Abstract
The objective of the present study was to determine the optimal incubation conditions for Gloiopeltis furcata culture. Three experiments, each lasting 30 days, were conducted to study the effects of photoperiod (6:18, 8:16, 12:12, 14:10, and 16:8 h light/dark), different wavelengths of LED light (blue, green, yellow, red, and white), and solar radiation filtered through different plastic films (blue, green, yellow, red, and white) on the growth and photosynthetic pigment content of G. furcata. The results of these experiments demonstrated that the growth rate of G. furcata was significantly higher under 14:10 and 16:8 light/dark than under 6:18 and 8:16 light/dark, while the pigment content of G. furcata was significant higher under 6:18 and 8:16 than under 14:10 and 16:8 light/dark. The growth rate of G. furcata was the lowest when the algae were exposed to blue LED and the highest under yellow LED illumination, while the phycobiliprotein content was the highest under blue LED and the lowest under yellow LED. Solar radiation filtered through different plastic films had no significant effect on the growth rate of G. furcata, but affected its pigment content. The results indicate that a photoperiod of 12 h or more of light and yellow LED are the optimal parameters for culturing of G. furcata thalli on land.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gloiopeltis furcata is a red alga widely distributed along the shorelines of China, Korea, Japan, and the Pacific coast of Russia (Tseng 1983). The species has been used extensively in Japan, China, and Korea as food, medicine, and a source of funoran, which has been traditionally employed as a sizing agent for textiles and a thickener in mortar and plaster (Xia 2004; Yu et al. 2007, 2010; Tuvikene et al. 2014). Most of the G. furcata harvest originates from wild stocks, and its cost of collection is approximately US$200–300 per kilogram dry weight on the Chinese and Japanese markets. A growing demand for G. furcata and the decline in its natural populations has sparked an increased interest in G. furcata culture (Chen et al. 2014a). Different aspects of G. furcata have been studied to develop successful culture management protocols, such as the effects of temperature and light intensity (Chen et al. 2011a, 2014b), salinity (Chen et al. 2013), nutrients (Zhang et al. 2016), and triacontanol (Chen et al. 2011b) on the growth and development of this species, as well as its spore culture in tanks (Chen et al. 2014b) and filament formation and differentiation (Yin et al. 2007).
Harvested thalli of G. furcata are only 4–10 cm in length and 4 mm in diameter, and its vegetative fragments are too short to be attached to ropes for culturing in the sea. Therefore, a culture technique using a designed device was devised for culturing of vegetative fragments of G. furcata on land (Chen et al. 2012, 2016). Light conditions are an important factor influencing the incubation of G. furcata thalli in land cultures. Although the effect of light intensity has been investigated, the effect of light quality and photoperiod on the growth of G. furcata thalli remains unknown.
Light quality and photoperiod affect algal growth and synthesis of photosynthetic pigments (Dring 1988; Katz et al 2000; Talarico and Maranzana 2000; Godínez-Ortega et al. 2008; Barufi et al. 2015; Green and Neefus 2015, 2016). The objective of the present study was to assess the effect of different light wavelengths and photoperiods on the growth and physiological status of G. furcata culture on land. To this end, we conducted a series of experiments designed to elucidate the responses to photoperiodic and light quality in thalli of G. furcata using different-colored LED lights and solar radiation filtered through plastic films of different colors.
Materials and methods
Young thalli of G. furcata (approximately 1 cm long) were collected from the seashore of Yangmeikeng, Shenzhen, China (22°32′52″ N; 114°34′28″ E) and transported to a laboratory, where they were cleaned and incubated under different photoperiod and light quality schemes.
Incubation of thalli under different photoperiods
The experiment was conducted in a laboratory with controlled temperature of 18 °C. Different photoperiods [6:18, 8:16, 12:12, 14:10, and 16:8 h light/dark (L/D)] were established using six separate incubation chambers (Chen et al. 2015, China, ZL201520216205.3). White LED (color temperature 6500 K) was used as the light source. In each incubation chamber, four Erlenmeyer flasks (each with 0.5 g of G. furcata thalli and 500 mL seawater at 18 °C) were placed under the same light intensity (105 μmol photons m−2 s−1). The light level was measured using a quantum meter (MQ-500; Apogee Instruments, Logan, UT, USA).
Incubation of thalli under LEDs of different colors
The experiment was conducted in a laboratory with controlled temperature of 18 °C. Different LED colors (red, blue, green, yellow, and white) were used as light source. Four Erlenmeyer flasks (each with 0.5 g of G. furcata thalli and 500 mL seawater at 18 °C) were placed under the same light intensity (105 μmol photons m−2 s−1) and a photoperiod of 12:12 L/D for each type of light. The wavelengths of the blue, green, yellow, and red LEDs were 450 nm, 520 nm, 585 nm, and 630 nm, respectively, and the color temperature of the white LED was 6500 K.
