Abstract
Age and growth of crimson sea bream Evynnis tumifrons were studied using samples collected from October 1999 to August 2013 off the southwestern coast of Kyushu, Japan. Ring marks (outer edges of opaque zones) on the 1805 transversely sectioned otoliths were counted and seasonality in their deposition was validated by marginal increment. Our results revealed that one ring mark was formed per year from late spring to early summer. Assuming December as the birth month, ages were assigned to every individual according to the number of ring marks and the value of marginal increments. Growth was estimated by fitting the von Bertalanffy growth function to the length-at-age and weight-at-age data. The estimated growth curves did not differ significantly between the sexes, and the growth curve of the pooled data was \(L_{\text{t}} = 271\left( {1 - { \exp }\left( { - 0.604\left( {t + 0.193} \right)} \right)} \right)\) for length-at-age and \(W_{\text{t}} = 519\left( {1 - { \exp }\left( { - 0.484\left( {t + 0.625} \right)} \right)} \right)^{3}\) for weight-at-age. The maximum age observed was 15 years for females and 16 years for males.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The sea breams of Sparidae are excellent food fish and are of high commercial importance (Iwatsuki 2009). This family belongs to the perch-like fishes (Perciformes) that comprise more than 100 species (Boufersaoui et al. 2018). Approximately 13 sea bream species are distributed in the coastal waters of Japan (Hayashi and Hagiwara 2013); one of these is the crimson sea bream Evynnis tumifrons. This species is endemic to the coastal waters of China, Hong Kong, Japan, South Korea and Taiwan (Akazaki 1962, 1984; Iwatsuki et al. 2007, 2014), and is found on the rocky reefs, gravel and sandy bottoms of the continental shelves (Hayashi and Hagiwara 2013). It feeds on a wide range of benthic invertebrates and fishes (Iwatsuki et al. 2014), and it was reported that its bathymetrical distribution varies according to the ontogenetic development, where the juveniles are commonly found at shallow depths (around 10 m) and the adult stages are in the deeper waters (50–100 m).
In Japan, E. tumifrons is a commercially important fish species (Kudoh and Yamaoka 2004) and is caught mainly by gill nets, surrounding seine nets and angling. Its age and growth have been studied in areas off Akita, Niigata, Fukuoka, Ibaraki, and Kochi and Miyazaki (Yamada et al. 2007). These localities are widely distributed from the northern to southern regions of Japan, but the southwestern region is not included.
Furthermore, the above-mentioned studies determined age and growth of E. tumifrons using scales, while some studies have criticized the use of scales due to the underestimation of ages in old fish by this character (Beamish and McFarlane 1983; Carlander 1987). On the other hand, transversely sectioned otoliths have been recommended as the age determination character because otoliths continue to grow towards the internal (proximal) side as fish age (Beamish and McFarlane 1983; Casselman 1987; Abecasis et al. 2008) and annuli on transversely sectioned otoliths are clearly exhibited (Masuda and Noro 2003; Masuda et al. 2003; Lee et al. 2009; Piddocke et al. 2015). This age determination character has been applied to many fish species such as Platycephalus indicus (Masuda et al. 2000), Rhabdosargus sarba (Radebe et al. 2002), Nemipterus bathybius (Granada et al. 2004), Sillago aeolus (Rahman and Tachihara 2005), Gerres equulus (Iqbal et al. 2006), Lutjanus fulviflammus (Shimose et al. 2009), Pseudopleuronectes yokohamae (Lee et al. 2009), Mora mora and Epigonus telescopus (Vieira et al. 2013), Scolopsis monogramma (Akita and Tachihara 2014) and Trachurus japonica (Yoda et al. 2014), but has not yet been applied to E. tumifrons. Hence, existing knowledge on the life history characteristics of E. tumifrons is insufficient.
The aim of the present study is to describe the age and growth of E. tumifrons using transversely sectioned otolith for the first time with samples collected off the southwestern coast of Kyushu, Japan. The results derived are indispensable for the development of measures to improve the management of this commercially important fish species.
