Abstract
Seaweed aquaculture in the Northwest Atlantic has been steadily increasing with five commercial kelp farms already established. Currently, kelp production is limited to winter and spring, and new seaweed crops need to be developed in order to supplement seasonal kelp production. Porphyra umbilicalis is a member of the most economically valuable group of seaweeds known by the Japanese name nori. It is an ideal candidate for aquaculture since it exhibits short production cycles, rapid growth, high nutrient uptake rates, and high pigment and protein content. Further, sexual reproduction appears to be absent in populations in the Northwest Atlantic, which considerably simplifies the production of seed stock. The goal of this study was to determine the conditions that optimize growth, photosynthetic efficiency of photosystem II (F v/F m), and pigment and protein content of P. umbilicalis. Cultured blades were grown under a matrix of temperatures (10, 15, and 20 °C), light levels (30, 60, 110, and 250 μmol photons m−2 s−1), and photoperiods (8:16, 12:12, and 16:8 light/dark) in a factorial design for 4 weeks. Growth rates were highest (>9 % day−1) in blades grown between 10 and 15 °C, with light levels ≥110 μmol photons m−2 s−1 and ≥12 h of light in the day. F v/F m was significantly affected by photoperiod with this effect dependent on light level; the overall range of F v/F m values was small. Here, we report detailed information on the growth rate, F v/F m,, and pigment and protein content of P. umbilicalis grown under 36 treatment combinations. These results provide physiological information on P. umbilicalis from the Northwest Atlantic that will aid in the development of P. umbilicalis aquaculture on a commercial scale.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The production of aquacultured seaweeds has been increasing steadily since the 1970s with an annual growth rate of 9.5 % during the 1990s and 7.4 % during the 2000s (FAO 2012). The total worldwide value of the seaweed aquaculture industry was estimated to be US$5.7 billion in 2010 (FAO 2012). Seaweed aquaculture is gaining intensity in the Northwest Atlantic, particularly in the northeastern United States which now has five commercial scale kelp farms currently established and several pending (C. Yarish, pers. comm.). With demand for seaweed products growing, there is a need for diversification of the industry by developing new species for cultivation. Nori (Porphyra, Pyropia, and other closely related red algal genera) is the most economically valuable maricultured seaweed in the world, serving as a source of food for humans (Pereira and Yarish 2010), and global demand is rising (Israel 2010). Currently Japan, China, and Korea are the major producers of nori (FAO 2012). Production of nori was over 1.5 million metric tons wet weight in 2010 (FAO 2012), valued at US$1.45 billion (Pereira et al. 2013).
An ideal nori species for aquaculture would have several attributes including a fast growth rate, high capacity for nutrient accumulation, high protein content, extended seasonality, and a life history that allows for easy propagation. In addition, it should be native to the locality where it will be grown. Porphyra umbilicalis Kützing is an excellent candidate for aquaculture in the Northwest Atlantic. Porphyra umbilicalis occurs year-round at rocky intertidal sites from Atlantic Canada to Long Island Sound (Blouin 2010). There are 19 foliose Bangiales species in the Northwest Atlantic (Mols-Mortensen et al. 2012; Mols-Mortensen et al. 2014). However, P. umbilicalis, for reasons that are still unclear, is the only species that appears to reproduce only asexually (Blouin et al. 2007; Blouin 2010; Blouin and Brawley 2012), making it particularly well suited for aquaculture. Reproduction occurs through neutral spores that germinate and grow into new blades when released, thus bypassing the microscopic conchocelis (sexual) phase of the life history of Porphyra (Brodie and Irvine 2003; Blouin et al. 2007). Porphyra umbilicalis also has high photosynthetic rates (Kraemer and Yarish 1999), nutrient uptake efficiency, and growth rates (Carmona et al. 2006; Kim et al. 2007). Furthermore, Blouin et al. (2006) showed no difference in the consumer acceptability between the currently marketed Pyropia yezoensis and Northwest Atlantic P. umbilicalis, making P. umbilicalis an excellent candidate for human consumption.
Although studies have examined important aspects of the physiology of P. umbilicalis including nutrient dynamics (Kraemer et al. 2004; Kim et al. 2007; Kim et al. 2013), photosynthesis (Kraemer and Yarish 1999; Sampath-Wiley et al. 2008), sporic ecology (Blouin et al. 2007), and environmental controls of growth rate (Fortes and Lüning 1980), there are still gaps in our knowledge that would facilitate the successful establishment of P. umbilicalis aquaculture in the Northwest Atlantic. Further, most previous studies have only investigated the effects of single variables on the physiology of P. umbilicalis (but see Kim et al. 2007). It is likely that there are complex interactions between environmental variables (e.g., photoperiod and light level) that significantly impact the physiology of this species. The objective of this study was to determine the conditions that optimize growth, photosynthetic efficiency of photosystem II (PSII), and pigment and protein content of P. umbilicalis. Blades produced via neutral spores from wild stocks were grown under a matrix of temperatures, light levels, and photoperiods to determine the individual and synergistic effects of these environmental parameters. We aimed to provide important missing information needed to spur a P. umbilicalis aquaculture industry and to acquire detailed information on the performance of P. umbilicalis under a wide range of conditions.