Incubation of thalli under solar radiation filtered through plastic films of different colors
Gloiopeltis furcata thalli (4 g) were placed in plastic tanks (size 24 cm × 17 cm × 16 cm), each filled with 4 L of seawater. The tanks were covered with plastic films (0.2 mm thick) of different colors (red, blue, green, yellow, or white). Eight replicates were prepared for each film color. The tanks were placed outdoors and exposed to the same solar radiation. During the experiment, the water temperature varied with air temperature (ranging from 16 to 23 °C), but the water temperature of all tanks was the same. The light transmittance of the plastic films and the percentage of different wavelengths of solar radiation passing through the films are shown in Table 1.
Incubation management
The three experiments described above were conducted for 30 days. During the experiments, water motion was provided by aeration. The incubation water was exchanged three times per week. A solution (1 mL L−1) composed of 10 g L−1 NaNO3 and 1 g L−1 KH2PO4 was added to the refreshed seawater. The seawater used in the experiments was filtered through fine sand and stored in the dark for 5 to 10 days. At the end of the experiment, the thalli from each tank were weighed. The relative growth rate (RGR) was calculated using the formula: RGR = [ln(Nt/No)/t] 100%, where N0 is the initial biomass and Nt is the biomass at day t (Korbee et al. 2005).
Pigment analysis
Pigment analysis was performed at the end of the experiment. Two hundred milligrams of thalli from each sample was ground in liquid nitrogen and extracted in 50 mM phosphate buffer (pH 7.8). Crude extracts were centrifuged at 4000 rpm for 20 min to obtain phycobiliproteins. Chlorophyll a (Chl-a) was extracted after dissolving the pellet in 90% acetone and centrifugation at 4000 rpm for 15 min. The pigments were quantified in a spectrophotometer (UV-1800; Shimadzu, Kyoto, Japan), and concentrations of phycobiliproteins (allophycocyanin [APC], phycocyanin [PC], and phycoerythrin [PE]) and Chl-a were calculated as described by Kursar et al. (1983) and Moran and Porath (1980), respectively.
Statistical analysis
Data were analyzed by one-way analysis of variance using SPSS 21 for Windows (IBM Corp., Armonk, NY, USA). The differences among the treatments were tested for significance using a least significant difference (LSD) multiple comparisons test (P < 0.05), and all the data were reported as mean ± SD.
Results
Thalli incubated under different photoperiods
After 30 days of incubation, the color of G. furcata under 16:8 L/D became yellow, while the color of G. furcata under 6:18 L/D was still reddish purple (Fig. 1).
The statistical analysis showed that the growth rate (% growth day−1) of G. furcata was significantly affected by the photoperiod (P < 0.05), and it increased with increasing illumination time from 6 to 14 h per day (Fig. 2). The LSD analysis revealed a significantly higher growth rate under 14:10 L/D and 16:8 L/D than under 6:18 L/D and 8:16 L/D photoperiods. There was no significant difference in growth rate among the treatments with 12:12L/D, 14:10 L/D, and 16:8 L/D photoperiods.
Similarly, the pigment content in G. furcata thalli was significantly affected by the photoperiod (P < 0.05). Based on the LSD analysis, the PE content was significantly higher under 6:18 L/D and 8:16 L/D than under 14:10 L/D and 16:8 L/D photoperiods (Fig. 3). There was no significant difference in the PE levels among the treatments with 12:12 L/D, 14:10 L/D, and 16:8 L/D photoperiods. The PC content was significantly higher under a 6:18 L/D than a 16:8 L/D photoperiod, and there was no significant difference in the PC levels among the treatments with other photoperiods (Fig. 3). The APC and Chl-a content were significantly higher in thalli subjected to a 6:18 L/D photoperiod than in treatments with any other photoperiod (Figs. 3 and 4).
Thalli incubated under different-colored LED lights
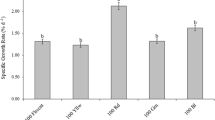
The different-colored LED lights significantly affected the growth rate and pigment content of G. furcata thalli (P < 0.05). The growth rate was the highest under yellow light and the lowest under blue light (Fig. 5). According to the LSD analysis, there was no significant difference in the growth rate of thalli among the treatments with white, green, and red light. The content of PE, PC, and APC was highest under blue light and lowest under yellow light (Fig. 6). The content of Chl-a was the highest under blue light, and there was no significant difference in Chl-a levels among the treatments with lights of other colors (Fig. 7).
