobin reductase activity. Aquat Toxicol 187:9–17. https://doi.org/10.1016/j.aquatox.2017.03.013
Graham JB (1997) Air-breathing fishes: evolution, diversity, and adaptation. Academic Press, p 299
Grosell M, Jensen FB (2000) Uptake and effects of nitrite in the marine teleostfish Platichthys flesus. Aquat Toxicol 50:97–107. https://doi.org/10.1016/s0166-445x(99)00091-0
Haya K (1989) Toxicity of pyrethroid insecticides to fish. Environ Toxicol Chem 5:567–576
Huong DTT, Gam LTH, Lek S, Ut VN, Phuong NT (2020) Effects of nitrite at different temperatures on physiological parameters and growth in clown knifefish (Chitala ornata, Gray 1831). Aquaculture 521:735060. https://doi.org/10.1016/j.aquaculture.2020.735060
IJC (International Joint Commission) (1977) New and revised Great lakes water quality objective. Great Lakes Basin. Windsor, IJC, Ottawa
Jensen FB (2003) Nitrite disrupts multiple physiological functions in aquatic animals. Comp Biochem Physiol A Mol Integr Physiol 135:9–24. https://doi.org/10.1016/S1095-6433(02)00323-9
Jensen FB (2007) Nitric oxide formation from nitrite in zebrafish. J Exp Biol 210:3387–3394. https://doi.org/10.1242/jeb.008748
Jensen FB (2009) The dual roles of red blood cells in tissue oxygen delivery: oxygen carriers and regulators of local blood flow. J Exp Biol 212:3387–3393. https://doi.org/10.1242/jeb.023697
Jensen FB, Rohde S (2010) Comparative analysis of nitrite uptake and hemoglobin-nitrite reactions in erythrocytes: sorting out uptake mechanisms and oxygenation dependencies. Am J Physiol Regul 298:R972–R982. https://doi.org/10.1152/ajpregu.00813.2009
Jensen FB, Andersen NA, Heisler N (1987) Effects of nitrite exposure on blood respiratory properties, acid-base and electrolyte regulation in the carp Cyprinus carpio. J Comp Physiol B 157:533–541. https://doi.org/10.1007/BF00700972
Johansen K (1968). Air-breathing fishes. Sci Am, 219: 102–111 https://www.jstor.org/stable/24927540
Kosaka H, Tyuma I (1987) Mechanism of autocatalytic oxidation of oxyhemoglobin by nitrite. Environ Health Perspect 73:147–151. https://doi.org/10.1289/ehp.8773147
Kroupova H, Machova J, Piackova V, Blahova J, Dobsikova R, Novotny L, Svobodova Z (2008) Effects of subchronic nitrite exposure on rainbow trout Oncorhynchus mykiss. Ecotox Environ Safe 71:813–820. https://doi.org/10.1016/j.ecoenv.2008.01.015
Kroupová HK, Valentová O, Svobodová Z, Šauer P, Máchová J (2018) Toxic effects of nitrite on freshwater organisms: a review. Rev Aquac 10:525–542. https://doi.org/10.1111/raq.12184
Kumaraguru AK, Beamish FWH (1983) Bioenergetics of acclimation to permethrin (NRDC-143) by rainbow trout. Comp Biochem Physiol 75:247–252
Lakshmi V, Alam MN (2009) Pollutional effect of a pesticide bayrusil on bimodal oxygen consumption in an air-breathing fish Heteropneustes fossilis (Bloch). Nat Environ Pollut Technol 8(3):529–532
Law on Animal Health, 2015. Vietnam National Assembly, No. 79/2015/QH13 dated on 10/7/2015
Lefevre S, Huong DTT, Wang T, Phuong NT, Bayley M (2011a) Hypoxia tolerance and partitioning of bimodal respiration in the striped catfish Pangasianodon hypophthalmus. Comp Biochem Physiol Part A Mol Integr Physiol 158:207–214. https://doi.org/10.1016/j.cbpa.2010.10.029
Lefevre S, Jensen FB, Huong DTT, Wang T, Phuong NT, Bayley M (2011b) Effects of nitrite exposure on functional haemoglobin levels, bimodal respiration, and swimming performance in the facultative air-breathing fish Pangasianodon hypophthalmus. Aquat Toxicol 104:86–93. https://doi.org/10.1016/j.aquatox.2011.03.019