Materials and methods
Culturing
Cultures were established from wild Porphyra umbilicalis blades collected from five sites in Maine (ME), Massachusetts (MA), and New Hampshire (NH), USA, including Nubble Light, York, ME (n = 2; 43°9′55.2″ N, 70°35′28.6″ W; collected in June 2011), Fort Constitution, New Castle, NH (n = 2; 43°4′16.7″ N, 70°42′37.1″ W; collected in July 2011), Fort Stark, New Castle, NH (n = 1; 43°3′27.1″ N, 70°42′44.3″ W; collected in February 2011), Hilton Park, Dover, NH (n = 4; 43°7′11.8″ N, 70°49′37.8″ W; collected in April 2011 and February 2012), and Gloucester, MA (n = 1; 42°36′32.1″ N, 70°37′58.4″ W; collected in May 2011). At each site, blades were collected from the mid- to high intertidal zone during low tide. Blades were placed on ice and transported to the laboratory where they were processed for spore release and isolation.
Using a sterile razor blade, the fertile portions of the blades were excised, cleaned, wrapped in sterile damp paper towels, and placed in the dark at 4 °C overnight. The following morning, the tissue was submerged in sterile seawater at 10 °C and 10–50 μmol photons m−2 s−1 light to induce spore release. Individual spores of P. umbilicalis were isolated using sterile Pasteur pipettes and placed into 12- or 24-well culture plates containing sterile Von Stosch enriched (VSE) seawater at 10 °C and 10–50 μmol photons m−2 s−1 under a neutral day photoperiod (12:12 L/D). Von Stosch enrichment was based on Ott (1966) with NH4Cl used as the source of nitrogen. Media was changed weekly. After spores germinated, blades were transferred to flasks with VSE seawater and held at 15 °C, 10–50 μmol photons m−2 s−1, and 12:12 L/D until they reached 1–2 cm in length when they were placed into the experimental microcosm (described below). A total of ten culture strains were used in the experiment (two different strains per trial with five trials conducted), collected from the various sites described above.
Genetic identification of cultured material
The genus Porphyra contains many species that have a very similar morphology, which makes them extremely hard to identify without the use of genetic markers. Therefore, DNA barcoding was used to confirm the identity of all cultures following the protocol of Green and Neefus (2015).
Microcosm description and experimental design
To determine the optimum conditions for growth, photosynthetic efficiency of PSII, and pigment and protein content, individual 1–2 cm blades of P. umbilicalis cultured from isolated spores were placed in 125 mL Erlenmeyer flasks (n = 1 blade per flask) and grown under a matrix of temperatures, light levels, and photoperiods for 4 weeks in a fully factorial split-split plot design. Blades were grown in sterile modified Von Stosch enriched seawater (30–32 ppt, 125 mL), replaced weekly, and supplied constant filter-sterilized air (1 μm, Pall Life Sciences, USA) using aquarium air pumps (Tetra Whisper 300).
In order to determine the individual and synergistic effects of photoperiod, temperature, and light level, blades were grown in a factorial split-split plot design. Photoperiods (8:16, 12:12, and 16:8 L/D; main plot) were controlled using three separate growth chambers (Percival Scientific, model E-30B). In each chamber, six independently controlled water baths were used to maintain temperature (two each at 10, 15, and 20 °C; subplot). Each water bath had a submersible aquarium heater (Marineland, 25 W) that was connected to a digital heater temperature controller (Finnex, HC-810M, ± 1.1 °C). Four flasks were placed in each water bath and individually wrapped with neutral density filter to achieve light levels (sub-subplot) of 250, 110, 60, and 30 μmol photons m−2 s−1. Light was supplied by eight cool white fluorescent bulbs in each chamber (Philips Alto II 17W, F17T8/TL741). Trials were repeated in a total of five times.
Growth and photosynthetic efficiency
The blotted-dry fresh weight (FW) of each blade was determined weekly and relative growth rate (RGR, hereafter referred to as growth rate) was calculated as RGR = 100 × ln [(L 2/L 1)/(t 2 − t 1)], where L 2 and L 1 are the blade weight at times t 2 and t 1, respectively. At the end of the 4-week trial period, blades were split into two and one half was used for phycobilin and protein analysis, while the other half was used for chlorophyll and carotenoid analyses (except in the third and fourth trials where only phycobilin and protein analyses occurred).
The photosynthetic efficiency of PSII (F v/F m) was determined weekly starting 1 week after the blades were placed in the microcosm. Measurements were taken using a white light PAM (pulse amplitude-modulated) fluorometer (Junior-PAM, Heinz Walz Germany) following a modified protocol from Figueroa et al. (1997) using a minimum of 10 min for dark adaptation and a far red pulse prior to measurement. All measurements occurred at the treatment temperatures and in the media that the blades were cultured in.