Thalli incubated under solar radiation filtered through different-colored plastic films
Solar radiation through different plastic films had no significant effect on the growth rate of G. furcata (Fig. 8), but it significantly affected the pigment content in its thalli (P < 0.05). The PE level was higher in thalli cultured under red plastic film than under white, blue, and yellow film. The content of PC and APC was higher under blue film than under green film and yellow film (Fig. 9). The content of Chl-a was the highest in thalli exposed to light passing through green plastic film (Fig. 10).
Discussion
Effect of photoperiod
Based on a previous study that examined the effects of light intensity on the growth of G. furcata, the optimal photon flux density for growth in this species was 105 μmol photons m−2 s−1 (Chen et al. 2014b). Therefore, in the present study, this optimal light intensity was implemented to investigate the effect of different photoperiods. The results showed that the growth rate of G. furcata was significantly higher when thalli were exposed to at least 12 h of light daily. Similar effects of the photoperiod on the growth rate were reported for Pyropia leucosticta and Porphyra umbilicalis (Green and Neefus 2015, 2016), whereas the growth rate of Chondrus ocellatus was the highest at a 12:12 h L/D photoperiod (Kim et al. 2006). The pigment content in G. furcata decreased with increasing growth rate under different light photoperiods. This decrease in pigment content may be due to the rapid growth of thalli, which effectively diluted the concentration of pigments (Green and Neefus 2015).
Effect of light quality
Light quality has a strong influence on the vegetative development, reproductive induction, and growth rate of macroalgae (Figueroa et al. 1995). The effect of light quality on growth has been found to vary among different algae species. For example, Gelidium sesquipedale exhibited higher growth rates under blue and red light than under white light (Carmona et al. 1996), Pyropia haitanensis experienced slower growth under red light than under white, blue, and green light (Wu 2016), and the growth rate of Kappaphycus alvarezii was higher under red light than under blue light (Thien et al. 2016). The growth rate of G. furcata was the lowest under blue light and the highest under yellow light. This result was in contrast to microalgae Amphora sp. (Romero-Romero and Sánchez-Saavedra 2017), which showed lower growth rates under yellow light, and it also differed from that of red alga Porphyra leucosticta incubated under blue, green, yellow, red, and white light. P. leucosticta did not exhibit a significantly higher growth rate under yellow light than under blue light (Korbee et al. 2005). There are few reports on the effect of yellow light on plant growth, which may be attributable to misclassification of light with 500–600 nm wavelength as green light by researchers (Dougher and Bugbee 2001). In the present experiment, wavelengths of 520 nm and 585 nm were considered to be green light and yellow light, respectively. There was a significant difference in the growth rate of G. furcata between the treatments with these two wavelengths. Therefore, it is necessary to separate yellow light from green light. Our experiments showed that the concentration of phycobiliproteins in G. furcata was the highest under blue LED and significantly different from those measured in other treatments. This was in accordance with the suggestion by Godínez-Ortega et al. (2008) that the accumulation of phycobiliproteins stimulated by blue light is a general feature in red algae. The lowest levels of phycobiliproteins in G. furcata thalli were measured under yellow LED, and they were significantly different from the values obtained under other LEDs. This may have resulted from diluted concentrations of these proteins as a consequence of the high growth rate of G. furcata observed under yellow light.
During the 30 days of the experiments, lower pigment content had not effect on the growth of G. furcata. However, the thalli with lower pigment content became white and decayed about 20 days after the end of experiments. This indicates that lower pigment content does affect the growth of G. furcata after a prolonged incubation period. Therefore, it is recommended that thalli be exposed to yellow light (wavelength of 585 nm) and a photoperiod of at least 12 h of light per day for no more than 30 days for optimal growth of G. furcata thalli in culture.
Effect of plastic films of different colors
Yellow LED can be used to provide the optimal light quality and photoperiod, although its use will add to production costs. A more economical alternative is the use of cheap colored plastic films to manipulate solar radiation and provide optimal light quality for G. furcata culture on land, especially under greenhouse conditions. Some studies showed that solar radiation through colored plastic films affected the growth and secondary metabolite accumulation in higher plants such as Chrysanthemum morifolium (Jin et al. 2011), Panax notoginseng seedlings (Kuang et al. 2014), Perilla frutescens (Grbic et al. 2016), and Capsicum annuum (Casierra-Posada et al. 2014). The use of plastic films of different colors to filter solar radiation before it reached G. furcata thalli had no significant effect on the growth rate of this alga, but it affected the content of the pigments significantly. The plastic films used in our experiment were not of the quality of optical filters, and solar radiation that passed through the monochrome plastic film was of multiple wavelengths (Table 1). This explains the difference between the effects of LED and plastic films of the same color on G. furcata growth. However, the use of optical filters in greenhouses is cost-prohibitive. Further studies should investigate other types of plastic films that will provide better control of filtered light and thus be suitable for the growth of G. furcata in cultures on land.