Materials and methods
Sampling and measurement
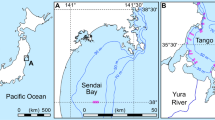
Samples of E. tumifrons were mainly collected at Eguchi Fisheries Cooperative, Hioki city, Kagoshima Prefecture, southern Japan, from April 2012 to August 2013. The fishers belonging to this cooperative caught the E. tumifrons using gill nets and surrounding seine nets in the area of 31°33′–31°39′ N and 130°13′–130°20′ E off Hioki city on the southwestern coast of Kyushu, Japan (Fig. 1a). After the fish were landed, the cooperative’s staff sorted them into eight different weight categories. We sampled specimens of various body sizes from these categories once a month, and a total of 1599 specimens (794 females and 805 males) were collected. In addition, to compensate for the insufficient number of the small-sized individuals collected at Eguchi Fisheries Cooperative, a total of 206 (73 females, 57 males and 76 sexually unknown) small fish caught by set net off Kasasa town (Fig. 1b) in 2004 and with Danish seine and gill nets off Ibusuki city (Fig. 1c) during the period from 1999 to 2004 were used for the analysis.
Fish collected were immediately chilled in ice and transported to the Laboratory of the Fisheries Biology of Faculty of Fisheries, Kagoshima University. Fish were measured for fork length (FL) on the measuring board to the nearest 1 mm and body weight (BW) on the electronic balance (UX6200H, Shimadzu, Kyoto, Japan) to the nearest 0.01 g, and then sexed through visual observation of the gonads.
Preparation of transversely sectioned otoliths
Otoliths were extracted from the fish head, washed with tap water and kept in a multi-well plate in a dried condition. The left otoliths were embedded in epoxy resin and transversely cut into 2-mm-thick sections through the focus using a micro-cutter (type MC-201, Maruto, Tokyo, Japan). The sectioned otoliths were polished using a grinder (type 9820, Makita, Tokyo, Japan) towards the focus, leaving a consequent thickness of 0.25 mm. The polished otoliths were then mounted on a slide glass with nail enamel (Masuda and Noro 2003; Granada et al. 2004; Iqbal et al. 2006).
Counting and validation of ring marks
The number of ring marks (outer edges of the opaque zones) on sectioned otoliths was counted from the focus to the dorsal tip of the sulcus acusticus using a microscope (Leica MZ 12.5, Leica Microsystems, Heerbrugg, Switzerland) under a transmitted light at 32× magnification (Fig. 2a). Otoliths that were difficult to count were excluded from the analysis.
a Counting and measuring area (within a rectangle) of ring marks on sectioned otolith of Evynnis tumifrons. F focus, S sulcus acusticus, V ventral side, D dorsal side. Open triangles indicate ring marks. b Ring marks (open triangles) on transverse sections of otoliths of the four representative specimens of Evynnis tumifrons. b1 135 mm fork length (FL) male with one ring mark; b2, 221 mm FL female with three ring marks; b3 260 mm FL male with five ring marks; b4, 255 mm FL female with seven ring marks. Bar = 1 mm
To validate the seasonality in the deposition of ring marks (outer edge of opaque zones) in sectioned otoliths, the marginal increment (MI), i.e., the distance between the outer edge of the outermost opaque zone and the periphery, was measured at 32× magnification and expressed as either (1) a proportion of the distance between the focus and the outer edge of the opaque zone when only one opaque zone was present, or (2) a proportion of the distance between the outer edges of the two outermost opaque zones when two or more opaque zones were present.
Assignment of age
Monthly changes in the gonadosomatic index and the occurrence of the mature or spawned females collected off the southwestern coast of Kyushu, Japan have shown that the spawning season lasts from November to May, with a peak in November or December (Havimana et al. 2020). Assuming that the birth month was December based on this result, ages were assigned to every individual according to the number of ring marks, the degree of MI values (i.e., high or low) and the time elapsed from the birth month to the capture month. For example, for the fish captured in June (6 months after December), an age of 0.5 (= 6/12) years was assigned to individuals with one new ring mark of low MI value or no ring mark, while for the individuals with a second new ring mark of low MI value or one old ring mark of high MI value, an age of 1.5 (= 1 + 6/12) years was assigned. The same assignment of age was applied for older fish. In the following month of July, 0.08 (= 1/12) was added to the age (Masuda et al. 2003).