Photosynthetic pigment measurements
Phycobilins (R-phycoerythrin, R-PE, and R-phycocyanin, R-PC) were extracted from samples (5–50 mg FW) in 1.5 mL of 0.1 M phosphate buffer (16.73 % NaH2PO4 anhydrous and 93.27 % Na2HPO4 12H2O, pH 6.8) at 4 °C for 24 h and determined per the methods of Sampath-Wiley and Neefus (2007) using a dual-beam UV-visible spectrophotometer (Helios Alpha).
Total chlorophyll and carotenoids were extracted from samples (5–50 mg FW) in 1.5 mL of 80 % acetone. The samples were centrifuged at 4 °C for 4 min at 17,000 rpm, and the supernatant was poured into 1.5 mL acetone-resistant microcuvettes. Absorbance was read at 470, 647, and 664 nm at room temperature using a dual-beam UV-visible spectrophotometer (Helios Alpha) against a blank containing 80 % acetone. Total chlorophyll and carotenoid concentrations were calculated using the equations of Lichtenhaler (1987).
Soluble and structural protein measurements
To measure soluble protein, 1.5 mL of Coomassie reagent (Bradford 1976) was added to 0.3 mL of supernatant from the phycobilin extraction and incubated at room temperature for 10 min. Absorbance was read at 595 nm using a dual-beam UV-visible spectrophotometer (Helios Alpha), and concentrations were calculated by means of standards made with bovine serum albumin (G-Biosciences 786-006).
To measure structural protein, 1.0 mL of 1.0 M NaOH was added to the pellet from the phycobilin extraction and samples were incubated at 4 °C for 24 h. Following incubation, 48 μL of concentrated HCl (12 N) was added to each sample to correct the pH (Korbee et al. 2005a). The above protein assay (Bradford 1976) was then performed on all samples.
Statistical analysis
Growth and photosynthetic efficiency were analyzed as a split-split-split plot analysis of variance (ANOVA) with photoperiod as the main plot, temperature as the subplot, light level as the sub-subplot, and week as the sub-sub-subplot (Federer and King 2007). Pigment and protein results were analyzed using a split-split plot ANOVA with photoperiod as the main plot, temperature as the subplot, and light level as the sub-subplot (Federer and King 2007). Blades in the third and fourth trials did not reach a size where both the phycobilin/protein and chlorophyll/carotenoid assays could be performed; therefore, only the phycobilin and protein assays were done for these trials (refer to methods). The response variables did not conform to the assumptions of normality and were rank transformed prior to analysis (Conover and Iman 1981). All post hoc comparisons were made using a Tukey’s HSD test, which has been shown to be effective on rank transformed data (Conover and Iman 1981). All analyses were performed in SYSTAT 13.00.05 (Systat, Inc.).
Results
Genetic identification of cultured material
Sequences were compared to the rbcL sequence of the P. umbilicalis neotype on GenBank (KF478756) using MegaAlign v. 7.1 (DNA Star Inc., USA) to confirm species identification. All culture strains used in this study were 100 % identical to the neotype.
Growth rate
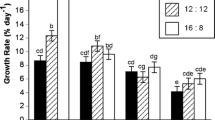
The effect of photoperiod on the growth rate of P. umbilicalis was dependent on light level (F 6.216 = 1.81, p < 0.001, Tables 1 and 2). Under all photoperiods, the growth increased with increasing light level up to 110 μmol photons m−2 s−1. The highest growth rates, which ranged from 9.04 ± 0.38 to 9.96 ± 0.38 % day−1, were recorded under neutral (12:12) and long (16:8) day photoperiods above 110 μmol photons m−2 s−1 (Fig. 1). Blades grew slowest under short-day (8:16) and 30 μmol photons m−2 s−1 conditions (0.80 ± 0.39 % day−1; Fig. 1).
Growth rate (% day−1) of Porphyra umbilicalis at three different photoperiods (8:16, 12:12, and 16:8 L/D) and four light levels (30, 60, 100, and 250 μmol photons m−2 s−1; mean ± SE). Temperature and week had no effect on this interaction and were averaged within each photoperiod × light level group. Bars with a letter in common are not significantly different (α = 0.05). Although analysis was performed on rank transformed data, original data and standard errors are graphed with letters derived from the post hoc analysis of rank transformed data
The effect of photoperiod on the growth rate of P. umbilicalis was also dependent on the week (F 6.1001 = 391.07, p < 0.001; Tables 1 and 2). During the first week, there was no difference in growth rates between the three photoperiods, with rates ranging from 6.77 ± 0.39 to 7.87 ± 0.38 % day−1 (Table 3). In the second week, growth under long-day conditions was higher than those under neutral and short day conditions. The differences between the photoperiods were less dramatic during the third and fourth weeks. Growth under short-day conditions declined rapidly from week 1 to week 2, but there was no difference in growth rate after week 2. In contrast, growth under long-day conditions peaked in the second week of the experiment, followed by a decline in weeks 3 and 4. Growth under neutral-day conditions showed a steady decline over time with growth during week 1 higher than growth during week 4 (Table 3).