References
Barufi JB, Figueroa FL, Plastino EM (2015) Effects of light quality on reproduction, growth and pigment content of Gracilaria birdiae (Rhodophyta: Gracilariales). Scientia Marina 79:15–24
Carmona R, Vergara JJ, Pérez-Lloréns JL, Figueroa FL, Niell FX (1996) Photosynthetic acclimation and biochemical responses of Gelidium sesquipedale cultured in chemostats under different qualities of light. Mar Biol 127:25–34
Casierra-Posada F, Matallana-Díaz YA, Zapata-Casierra E (2014) Growth of bell pepper plants (Capsicum annuum) affected by coloured covers. Gesunde Pflanzen 66:149–155
Chen SW, Wu JF, Chen LX, Zhu CB (2011a) Effects of light and temperature on the attachment and development of Gloiopeltis tenax and Gloiopeltis furcata tetraspores. J Appl Phycol 23:1045–1051
Chen SW, Wu JF, Chen LX, Cheng SL, Xiao RH (2011b) The effect of triacontanol on the germination of spore and the growth of germling and frond of Gloiopeltis furcata. J South China Agric Univ 32:78–82 (In Chinese with English abstract)
Chen SW, Chen LX, Huang Z, Zhu CB (2012) Method and device for culture Gloiopeltis thalli on land: Chinese patent, ZL201210124825.5 (In Chinese)
Chen LX, Wu JF, Chen SW, Xie XY, Zhu CB, Guo YH (2013) Effect of salinity on attachment, development and survival of Gloiopeltis furcata spore. South China Fish Sci 9:53–57 (In Chinese with English abstract)
Chen SW, Chen LX, Zhu CB, GuoYJ GYH (2014a) Spore culture of red seaweed, Gloiopeltis furcata. J World Aquac Soc 45:487–492
Chen SW, Chen LX, Zhu CB, Su L (2014b) Effects of environmental factors on growth and survival of Gloiopeltis furcata thalli. South China Fish Sci 10:92–96 (In Chinese with English abstract)
Chen SW, Chen LX, Zhang WW, Zhu CB, Li JW, Guo YJ, Xie XY (2015) Device for be used for experiment of marine alga photoperiod: Chinese patent, ZL2015202 16205.3 (In Chinese)
Chen SW, Guo YJ, Li JW, Xie XY, Zhu CB (2016) Studies on culture and storage of Gloiopeltis furcata thalli. Prog Fish Sci 37:108–113 (In Chinese with English abstract)
Dougher TAO, Bugbee B (2001) Evidence for yellow light suppression of lettuce growth. Photochem Photobiol 73:208–212
Dring MJ (1988) Photocontrol of development in algae. Ann Rev Plant Physiol Plant Mol Biol 39:157–174
Figueroa F, Aguilera J, Xavier Niell F (1995) Red and blue light regulation of growth and photosynthetic metabolism in Porphyra umbilicalis (Bangiales, Rhodophyta). Eur J Phycol 30:11–18
Godínez-Ortega JL, Snoeijs P, Robledo D, Freile-Pelegrín Y, Pedersén M (2008) Growth and pigment composition in the red alga Halymenia floresii cultured under different light qualities. J Appl Phycol 20:253–260
Grbic N, Paschko K, Pinker I, Böhme MH (2016) Effect of different light spectra by using coloured plastic films on growth, fresh and dry matter, nutrient solution uptake and secondary metabolites of Perilla frutescens (L.) Britt. Sci Hortic 210:93–98
Green LA, Neefus CD (2015) Effects of temperature, light level, photoperiod, and ammonium concentration on Pyropia leucosticta (Bangiales, Rhodophyta) from the Northwest Atlantic. J Appl Phycol 27:1253–1261
Green LA, Neefus CD (2016) Effects of temperature, light level, and photoperiod on the physiology of Porphyra umbilicalis Kützing from the Northwest Atlantic, a candidate for aquaculture. J Appl Phycol 28:1815–1826
Jin M, Zhu Z, Guo Q, Shen H, Wang Y (2011) Growth and accumulation of bioactive compounds in medicinal Chrysanthemum morifolium Ramat. Cv. ‘Chuju’ under different coloured shade polyethylene. J Med Plant Res 6:398–404
Katz S, Kizner Z, Dubinsky Z, Friedlander M (2000) Responses of Porphyra linearis (Rhodophyta) to environmental factors under controlled culture conditions. J Appl Phycol 12:535–542