Growth analysis
The 76 individuals whose sex was unknown were alternatively treated as female or male under the assumption of a sex ratio of 1:1, and were incorporated into sexually known female or male data. The von Bertalanffy growth equation was fitted to the length-at-age and weight-at-age data based on the least-squares method using the curve-fitting function of the computer software (DeltaGraph 7, Salt Lake City, UT, USA). The equation was expressed as \(L_{\text{t}} = L_{\infty } ( {1 - { \exp }( { - K( {t - t_{0} } )} )} )\;{\text{for length \,\,and}}\,\, W_{\text{t}} = W_{\infty } (1 - \exp ( { - K( {t - t_{0} } ))} )^{3} \;{\text{for weight}},\) where Lt and Wt are the fork length (mm) and body weight (g) at age t (year), L∞ and W∞ are the asymptotic fork length and body weight, K is the growth coefficient and t0 is the hypothetical age when length or weight will be zero.
The F test was conducted to compare the growth curves between the sexes using the formula provided by Akamine (2010):
where Sp is the residual sum of squares (RSS) for both sexes (pooled data), Sf is the RSS for females, Sm is the RSS for males, nf is the sample size of females, nm is the sample size of males and r is the number of parameters.
Results
Ring formation period
Clear opaque and translucent zones were observed alternately from the core region to the terminal edge of the transversely sectioned otoliths (Fig. 2b). The opaque zones of E. tumifrons were classed as type A as described by Katayama (2018), and the distance between the ring marks (outer edge of the opaque zones) appeared to be smaller from the focus towards the otolith’s margin. A total of 1790 out of 1805 (99.2%) otoliths had countable ring marks; thus, the transversely sectioned otolith was regarded as a good calcified structure from which to estimate the age of E. tumifrons.
The monthly changes in marginal increment (MI) were analyzed to estimate the ring formation period. The occurrence of low MI values suggested that the new ring marks were recently formed, and high values suggested that new ring marks were not yet formed. The period of coexistence of low and high MI values was therefore considered as the ring formation period. Looking at the results for females (Fig. 3a), the coexistence period of low and high MI values was observed in June in the one-ring group and from May to July in the two-ring group. In the three-, four- and 5–15-ring groups, it was from June to July. For males (Fig. 3b), a similar trend was observed in June in the one-ring group, from May to July in the two-ring group, from June to July in the three-ring group, and in July in the four-ring group. Based on this information, rings were estimated to form from May to July and were considered as annuli in E. tumifrons.
Length–frequency distribution
The monthly changes in length–frequency distribution by age for both sexes are shown in Fig. 4. Assuming that the birth month was December, monthly occurrences of the assigned ages (0–6 ≤ years old) against the fork length were demonstrated. Clear peaks were observed in the young age groups of 0, 1 and 2 years but not in age ≥ 3 years. The 0-year-old fish first appeared in March, with fork length ranging from 36 to 46 mm. In December, they turned 1 year old, with FL ranging from 107 to 143 mm, and became the major component of the catches in April and subsequent months. In the following December, these 1-year-old fish turned 2 years old, and FL ranged from 180 to 233 mm; this transition of age continues throughout the life of the fish.
Growth
The von Bertalanffy growth function estimated from the length-at-age of the females was \(L_{\text{t}} = 272\left( {1 - \exp \left( { - 0.599\left( {t + 0.189} \right)} \right)} \right)\) (n = 896, r2 = 0.907) and that of the males was \(L_{\text{t}} = 270\left( {1 - \exp \left( { - 0.610\left( {t + 0.197} \right)} \right)} \right)\) (n = 894, r2 = 0.909), with no significant difference between the sexes (P > 0.05). For the body weight-at-age, the estimate was \(W_{\text{t}} = 527\left( {1 - { \exp }\left( { - 0.479\left( {t + 0.621} \right)} \right)} \right)^{3}\) (n = 896, r2 = 0.856) for the females and \(W_{\text{t}} = 509\left( {1 - { \exp }\left( { - 0.493\left( {t + 0.618} \right)} \right)} \right)^{3}\) (n = 894, r2 = 0.852) for the males, with no significant difference between the sexes (P > 0.05). Hence, the von Bertalanffy growth function of the pooled data of the length-at-age was \(L_{\text{t}} = 271\left( {1 - \exp \left( { - 0.604\left( {t + 0.193} \right)} \right)} \right)\) (n = 1790, r2 = 0.862) (Fig. 5a) and that of the weight-at-age was \(W_{\text{t}} = 519\left( {1 - { \exp }\left( { - 0.484\left( {t + 0.625} \right)} \right)} \right)^{3}\) (n = 1790, r2 = 0.854) (Fig. 5b). The maximum age of female fish was 15 years (fork length = 310 mm; body weight = 523 g) and that of male fish was 16 years (fork length = 276 mm; body weight = 445 g).