The effect of temperature on the growth rate of P. umbilicalis was dependent on the week (F 6.1001 = 4.15, p < 0.001; Tables 1 and 2). There were no differences in growth rate between the temperatures within each week (e.g., no difference between growth at 10, 15, or 20 °C in week 1; Fig. 2). Growth at 10 °C showed no differences across the 4 weeks. However, growth at 15 °C was highest during weeks 1 and 2 and growth during week 1 was higher than growth during weeks 3 and 4. Similarly, growth at 20 °C was not different between weeks 1 and 2, but growth during both weeks was higher than growth during weeks 3 and 4 (Fig. 2; Table 2).
Growth rate (% day-1) of blades of Porphyra umbilicalis under three different temperatures (10, 15, and 20 °C) over a period of 4 weeks (mean ± SE). Photoperiod and light level had no effect on this interaction and were averaged within each temperature. Bars with a letter in common are not significantly different (α = 0.05). Although analysis was performed on rank transformed data, original data and standard errors are graphed with letters derived from the post hoc analysis of rank transformed data
Overall, blades of P. umbilicalis grew best at temperatures between 10 and 15 °C, light levels ≥110 μmol photons m−2 s−1 and ≥12 h of light in a day, although growth generally decreased over time under all conditions (Table 2).
Photosynthetic efficiency of PSII
The effect of photoperiod on photosynthetic efficiency of PSII (F v/F m) was dependent on light level (F 6.216 = 7.85, p < 0.001; Table 1), with a slight decrease in F v/F m at high light levels under neutral and long-day conditions (Fig. 3; Table 4). F v/F m was also affected by week and decreased over time (F 1.1005 = 6.54, p < 0.001).
Photosynthetic efficiency (F v/F m) of Porphyra umbilicalis at three different photoperiods (8:16, 12:12, and 16:8 L/D) and four light levels (30, 60, 100, and 250 μmol photons m−2 s−1; mean ± SE). Temperature and week had no effect on this interaction and were averaged within each photoperiod × light level group. Bars with a letter in common are not significantly different (α = 0.05). Although analysis was performed on rank transformed data, original data and standard errors are graphed with letters derived from the post hoc analysis of rank transformed data
Phycobilins
The effect of photoperiod on R-phycoerythrin (R-PE) content was dependent on light level (F 6.243 = 3.94, p = 0.001; Tables 1 and 5). Post hoc analysis revealed no differences between the photoperiods at low light levels (≤60 μmol photons m−2 s−1; Table 6). At high light levels (≥110 μmol photons m−2 s−1), R-PE content was higher under short than long-day conditions. R-PE content increased with increasing light level under short-day conditions, while under long-day conditions R-PE content was lower at 110 μmol photons m−2 s−1 than all other light levels. There was no change in R-PE content under neutral day conditions across the four light levels (Table 6).
The effect of photoperiod on R-PC content was also dependent on light level (F 6.233 = 3.38, p = 0.003; Tables 1 and 5). Post hoc analysis revealed no differences between the three photoperiods at 30, 60, or 110 μmol photons m−2 s−1 (Table 6). However, at 250 μmol photons m−2 s−1 R-PC content under short-day conditions was higher than under long-day conditions. Similar to R-PE content, R-PC content increased with increasing light level under short-day conditions but showed no change with light level under either neutral or long-day conditions (Table 6).
Chlorophyll and carotenoids
The total chlorophyll content was affected by light level (F 3.98 = 7.46, p < 0.001; Tables 1 and 5) and was independent of photoperiod. Chlorophyll content decreased with increasing light level. Post hoc analysis showed that blades grown at 30 μmol photons m−2 s−1 had more chlorophyll (0.76 ± 0.05 mg g−1 FW) than blades grown at 110 (0.56 ± 0.04 mg g−1 FW) and 250 (0.57 ± 0.03 mg g−1 FW), but not blades grown at 60 μmol photons m−2 s−1 (0.78 ± 0.05 mg g−1 FW). There were no differences in total carotenoid content between any treatment groups (Tables 1 and 5).
Soluble and structural protein
The soluble protein content of P. umbilicalis blades was affected by light level (F 3.245 = 16.2, p < 0.001; Tables 1 and 7). Soluble protein was higher at 30 (28.26 ± 3.51 mg g−1 FW) and 60 μmol photons m−2 s−1 (15.64 ± 3.53 mg g−1 FW) than at 110 (11.41 ± 3.42 mg g−1 FW) and 250 μmol photons m−2 s−1 (11.26 ± 3.42 mg g−1 FW).