Kim YS, Choi HG, Nam KW (2006) Phenology of Chondrus ocellatus in Cheongsapo near Busan. Korea J Appl Phycol. 18:551–556
Korbee N, Figueroa FL, Aguilera J (2005) Effect of light quality on the accumulation of photosynthetic pigments, proteins and mycosporine-like amino acids in the red alga Porphyra leucosticta (Bangiales, Rhodophyta). J Photochem Photobiol, B 80:71–78
Kuang SB, Xu XZ, Yang SC, Zhang GH, Meng ZG, Long GQ, Chen ZJ, Chen JW (2014) Effects of different light qualities and transmittances on growth of Panax notoginseng seedlings. J South Agric 45:1935–1942
Kursar TA, Meer JVD, Alberte RS (1983) Light-harvesting system of the red alga Gracilaria tikvahiae. I. Biochemical analysis of pigment mutations. Plant Physiol 73:353–360
Moran R, Porath D (1980) Chlorophyll determination in intact tissues using n, n-dimethylformamide. Plant Physiol. 65:478–479
Romero-Romero CC, Sánchez-Saavedra MP (2017) Effect of light quality on the growth and proximal composition of Amphora sp. J Appl Phycol 29:1203–1211
Talarico L, Maranzana G (2000) Light and adaptive responses in red macroalgae: an overview. J Photochem Photobiol B 56:1–11
Thien VY, Rodrigues KF, Wong CMVL, Yong WTL (2016) Investigation of growth rate and phycocolloid content from Kappaphycus alvarezii (Rhodophyta, Solieriaceae) under different light conditions using vibrational spectroscopy. J Appl Biotechnol 4:1–9
Tseng CK (1983) Common seaweeds of China. Science Press, Beijing, p 92
Tuvikene R, Robal M, Fujita D, Saluri K, Truus K, Tashiro Y, Ogawa H, Matsukawa S (2014) Funorans from Gloiopeltis species. Part I. Extraction and structural characteristics. Food Hydrocoll 43:481–492
Wu HY (2016) Effect of different light qualities on growth, pigment content, chlorophyll fluorescence, and antioxidant enzyme activity in the red alga Pyropia haitanensis (Bangiales, Rhodophyta). Biomed Res Int Article ID 7383918, 8 pp https://dx.doi.org/10.1155/2016/73839187383918
Xia BM (2004) Flora Algarum Marinarum Sinicarum (Tomus II) Rhodophyta (No. III) Gelidiales Cryptonemiales Hildenbrandiales. Science Press, Beijing, pp. 50–53 (In Chinese)
Yin MY, Hu XY, Tseng CK (2007) Filament formation and differentiation in seven species of red algae. Bot Mar 50:113–118
Yu J, Xu ZC, Yan LL, Cheng SJ (2007) Studies on the anti-mutagenic and anti-tumor effects of the polysaccharide of Gloiopeltis furcata. J Shantou Univ 22:59–63 (In Chinese with English abstract)
Yu GL, Hu Y, Yang NB, Zhao X, Wang PP, Wu JD, Guan HS (2010) Extraction, isolation and structural characterization of polysaccharides from a red alga Gloiopeltis furcata. J Ocean Univ China 9:193–197 (In Chinese with English abstract)
Zhang WW, Guo YJ, Li JW, Zhu CB, Chen SW, Xie XY (2016) Effect of nutrient on growth and biochemical composition of Gloiopeltis furcate. South China Fish Sci 12:30–35 (In Chinese with English abstract)
Acknowledgements
This work was supported by the Central Public-interest Scientific Institution Basal Research Fund, CAFS (Grant number 2014A07XK01); the Technology Project and Fishery Technology Popularization Programs of Guangdong Province (Grant numbers B201601-09, Z2015013); and the Central Public-interest Scientific Institution Basal Research Fund, South China Sea Fisheries Research Institute, CAFS (Grant number 2013ZD01).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, W., Zhu, C. & Chen, S. Effects of light quality and photoperiod on growth and photosynthetic pigment content of a Rhodophyta, Gloiopeltis furcata. Fish Sci 86, 367–373 (2020). https://doi.org/10.1007/s12562-020-01400-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-020-01400-w