Discussion
Age estimation in sparid fish is complicated due to the phenomenon of stacking of ring marks towards the otolith’s margin, particularly in older fish (Van der Walt and Beckley 1997; Pajuelo and Lorenzo 2002). However, in E. tumifrons, clear ring marks on the internal (proximal) side of the sectioned otoliths enabled age determination with relative ease (99.2% of the otoliths were countable). The oldest age in the present study was 16 years, and the stacking phenomenon was not observed.
To confidently assign the age from the count of ring marks (outer edges of opaque zones) on the transversely sectioned otoliths, the periodicity of the increment formation must be determined (Beamish and McFarlane 1983; Campana 2001; Piddocke et al. 2015), and this is typically done through the analysis of monthly changes in the marginal increment. Our results revealed that one ring mark was deposited each year in the sagittal otolith, owing to the alternating period of fast and slow growth (Hou et al. 2008). Therefore, each ring mark was confirmed to be an annulus with a formation period that lasted from late spring to early summer season (Fig. 3a, b). The accuracy and reliability of an annulus on transversely sectioned otoliths has been demonstrated in Pagrus auratus, where young individuals captured in the wild were injected with tetracycline and were reared on a natural food in a large pool with flowing seawater under ambient marine conditions (Ferrell et al. 1992). An opaque zone was observed between July and October in the two successive years of the investigation. In Rhabdosargus holubi, individuals were captured in the wild, tagged and injected with oxytetracycline, and later released into an artificial saltwater impoundment that contained seawater pumped from the inshore surf zone (Farthing et al. 2016). The study reported that the two individuals recaptured showed one opaque zone deposited annually on the otoliths. In the flathead Platycephalus indicus, annuli counts from the cultivated fish were concordant with their known ages (Masuda et al. 2000).
The FLs calculated at each age of E. tumifrons in different coastal waters of Japan are shown in Fig. 6. The length at age 1 year in the present study, conducted in the southernmost location off the southwestern coast of Kyushu, Japan (latitude, 31º33′–31º39′ N), was 139 mm FL, and this estimate appears to be the largest when compared with those of previous studies. A trend was also realized wherein the size of the young E. tumifrons decreased with increasing latitudinal area. In the coastal waters of southern Japan, fish size at age 1 was 107 mm FL off Fukuoka Prefecture (latitude, 33°50′ N) and 132 mm FL off Kochi and Miyazaki Prefectures (latitudes, 33º20′ N and 32º10′N). In the northernmost areas, off Akita Prefecture (approximate latitude, 39º39′N), it was 52 mm FL, off Niigata Prefecture (latitude, 37º50′ N) it was 69 mm FL, and off Ibaraki Prefecture (latitude, 36º20′ N) it was 109 mm FL. Based on these observations, the growth of fish at an early age differs according to the populations found across the coastal waters of Japan. Ochiai and Tanaka (1986) observed a similar phenomenon in Pagrus major, a related red sea bream in the coastal waters of Japan, where initial growth was highest in the southern waters of Kagoshima Bay (Kagoshima Prefecture), followed by Kii Channel (Tokushima and Wakayama Prefectures) and Kitakyushu (Fukuoka Prefecture), and that in the Sea of Japan, while that in the Seto Inland Sea was generally low. The authors reported that the factor responsible for this variation is the difference in water temperature among the localities, where warmer temperatures favor a high initial growth. One-year-old butterfish Odax pullus in the coastal waters of New Zealand showed a similar phenomenon, where growth was higher in the Hauraki Gulf of northern New Zealand (latitude, 36.3º S) than in the Stewart Islands of southern New Zealand (latitude, 47º S). In Odax pullus, the factor responsible for the variation in growth was the water temperature difference between the two localities (Trip et al. 2014). Water temperature differences over latitudinal gradients has been reported as one of the major factors influencing growth in fish (Conover 1992; Trip et al. 2014). Our results revealed that the fork lengths in 1-year-old E. tumifrons decreased with an increase in latitude. Hence, the observed variations in the growth of the young fish are likely caused by water temperature differences. However, other factors such as feeding regime and reproductive cycle are possible influences as well, and to elucidate the degrees to which these factors contribute to these changes, future laboratory experimental work in these populations will be needed.