The structural protein content was also affected by light level (F 3.249 = 32.1, p < 0.001; Tables 1 and 7). It was also highest at 30 μmol photons m−2 s−1 (53.12 ± 3.68 mg g−1 FW) and decreased with increasing light level to 31.68 (± 3.65) mg g−1 FW at 60 μmol photons m−2 s−1, 23.64 (± 3.62) mg g−1 FW at 110 μmol photons m−2 s−1 , and 22.52 (± 3.62) mg g−1 FW at 250 μmol photons m−2 s−1.
Photoperiod also affected structural protein content (F 2.8 = 29.7, p < 0.001) and was higher in blades grown under short-day conditions (44.27 ± 3.15 mg g−1 FW) than neutral (23.07 ± 3.15 mg g−1 FW) and long-day conditions (30.88 ± 3.15 mg g−1 FW).
Ratio of phycobilin to soluble protein content
Light affected the ratio of phycobilin to soluble protein content (PB/SP; F 3.238 = 9.52, p < 0.001; Tables 1 and 7). Post hoc analysis revealed that the PB/SP was lower in blades grown under 30 μmol photons m−2 s−1 (0.24 ± 0.02) than under 60 (0.31 ± 0.02), 110 (0.34 ± 0.02) and 250 μmol photons m−2 s−1 (0.34 ± 0.02).
Discussion
Overall, these results indicate that it is important to look at the synergistic effects of environmental factors when determining the conditions for optimum growth, photosynthetic efficiency of PSII, and pigment and protein content of seaweeds. Many studies (see Fortes and Lüning 1980; Blouin et al. 2007) have looked at growth and other physiological measures in P. umbilicalis, but none have looked at the suite of variables measured here under a fully factorial matrix of photoperiods, temperatures, and light levels. Such results will allow potential seaweed growers to select the best combination of conditions for growing P. umbilicalis based on their intended use (Tables 2, 4, 5, and 7).
Maximum growth rates (>9 % day−1) were observed when blades were grown at 10 to 15 °C with at least 12 h of light in the day and ≥110 μmol photons m−2 s−1 (Fig. 1), which is consistent with previously published information on P. umbilicalis from both Fortes and Lüning (1980) and Kim et al. (2007). Fortes and Lüning (1980) further reported that growth increased with increasing day length, a phenomenon that we also documented. We also found that the growth rate of P. umbilicalis decreased over the 4-week duration of the experiment with the most dramatic reductions occurring under short-day conditions (Fig. 2, Table 1). Previous studies with Fucus distichus Linnaeus have indicated that young thalli have nitrogen uptake rates 8–40 times higher than mature thalli, leading to faster growth of younger, smaller blades (Thomas et al. 1985). Additionally, Kim (2008) reported a decrease in growth rate over a similar time period with blades of a closely related species of nori, Wildemania amplissima (Kjellman) Foslie. Overall, our results have important implications for P. umbilicalis aquaculture and indicate that blades should be harvested frequently to ensure optimal production (approximately every 4 weeks). Similarly, Israel et al. (2006) suggested harvesting cycles of 2–3 weeks for Porphyra grown in outdoor tank cultivation systems.
The significant reduction in photosynthetic efficiency of PSII (F v/F m), with increasing light levels that we documented, suggests that even at light levels as low as 110 μmol photons m−2 s−1, there was an energetic cost to dealing with excess light energy (Fig. 3). Macroalgal species that live in the high intertidal zone have mechanisms to limit the amount of damage from photoinhibition (Herbert 1990), although the mechanisms remain poorly understood (Blouin et al. 2011). Some researchers have suggested that there is a protective mechanism associated with the photoinhibition-sensitive site of PSII that dissipates excess light energy (Herbert 1990), while others have suggested that depressions in F v/F m at high irradiance are a protective response to limit damage by limiting the number of available reaction centers (Kokubu et al. 2015). While the reduction in F v/F m we observed was statistically significant, F v/F m was at or near 0.6 under all conditions. These values are consistent with the maximum F v/F m levels reported for P. umbilicalis in nature (Sampath-Wiley et al. 2008), suggesting that despite the reduction, F v/F m remained high in all blades. Therefore, we hypothesize that the reduction in F v/F m above 110 μmol photons m−2 s−1 under long-day conditions was a photoprotective response, whether from a reduction in available reaction centers or through a permanent photoprotective mechanism, in the high intertidal P. umbilicalis. Further studies are necessary to validate this hypothesis. The pattern of F v/F m reduction may be more clearly demonstrated at light levels closer to those observed in nature which range from 100 to 600 μmol photons m−2 s−1 at high tide to 2000 μmol photons m−2 s−1 at low tide (Blouin et al. 2011), depending on cloud cover and time of day.
Interestingly, we saw no effect of temperature on F v/F m, although it has been suggested that F v/F m is temperature dependent (Dongsansuk et al. 2013). However, the range of temperatures tested here (10–20 °C) was relatively small. Other studies have shown no change or very small changes in F v/F m in other Bangiales over a similar temperature range (see Watanabe et al. 2014; Green and Neefus 2015). Future studies should incorporate a wider temperature range in order to determine if photosynthetic efficiency in P. umbilicalis is temperature dependent.
