Abstract
Ovarian maturation, size at sexual maturity and the annual reproductive cycle of female crimson sea bream Evynnis tumifrons were studied using samples collected from April 2012 to June 2014 off the southwestern coast of Kyushu, Japan. A total of 801 ovaries were examined histologically to estimate the degree of ovarian maturation. E. tumifrons showed an asynchronous ovary signifying multiple spawning in a single reproductive season. Ovarian maturity stages were classified into six categories based on the appearance of the most advanced oocytes, post-ovulatory follicles and atretic oocytes in the ovary as follows: immature, maturing, mature, spawned, spent and resting. Females with ovaries at maturing, mature, spawned or spent stages were defined as sexually mature individuals, and the size at sexual maturity was estimated to be 179-mm fork length based on 50% maturity size. Monthly changes in the gonadosomatic index and the occurrence of mature or spawned maturity stages showed that the spawning season lasts from November to May with an intermission in March 2013. The factor responsible for this intermission was considered to be the low water temperature that occurred in the preceding month.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The crimson sea bream Evynnis tumifrons (Sparidae) is distributed in the coastal waters of China, Hong Kong, Japan, South Korea and Taiwan (Akazaki 1962, 1984; Youn 2002; Iwatsuki et al. 2007, 2014). This species inhabits rocky reefs, gravel and sandy bottoms on continental shelves (Hayashi 2002). Its bathymetrical distribution is determined by its ontogenetic development where juveniles are commonly found at shallow depths (~ 10 m) and adult stages mostly in deeper waters (50–100 m) (Iwatsuki et al. 2014).
Evynnis tumifrons is a commercially important fish species in the coastal waters of Japan (Kudoh and Yamaoka 2004), and its population biology has been studied in various localities (prefectures). Mio (1961, 1962) reported the age and growth, and spawning season and fecundity, of this species off Fukuoka. Toriyama et al. (1975) estimated the distribution and spawning season of E. tumifrons, and Toriyama and Kudo (1976) determined its age and growth off Kochi and Miyazaki. Yamahora (1983) compared the life history of E. tumifrons and Pagrus major off Yamagata. In addition, Shibuya (1985) studied the age and growth of E. tumifrons off Akita, while Anzawa et al. (1987) examined its spawning season and age and growth off Niigata.
Among these biological studies, those on the reproductive biology of the species are important for the estimation of the reproductive potential of stock and for the construction of appropriate management models for sustainable harvesting (Farley et al. 2015). The reproductive strategies of a fish also play a major role in its geographical distribution and population dynamics. Therefore, reliable information and sufficient understanding of a fish’s reproduction are essential. In previous studies, however, the spawning season of E. tumifrons was estimated mainly from monthly changes in its gonadosomatic index (GSI), and its size at sexual maturity has only been examined in Niigata Prefecture (Anzawa et al. 1987). Some studies have suggested that the estimation of the reproductive characteristics of a fish can be improved through histological observation of its ovaries (West 1990; Tyler and Sumpter 1996; Blazer 2002; Alejo-Plata et al. 2011). Histological techniques have been used successfully in other related species, e.g. Pagrus major, to determine the diurnal rhythm of oocyte development during the spawning season (Matsuyama et al. 1988), and e.g. in Dentex hypselosomus, to clarify gonadal development and the annual and/or diurnal reproductive cycle (Oki and Tabeta 1998; Tominaga et al. 2005; Yoda and Yoneda 2009), but up to now histological techniques have been infrequently used to determine the fecundity E. tumifrons (Mio 1962).
Furthermore, the localities of previous studies range widely from the northern to southern regions of Japan, but did not include the southwestern region, where E. tumifrons is targeted by a variety of commercial fisheries, e.g. using gillnets, surrounding seine nets and angling. In addition to these limitations, the duration of the spawning season, the percentage of females spawning per day (spawning frequency) and the number of oocytes per spawning (batch fecundity), which are essential for the estimation of the annual fecundity of a multi-spawning fish with indeterminate fecundity (Yoda and Yoneda 2009), should be determined so that appropriate and timely management approaches can be established to protect sexually mature populations of E. tumifrons and to allow a sufficient proportion of sexually mature fish to spawn.
The focus of the present study is on the elucidation of the spawning season of E. tumifrons. Hence, the objectives of this study were to describe ovarian maturation, size at sexual maturity and the annual reproductive cycle of female E. tumifrons off the southwestern coast of Kyushu, Japan using an histological approach. The obtained results are indispensable for the estimation of the reproductive potential of this species and for the development of appropriate management measures.