Comparison of age–FL relationships of Evynnis tumifrons at six different localities in Japan. Data for the previous studies were cited from Yamada et al. (2007)
The steepness of the curve (growth rate) after the age of 1 year also appeared to differ among localities (Fig. 6). The least steep curve was obtained in the present study, followed by that of Niigata Prefecture, Ibaraki Prefecture, Akita Prefecture, Fukuoka Prefecture, and Kochi and Miyazaki Prefectures. These differences might be attributed to the estimated maximum ages, i.e., steepness of the curve tended to be inversely proportional to the maximum age. The maximum age in the present study (16 years) is 7 years older than that reported in Akita and Niigata Prefectures (Yamada et al. 2007). In the present study, we used the transversely sectioned otoliths, while scales were used in the previous studies. Some researchers have criticized the use of scales, because they cease to grow as the fish ages (Beamish and McFarlane 1983; Casselman 1987), and the age of old fish is frequently underestimated (Beamish and McFarlane 1987; Carlander 1987). The superiority of transversely sectioned to whole otolith or other aging structures has been demonstrated as well (e.g., Beamish 1979; Erickson 1983; Hyndes et al. 1992; Masuda and Noro 2003; Masuda et al. 2003). Hence, previous studies on E. tumifrons, which used scales for age determination, possibly underestimated the maximum age, and this may be one of the factors that caused the differences in steepness of the growth curves between localities.
In the present study, we aimed to promote a better understanding of age and growth of E. tumifrons off the southwestern coast of Kyushu, Japan using transversely sectioned otoliths, and to validate the periodicity of ring marks deposited on the otoliths. The accuracy and reliability of age information is important for proper stock assessment and management of any commercially exploited species. Our findings, which revealed the possibility of the underestimation of age by previous studies, may now challenge the authenticity of any existing management measures targeting this species. The finding that initial growth at a young age (1 year) varied in each population distributed across the coastal waters of Japan is interesting from the viewpoint of life strategy, because it suggests that growth parameters and size at sexual maturity are likely to vary among the populations. It is therefore of paramount importance that any existing management measures are updated in pursuit of sustainable management of the resource, taking into account the species biology and the anthropogenic factors impacting each of the different geographical populations.