Decreasing phycobilin content with increasing light level, which we found under long-day conditions, has been previously documented (Jahn et al. 1984). Sampath-Wiley et al. (2008) found that differences in phycobilin content of sun and shade blades of P. umbilicalis were dependent on season, with differences during summer months, but not in the winter months when photoperiods were shorter. Further, the phenomenon of decreasing chlorophyll content with increasing light levels, which we found, has been documented in land plants (Cooper and Quails 1967) and algae (Sampath-Wiley et al. 2008). Studies by the latter authors have also shown no difference in total carotenoid content in blades of P. umbilicalis exposed to varying light levels.
Phycobilin content under short-day conditions increased with increasing light level. This phenomenon has been previously documented in the closely related Pyropia leucosticta (Green and Neefus 2015) and could have two explanations. First, blades grown under short-day conditions could have higher phycobilin content because of their lower growth rates compared to blades grown under neutral or long-day conditions (Fig. 1). Blades that are growing more rapidly may effectively dilute the concentration of pigments (mg g−1 FW of tissue), thus showing lower pigment content (Day 2008). Second, blades grown under short-day conditions may have been light limited by day length. Under these conditions, the blades may have been adjusting the size and number of photosynthetic units (Waaland et al. 1974; Mishkind and Mauzerall 1980) to optimize light capture.
Although the optimum conditions for increased phycobilin content (mg g−1 FW) were observed in blades grown under short-day conditions, from an aquaculture standpoint, the reduction in growth rate under these conditions makes it impractical to grow them this way simply to yield high phycobilin content. Phycobilin content was also high in blades grown under neutral and long-day conditions with low light (≤60 μmol photons m−2 s−1). We hypothesize that a brief period of growth (1–7 days) under low light, neutral/long-day conditions or high light, short-day conditions prior to harvest will increase pigment content, although this hypothesis warrants further investigation. Additionally, if the overall production of phycobilins (mg blade−1 or tank−1) is the goal, then growth under neutral and long-day conditions will be the best choice. Faster growth rates under neutral and long-day conditions outweigh the lower pigment content (mg g−1 FW), leading to higher total phycobilin production (mg blade−1, data not shown).
Protein content is an important determinate of the nutritional value of seaweed for aquaculture feeds (Shpigel et al. 1999), as many finfish require high protein content in their diets (Wilson 2002). Walker et al. (2009) showed that P. umbilicalis grown in a recirculating IMTA system could be used to replace up to 30 % of the fish meal in juvenile cod diets without deleterious effects on their growth. Further, a relationship between phycobilin and soluble protein content has been suggested in several studies (Hernández et al. 1993; Korbee et al. 2005b), since proteins associated with phycobilins can make up a large portion of the soluble protein in red seaweeds (Gantt 1975); phycobilins in the current study accounted for up to 59 % of the soluble protein. Our results did not show a linear relationship between phycobilin (PB) and soluble protein (SP) content (data not shown), but there was an effect of light level on PB/SP. Above 60 μmol photons m−2 s−1 PB/SP remained stable, while at the lowest light level, accumulation of non-phycobilin soluble proteins lead to a significant decrease in PB/SP.
Conclusions
Ultimately, the conditions that a seaweed grower chooses for growing P. umbilicalis will depend on several things. Here we have reported the growth rate, photosynthetic efficiency of PSII, and pigment and protein content of blades of P. umbilicalis grown under 36 different combinations of photoperiods, temperatures, and light levels. Growth was optimized at temperatures between 10 and 15 °C, light levels ≥110 μmol photons m−2 s−1, and ≥12 h of light in a day. It should be noted, however, that shading at high stocking densities would require an increase in light levels and that these levels need to be confirmed in a larger-scale trial. Because growth rates decrease over time under all conditions, we suggest a relatively short production cycle, which is consistent with previous commercial scale trials on Porphyra (Israel et al. 2006). Maximum phycobilin content was achieved at high light levels and short-day conditions, but keep in mind that the maximum light levels used in this study are lower than those in a greenhouse or open coastal aquaculture facility. Our data suggests that growing P. umbilicalis under low light (≤60 μmol photons m−2 s−1), neutral/long-day conditions will not only result in higher pigment content, but also in higher protein content, making the blades more suitable for either an aquaculture feed substitute or a human food product. Given that the growth rate under low light is significantly less than that at high light, we hypothesize that a “finishing off” period of 1–7 days prior to harvest when the light levels are decreased will result in increased protein and pigment content. Validation of this hypothesis requires further research. We hope that the results of this study will encourage potential growers to develop P. umbilicalis as a commercial aquaculture crop in the northeastern United States.