Lefevre S, Huong DTT, Phuong NT, Wang T, Bayley M (2012a) Effects of hypoxia on the partitioning of oxygen uptake and the rise in metabolism during digestion in the air-breathing fish Channa striata. Aquaculture 364–365:137–142. https://doi.org/10.1016/j.aquaculture.2012.08.019
Lefevre S, Jensen FB, Huong DTT, Wang T, Phuong NT, Bayley M (2012b) Haematological and ion regulatory effects of nitrite in the air-breathing snakehead fish Channa striata. Aquat Toxicol 118–119:48–53. https://doi.org/10.1016/j.aquatox.2012.03.011
Lefevre S, Wang T, Jensen A, Cong NV, Huong DTT, Phuong NT, Bayley M (2014) Air-breathing fishes in aquaculture. What can we learn from physiology? J Fish Biol 84:705–731. https://doi.org/10.1111/jfb.12302
Lefevre S, Findorf I, Bayley M, Huong DTT, Wang T (2016) Increased temperature tolerance of the air-breathing Asian swamp eel Monopterus albus after high-temperature acclimation is not explained by improved cardiorespiratory performance. J Fish Biol 88:418–432. https://doi.org/10.1111/jfb.12696
Lewis WM Jr, Morris DP (1986) Toxicity of nitrite to fish: a review. Trans Am Fish Soc 115:183–195. https://doi.org/10.1577/1548-8659(1986)115%3c183:TONTF%3e2.0.CO;2
Lingaraja T, Venugopalan VK (1978) Pesticide induced Physiological and behavioral changes in an Estuarine teleost Therapon jarbua (Forsk). Fish Technol 15(2):115–119
Logaswamy S, Remia KM (2009) Impact of cypermethrin and ekalux on respiratory and some biochemical activities of a freshwater fish Tilapia mossambica. J Curr Biotica 3(1):65–73
Lokhande MV (2017) Oxygen consumption and behaviour surveillance in the freshwater fish Rasbora daniconius exposed to dimethoate. Int J Fish Aqua Stu 5(2):712–716
Lv X, Xie H, Xia D, Shen C, Luo Y (2018) Mass scaling of the resting and maximum metabolic rates of the black carp. J Comp Physiol B 188:591–598. https://doi.org/10.1007/s00360-018-1154-5
Magesh S, Kumaraguru AK (2006) Acute toxicity of endosulfan to the milkfish, Chanos chanos, of the Southeast Coast of India. Bull Environ Contam Toxicol 76:622–628. https://doi.org/10.1007/s00128-006-0965-3
Maharajan A, Usha R, Ruckmani PSP, Vijaykumar BS, Ganapiriya V, Kumarasamy P (2013) Sublethal effect of profenofos on oxygen consumption and gill histopathology of the Indian major carp, Catla catla (Hamilton). Int J Pure Appl Zool 1(1):196–204
Marigoudar SR, Ahmed RN, David M (2009) Cypermethrin induced respiratory and behavioural responses of the freshwater teleost, Labeo rohita (Hamilton). Veterinarski Arhiv 79(6):583–590
Multappa K, Reddy HRV, Rajesh M, Padmanabha A (2014) Quinalphos induced alteration in respiratory rate and food consumption of freshwater fish Cyprinus carpio. J Env Biol 35(2):395–398
Nwani CD, Nagpure NS, Kumar R, Kushwaha B, Kumar P, Lakra WS (2010) Lethal concentration and toxicity of carbosulfan, glyphosate, and atrazine to freshwater air-breathing fish Channa punctatus (Bloch). Int Aquat Res 2:105–111
Nwani CD, Ama IU, Okoh F, Oji UO, Ogbonyealu RC, Ibiam AA, Ibiam OU (2013a) Acute toxicity of the chloroacetanilide herbicide butachlor and its effects on the behavior of the freshwater Tilapia zillii. Afr J Biotechnol 12(5):499–503. https://doi.org/10.5897/AJB122433
Nwani CD, Ibiam UA, Ibiam OU, Nworie O, Onyishi G, Atama C (2013b) Investigation on acute toxicity and behavioral changes in Tilapia zillii due to glyphosate-based herbicide, forceup. J Anim Plant Sci 23(3):888–892
Patil VK, David M (2008) Behaviour and respiratory dysfunction as an index of malathion toxicity in the freshwater fish, Labeo rohita (Hamilton). Tuk J Fish Aqua Sci 8:233–237