References
Abecasis D, Bentes L, Coelho R, Correia C, Lino PG, Monteiro P, Goncalves JMS, Ribeiro J, Erzini K (2008) Ageing seabreams: a comparative study between scales and otoliths. Fish Res 89:37–48
Akamine T (2010) Introduction to data analysis for fisheries resources. Kouseisha-kouseikaku Corp, Tokyo (in Japanese)
Akazaki M (1962) Studies on the spariform fishes. Anatomy, phylogeny, ecology and taxonomy, vol 1. Misaki Marine Biology Institute, Kyoto University Special Report, pp 1–368 (in Japanese with English abstract)
Akazaki M (1984) Sparidae. In: Masuda H et al (eds) The fishes of Japanese Archipelago, English edn. Tokai University Press, Tokyo, pp 170–171
Akita Y, Tachihara K (2014) Age, growth, maturity and sex changes of monogrammed monocle bream Scolopsis monogramma in the waters around Okinawa-jima Island, Japan. Fish Sci 80:679–685
Beamish RJ (1979) Differences in the age of Pacific hake Merluccius productus using whole otoliths and sections of otoliths. J Fish Res Board Can 36:141–151
Beamish R, Mcfarlane G (1983) The forgotten requirement for age validation in fisheries biology. Trans Am Fish Soc 112:735–743
Beamish RJ, McFarlane GA (1987) Current trends in age determination methodology. In: Summerfelt RC, Hall GE (eds) Age and growth of fish. Iowa State University Press, Ames, pp 15–42
Boufersaoui S, Kassar A, Mokrane Z, Elleboode R, Mahe K (2018) Age and growth of the striped seabream, Lithognathus mormyrus (Actinopterygii: Perciformes: Sparidae), in the central coast of Algeria, Mediterranean sea. Acta Ichthyol Piscat 48:319–328
Campana SE (2001) Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. J Fish Biol 59:197–242
Carlander KD (1987) A history of scale age and growth studies of northern American freshwater fish. In: Summerfelt RC, Hall GE (eds) Age and growth of fish. Iowa State University Press, Ames, pp 3–14
Casselman JM (1987) Determination of age and growth. In: Weatherley AH, Gill HS (eds) The biology of fish growth. Academic Press, London, pp 209–242
Conover DO (1992) Seasonality and the scheduling of life history at different latitudes. J Fish Biol 41:161–178
Erickson CM (1983) Age determination of Manitoban walleyes using otoliths, dorsal spines, and scales. N Am J Fish Manag 3:176–181
Farthing MW, James NC, Potts WM (2016) Age and growth of cape stumpnose Rhabdosargus holubi (Pisces: Sparidae) in the eastern Cape, South Africa. Afr J Mar Sci 38:65–71
Ferrell DJ, Henry GW, Bell JD, Quartararo N (1992) Validation of annual marks in the otoliths of young snapper, Pagrus auratus (Sparidae). Aust J Mar Freshw Res 43:1051–1055
Granada PV, Masuda Y, Matsuoka T (2004) Age and growth of the yellowbelly threadfin bream Nemipterus bathybius in Kagoshima Bay, southern Japan. Fish Sci 70:497–506
Havimana L, Ohtomi J, Masuda Y, Vazquez Archdale M (2020) The reproductive biology of female crimson sea bream Evynnis tumifrons off the southwestern coast of Kyushu. Japan. Fish Sci 86:65–75
Hayashi M, Hagiwara K (2013) Sparidae. In: Nakabo T (ed) Fishes of Japan with pictorial keys to the species. Tokai University Press, Tokyo, pp 955–959 (in Japanese)
Hou G, Feng B, Lu H, Zhu J (2008) Age and growth characteristics of crimson sea bream Paragyrops edita Tanaka in Beibu Gulf. J Ocean Univ China 7:457–465
Hyndes GA, Loneragan NR, Potter IC (1992) Influences of sectioning otoliths on marginal increment trends and age and growth estimates for the flathead Platycephalus speculator. Fish Bull 90:276–284
Iqbal KM, Masuda Y, Suzuki H, Shinomiya A (2006) Age and growth of the Japanese silver-biddy Gerres equulus, in western Kyushu, Japan. Fish Res 77:45–52
Iwatsuki Y (2009) Sparidae. In: Kimura S et al (eds) Fishes of Andaman Sea West coast of southern Thailand. Natl Mus Nat Sci, Tokyo, pp 165–166
Iwatsuki Y, Akazaki M, Taniguchi N (2007) Review of the species of the genus Dentex (Perciformes: Sparidae) in the western Pacific defined as the D. hypselosomus complex with the description of a new species, Dentex abei and a redescription of Evynnis tumifrons. Bull Natl Mu Nat Sci Ser A Supply 1:22–49