References
Blouin N (2010) Asexual reproduction in Porphyra umbilicalis Kützing (Rhodophyta) and its development for use in mariculture. PhD Thesis. University of Maine, School of Marine Sciences, USA pp. 150
Blouin NA, Brawley SH (2012) An AFLP-based test of clonality in widespread, putatively asexual populations of Porphyra umbilicalis (Rhodophyta) in the Northwest Atlantic with an in silico analysis for bacterial contamination. Mar Biol 159:2723–2729
Blouin N, Calder BL, Perkins B, Brawley SH (2006) Sensory and fatty acid analyses of two Atlantic species of Porphyra (Rhodophyta). J Appl Phycol 18:79–85
Blouin N, Xiugeng F, Peng J, Yarish C, Brawley SH (2007) Seeding nets with neutral spores of the red alga Porphyra umbilicalis (L.) Kützing for use in integrated multi-trophic aquaculture (IMTA). Aquaculture 270:77–91
Blouin NA, Brodie JA, Grossman AC, Xu P, Brawley SH (2011) Porphyra: a marine crop shaped by stress. Trends Plant Sci 16:29–37
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brodie J, Irvine LM (2003) Seaweeds of the British Isles, volume 1 Rhodophyta, part 3b Bangiophycidae. The National History Museum, London, p 167
Carmona R, Kraemer GP, Yarish C (2006) Exploring Northeast American and Asian species of Porphyra for use in an integrated finfish-algal aquaculture system. Aquaculture 252:54–65
Conover WJ, Iman RL (1981) Rank transformation as a bridge between parametric and nonparametric statistics. Am Stat 35:124–129
Cooper CS, Quails M (1967) Morphology and chlorophyll content of shade and sun leaves of two legumes. Crop Sci 7:672–673
Day JP (2008) The development of a modular integrated recirculating aquaculture system using Porphyra (nori) for the bioremediation of marine finfish effluent. PhD Thesis, University of New Hampshire, USA
Dongsansuk A, Lütz C, Neuner G (2013) Effects of temperature and irradiance on quantum yield of PSII photochemistry and xanthophyll cycle in a tropical and a temperate species. Photosynthetica 51:13–21
FAO (Food and Agricultural Organization of the United Nations) (2012) The State of the World Fisheries and Aquaculture. FAO Fisheries and Aquaculture Department, Rome
Federer WT, King F (2007) Variations on split plot and split block experimental designs. Wiley, Hoboken, p 270
Figueroa FL, Salles S, Aguilera J, Jimenez C, Mercado J, Vinegla B, Flores-Moya A, Altamirano M (1997) Effects of solar radiation of photoinhibition and pigmentation in the red alga Porphyra leucosticta. Mar Ecol Prog Ser 151:81–90
Fortes MD, Lüning K (1980) Growth rates of North Sea macroalgae in relation to temperature, irradiance and photoperiod. Helgol Meeresun 34:15–29
Gantt E (1975) Phycobilisomes: light-harvesting pigment complexes. Bioscience 25:781–788
Green LA, Neefus CD (2015) Effects of temperature, light level, photoperiod, and ammonium concentration on Pyropia leucosticta (Bangiales, Rhodophyta) from the Northwest Atlantic. J Appl Phycol 27:1253–1261
Herbert SK (1990) Photoinhibition resistance in the red alga Porphyra perforata. Plant Physiol 92:514–519
Hernández I, Corzo A, Gordillo FJ, Robles MD, Saez E, Fernández JA, Niell FX (1993) Seasonal cycle of the gametophytic form of Porphyra umbilicalis: nitrogen and carbon. Mar Ecol Prog Ser 99:301–311
Israel A (2010) The extreme environments of Porphyra, a fast growing and edible red marine macroalga. In: Seckbach J, Chapman DJ (eds) Red algae in the genomic age, cellular origins, life in extreme habitats and astrobiology, vol 13. Springer, NY, pp 61–75
Israel A, Levy I, Friedlander M (2006) Experimental tank cultivation of Porphyra in Israel. J Appl Phycol 18:235–240
Jahn W, Steinbiss K, Zetsche K (1984) Light intensity adaptation of the phycobiliprotein content of the red alga Porphyridium. Planta 161:536–539
Kim JK (2008) Mechanism of nitrogen assimilation of Porphyra from New England. Ph.D. Dissertation, University of Connecticut, USA
Kim JK, Kraemer GP, Neefus CD, Chung IK, Yarish C (2007) Effects of temperature and ammonium on growth, pigment production and nitrogen uptake by four species of Porphyra (Bangiales, Rhodophyta) native to the New England coast. J Appl Phycol 19:431–440
Kim JK, Kraemer GP, Yarish C (2013) Emersion induces nitrogen release and alteration of nitrogen metabolism in the intertidal genus Porphyra. PLoS One 8(7):e69961
Kokubu S, Nishihara N, Watanabe Y, Tsuchiya Y, Amamo Y, Terada R (2015) The effect of irradiance and temperature on the photosynthesis of a native alga Sargassum fusiforme (Fucales) from Kagoshima, Japan. Phycologia 54:235–247