Perrone SJ, Meade TL (1977) Protective effect of chloride on nitrite toxicity to coho salmon (Oncorhynchus kisutch). J Fish Res Board Can 34:486–492. https://doi.org/10.1139/f77-079
Rahman Q, Sadhu DN (2009) Effects of pesticides on aquatic and aerial oxygen consumption in an air-breathing murrel fish Channa gachua. Nat Environ Pollut Technol 8(3):603–608
Saoud PI, Naamani S, Ghanawi J (2014) Effects of acute and chronic nitrite exposure on rabbitfish Siganus rivulatus growth, hematological parameters, and gill histology. J Aquac Res Development 5(6):263–272. https://doi.org/10.4172/2155-9546100063
Schmidt-Nielsen K (1997) Animal physiology: adaptation and environment, 5th edn. Cambridge University Press, p 607
Siikavuopio SI, Sæther BS (2006) Effects of chronic nitrite exposure on growth in juvenile Atlantic cod, Gadus morhua. Aquaculture 255:351–356. https://doi.org/10.1016/j.aquaculture.2005.11.058
Somdare PO, Nwani CD, Nwadinigwe AO, Nwani JC, Odo GE, Ugbor ON, Ukonze JA, Ezeibe ABCA (2015) Fenthion induced toxicity and histopathological changes in gill tissue of freshwater African catfish, Clarias gariepinus (Burchell, 1822). Afr J Biotechnol 14(25):2103–2113. https://doi.org/10.5897/AJB2015.14696
Stormer J, Jensen FB, Rankin JC (1996) Uptake of nitrite, nitrate, and bromide in rainbow trout Oncorhynchus mykiss: Effects on ionic balance. Can J Fish Aquat Sci 53:1943–1950. https://doi.org/10.1139/f96-142
Subramanian MA (2004) Toxicology. MJP Publishers, Chennai
Tiwari M, Nagpure NS, Saksena DN, Kumar R, Singh SP, Kushwaha B, Lakra WS (2011) Evaluation of acute toxicity levels and ethological responses under heavy metal cadmium exposure in freshwater teleost, Channa punctata (Bloch). Int J Aqu Sci 2(1):36–47
Tomasso J (1994) Toxicity of nitrogenous wastes to aquaculture animals. Rev Fish Sci Aquac 2:291–314. https://doi.org/10.1080/10641269409388560
Tuong DD, Ngoc TB, Huynh VTN, Huong DTT, Phuong NT, Hai TN, Wang T, Bayley M (2018) Clown knifefish Chitala ornata oxygen uptake and its partitioning in present and future temperature environments. Comp Biochem Physiol Part A Mol Integr Physiol 216:52–59. https://doi.org/10.1016/j.cbpa.2017.11.018
Vleeming W, Van De Kuil A, te Biesebeek JD, Meulenbelt J, Boink ABTJ (1997) Effect of nitrite on blood pressure in anaesthetized and free-moving rats. Food Chem Toxicol 35:615–619. https://doi.org/10.1016/S0278-6915(97)00015-X
Voslárová E, Pistekova V, Svobodova Z, Bedanova I (2008) Nitrite toxicity to Danio rerio: Effects of subchronic exposure on fish growth. Acta Vet Brno 77:455–460. https://doi.org/10.2754/avb200877030455
Yager TK, Summerfelt RC (1993) Effects of fish size and feeding frequency on metabolism of juvenile walleye. Aquacult Eng 12:19–36. https://doi.org/10.1016/0144-8609(93)90024-6
Zhang Y, Huang Q, Liu S, He D, Wei G, Luo Y (2014) Intraspecific mass scaling of metabolic rates in grass carp Ctenopharyngodon idellus. J Comp Physiol B 184:347–354. https://doi.org/10.1007/s00360-014-0802-7
Acknowledgements
This study was funded in part by the Can Tho University Improvement Project VN14-P6, supported by a Japanese ODA loan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Phu, T.Q., Hang, B.T.B., Tuong, D.D. et al. Effects of size and nitrite exposure on respiration, oxygen partitioning, and growth of obligate air-breathing fish Channa striata. Fish Sci 88, 149–159 (2022). https://doi.org/10.1007/s12562-021-01562-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-021-01562-1