Iwatsuki Y, Russell B, Pollard D, Mann BQ, Carpenter KE, Buxton CD, Shao K, Liao W. (2014) Evynnis tumifrons. The IUCN red list of threatened species 2014: e.T1702212A1294201. http://dx.doi.org/10.2305/IUCN.UK.2014-3.RLTS.T170212A1294201.en. Accessed 25 Apr 2016
Katayama S (2018) A description of four types of otolith opaque zone. Fish Sci 84:735–745
Kudoh T, Yamaoka K (2004) Territorial behavior in juvenile red sea bream Pagrus major and crimson sea bream Evynnis japonica. Fish Sci 70:241–246
Lee JH, Kodama K, Kume G, Oyama M, Katayama S, Takao Y, Horiguchi T (2009) Comparison between surface-reading and cross-section methods using sagittal otolith for age determination of the marbled sole Pseudopleuronectes yokohamae. Fish Sci 75:379–385
Masuda Y, Noro T (2003) Recommendation of ageing fish with transverse sections of otoliths. Mem Fac Fish Kagoshima Univ 52:51–56 (in Japanese with English abstract)
Masuda Y, Ozawa T, Onoue S, Hamada T (2000) Age and growth of the flathead Platycephalus indicus from the coastal waters of west Kyushu, Japan. Fish Res 46:113–121
Masuda Y, Sako T, Matsushita G, Shiraishi T, Kirizushi J, Kamimura Y, Ozawa T (2003) Age, growth and year-class composition of brown barracuda Sphyraena pinquis in Kagoshima Bay, southern Japan. Nippon Suisan Gakkaishi 69:709–716 (in Japanese with English abstract)
Ochiai A, Tanaka M (1986) Pagrus major (Temmink et Schlegel). Ichthyology, vol 2. Kouseisya-kouseikaku Corp, Tokyo, pp 736–750 (in Japanese)
Pajuelo JG, Lorenzo JM (2002) Growth and age estimation of Diplodus sargus cadenati (Sparidae) off the Canary Islands. Fish Res 59:93–100
Piddocke TP, Butler GL, Butcher PA, Purcell SW, Bucher DJ, Christidis L (2015) Age validation in the Lutjanidae: a review. Fish Res 167:48–63
Radebe PV, Mann BQ, Beckley LE, Govender A (2002) Age and growth of Rhabdosargus sarba (Pisces: Sparidae), from KwaZulu-Natal, South Africa. Fish Res 58:193–201
Rahman H, Tachihara K (2005) Age and growth of Sillago aeolus in Okinawa Island, Japan. J Oceanogr 61:569–573
Shimose T, Tanabe T, Chen KS, Hsu CC (2009) Age determination and growth of Pacific bluefin tuna Thunnus orientalis off Japan and Taiwan. Fish Res 100:134–139
Trip EDL, Clements KD, Raubenheimer D, Choat JH (2014) Temperature-related variation in growth rate, size, maturation and life span in a marine herbivorous fish over a latitudinal gradient. J Anim Ecol 83:866–875
Van der Walt BA, Beckley LE (1997) Age and growth of Sarpa salpa (Pisces: Sparidae) off the east coast of South Africa. Fish Res 31:241–248
Vieira AR, Figueiredo I, Figueiredo C, Menezes GM (2013) Age and growth of two deep-water fish species in the Azores Archipelago: Mora moro (Risso, 1810) and Epigonus telescopus (Risso, 1810). Deep Sea Res Part 2 Top Stud Oceanogr 98:148–159
Yamada U, Tokimura M, Horikawa H, Nakabo T (2007) Evynnis japonica Tanaka. Fishes and Fisheries of the East China and Yellow Seas. Tokai Univeristy Press, Tokyo, pp 753–758 (in Japanese)
Yoda M, Shiraishi T, Yukami R, Ohshimo S (2014) Age and maturation of jack mackerel Trachurus japonicus in the East China Sea. Fish Sci 80:61–68
Acknowledgements
We would like to acknowledge the tireless work and cooperation of the commercial fishers and staff of Eguchi, Kasasa and Ibusuki Fisheries Cooperatives and the students of the Laboratory of Fisheries Biology of the Faculty of Fisheries, Kagoshima University, during the collection and measuring of the samples. Our sincere gratitude is also rendered to Mr. Mitsuhiro Nakamura for his assistance in making the otolith sections.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Havimana, L., Ohtomi, J., Masuda, Y. et al. Age and growth of crimson sea bream Evynnis tumifrons off the southwestern coast of Kyushu, Japan. Fish Sci 86, 319–327 (2020). https://doi.org/10.1007/s12562-020-01399-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-020-01399-0