Korbee N, Figueroa FL, Aguilera J (2005a) Effect of light quality on the accumulation of photosynthetic pigments, proteins and mycosporine-like amino acids in the red algae Porphyra leucosticta (Bangiales, Rhodophyta). J Photochem Photobiol B 80:71–78
Korbee N, Huovinen P, Figueroa FL, Aguilera J, Karsten U (2005b) Availability of ammonium influences photosynthesis and the accumulation of mycosporine-like amino acids in two Porphyra species (Bangiales, Rhodophyta). Mar Biol 146:645–654
Kraemer GP, Yarish C (1999) Preliminary comparison of the mariculture potential of Porphyra purpurea and Porphyra umbilicalis. J Appl Phycol 11:473–477
Kraemer GP, Carmona R, Chopin T, Neefus C, Tang X, Yarish C (2004) Evaluation of the bioremediatory potential of several species of the red alga Porphyra using short-term measurements of nitrogen uptake as a rapid bioassay. J Appl Phycol 16:489–497
Lichtenhaler HK (1987) Chlorophyll and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Mishkind M, Mauzerall D (1980) Kinetic evidence for common photosynthetic step in diverse seaweeds. Mar Biol 56:264–265
Mols-Mortensen A, Neefus CD, Nielsen R, Gunnarsson K, Egilsdóttir S, Pedersen PM, Brodie J (2012) New insights into the biodiversity and generic relationships of foliose Bangiales (Rhodophyta) in Iceland and the Faroe Islands. Eur J Phycol 47:146–159
Mols-Mortensen A, Neefus CD, Pedersen PM, Brodie J (2014) Diversity and distribution of foliose Bangiales (Rhodophyta) in West Greenland: a link between the North Atlantic and North Pacific. Eur J Phycol 49:1–10
Ott FD (1966) A selected listing of xenic cultures. Systematics-ecology program No. 72 (Mar. Biol. Lab., Woods Hole, MA), pp. 1–45
Pereira R, Yarish C (2010) The role of Porphyra in sustainable culture systems: physiology and applications. In: Israel A, Einav R (eds) Role of seaweeds in a globally changing environment. Springer, NY, pp 339–354
Pereira R, Yarish C, Critchley AT (2013) Seaweed aquaculture for human foods in land-based and IMTA systems. In: Christou P, Savin R, Costa-Pierce BA, Misztal I, Whitelaw BA (eds) Sustainable food production. Springer, NY, pp 1405–1424
Sampath-Wiley P, Neefus CD (2007) An improved method from estimating R-phycoerythrin and R-phycocyanin contents from crude aqueous extracts of Porphyra (Bangiales, Rhodophyta). J Appl Phycol 19:123–129
Sampath-Wiley P, Neefus CD, Jahnke LS (2008) Seasonal effects of sun exposure and emersion on intertidal seaweed physiology: fluctuations in antioxidant contents, photosynthetic pigments, and photosynthetic efficiency in the red alga Porphyra umbilicalis Kützing (Rhodophyta, Bangiales). J Exp Mar Biol Ecol 361:83–91
Shpigel M, Ragg NL, Lupatsch I, Neori A (1999) Protein content determines the nutritional value of the seaweed Ulva lactuca L. for the abalone Haliotis tuberculata L. and H. discus hannai Ino. J Shellfish Res 18:227–233
Thomas TE, Harrison PJ, Taylor EB (1985) Nitrogen uptake and growth of the germlings and mature thalli of Fucus distichus. Mar Biol 84:267–274
Waaland JR, Waaland SD, Bates G (1974) Chloroplast structure and pigment composition in the red alga Griffithsia pacifica: regulation by light intensity. J Phycol 10:193–199
Walker AB, Fournier HR, Neefus CD, Nardi GC, Berlinsky DL (2009) Partial replacement of fish meal with laver Porphyra spp. in diets for Atlantic cod. Aquaculture 71:39–45
Watanabe Y, Nishihara GN, Tokunaga S, Terada R (2014) Effect of irradiance and temperature on the photosynthesis of a cultivated red alga, Pyropia tenera (= Porphyra tenera), at the southern limit of distribution in Japan. Phycol Res 62:187–196
Wilson RP (2002) Amino acids and proteins. In: Halver JE, Hardy RW (eds) Fish nutrition, 3rd edn. Academic, San Diego, pp 144–175
Acknowledgments
We would like to acknowledge Drs. Leland Jahnke, Arthur Mathieson, Charles Yarish, and David Berlinsky, and two anonymous reviewers for their valuable feedback on this manuscript. Partial funding was provided by the New Hampshire Agricultural Experiment Station. This is Scientific Contribution Number 2627. This work was supported by the USDA National Institute of Food and Agriculture Hatch Project 223365. This research was also funded by a grant from New Hampshire Sea Grant (R/CFR-14, C.D. Neefus).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Green, L.A., Neefus, C.D. Effects of temperature, light level, and photoperiod on the physiology of Porphyra umbilicalis Kützing from the Northwest Atlantic, a candidate for aquaculture. J Appl Phycol 28, 1815–1826 (2016). https://doi.org/10.1007/s10811-015-0702-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-015-0702-6