Materials and methods
Sampling and measurement
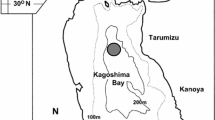
Monthly sampling of E. tumifrons was conducted at Eguchi Fishermen’s Cooperative, Hioki City, Kagoshima Prefecture, southern Japan from April 2012 to June 2014. Fishers belonging to this cooperative caught E. tumifrons using gillnets and surrounding seine nets from 31°33′ to 31°39′N and from 130°13′ to 130°20′E off Hioki City, on the southwestern coast of Kyushu, Japan (Fig. 1). After landing, fish were sorted into eight categories according to their weight by the cooperative’s staff. We sampled specimens of various size from these categories once a month; a total of 1101 females were collected. Fish specimens were immediately chilled on ice and taken to the Laboratory of Fisheries Biology of Faculty of Fisheries, Kagoshima University.
For each specimen, fork length (FL) was measured on a measuring board to the nearest 1 mm, body weight (BW) on an electronic balance (UX6200H; Shimadzu) to the nearest 0.01 g and ovarian weight (OW) on an electronic balance (UX620H; Shimadzu) to the nearest 0.001 g. Ovaries were fixed in 10% formalin until they were histologically examined. The GSI was calculated as:
Histological analysis
A total of 801 ovaries were examined histologically to estimate the degree of ovarian maturation. A small piece of tissue was taken from the middle part of a female’s gonad, embedded in paraffin, sectioned into 6-μm thickness, and stained with Mayer’s haematoxylin and eosin. The developmental stages of oocytes were classified according to Yamamoto (1956) and Granada et al. (2004) with a few modification for this species, and the histological classification of atretic oocytes and post-ovulatory follicles followed Hunter and Macewicz (1985). Ovarian maturity stages were estimated from the appearance of the most advanced oocytes, post-ovulatory follicles and atretic oocytes (Granada et al. 2004; Alejo-Plata et al. 2011; Sun et al. 2015; Okochi et al. 2016).
Size at sexual maturity
In the present study, the FL at which 50% of females are sexually mature (FL50) was estimated as the mean length at sexual maturity (King 2007). Sexually mature individuals were defined as females with ovaries at maturing, mature, spawned or spent stages which had yolked oocytes. The percentage of sexually mature females (PMF) was plotted against FL and size at 50% maturity was estimated by fitting a logistic function based on the least-squares method using the software DeltaGraph 7 (Red Rock Software, Salt Lake City, UT). The logistic equation was expressed as:
where r is slope of the curve and FLm is the median value of each FL class.
Annual reproductive cycle
Annual reproductive cycle was analysed from the monthly changes in the GSI and the occurrence of each ovarian maturity stage. Fish smaller than the minimum size of the sexually mature females were excluded from this analysis.
Condition factor
The monthly change in condition factor (CF) was analysed to examine the seasonal change of the body condition of the fish throughout the year. The formula used was based on Zhu et al. (1989):
where BW is in grams, OW is in grams and FL is in millimetres.
Results
Developmental stages of oocytes
The following developmental stages of the oocytes were observed. Peri-nucleolus stage (Fig. 2a): the nucleus is relatively large and multiple nucleoli can be seen around its periphery. The cytoplasm is basophilic and stained dark blue by hematoxylin. Oocyte diameter ranges from 60 to 120 µm. Pre-vitellogenic stage (Fig. 2b): yolk vesicle- or oil droplet-like granules appear in the cytoplasm and gradually increase in number and size. The cytoplasm is basophilic and oocyte diameter ranges from 90 to 275 µm. Early yolk globule stage (Fig. 2c): small acidophilic yolk globules start to appear in the cytoplasm. Oocyte diameter ranges from 195 to 310 µm. Late yolk globule stage (Fig. 2d): yolk globules increase in size and cover the whole cytoplasm. Oil droplets around the nucleus increase in size. Oocyte diameter ranges from 295 to 450 µm. Migratory nucleus stage (Fig. 2e): the nucleus migrates towards the animal pole where smaller yolk globules occur and the yolk globules begin to coalesce. Oocyte diameter ranges from 370 to 485 µm. Hydrated stage (Fig. 2f): yolk globules completely undergo coalescence and form a single translucent yolk mass. Oocyte diameter ranges from 385 to 650 µm. Early atretic stage (Fig. 2g): the nucleus and yolk globules disintegrate and are reabsorbed, leaving only the follicular layers. This stage corresponds with alpha stage atresia described by Hunter and Macewicz (1985). Late atretic stage (Fig. 2h): the follicle decreases in size and is characterized by numerous disorganized granulosa cells and intracellular vacuoles. This stage corresponds with beta stage atresia described by Hunter and Macewicz (1985). In addition, the post-ovulatory follicle (Fig. 2i), which is characterised by a granulosa cell layer, coils up within a less irregular thecal cell layer covering, and is frequently observed during the spawning season.
Oocyte developmental stages and post-ovulatory follicle of female E. tumifrons: a peri-nucleolus, b pre-vitellogenic, c early yolk globule, d late yolk globule, e migratory nucleus, f hydrated follicle, g early atretic follicle, h late atretic follicle, and i post-ovulatory follicle. n Nucleus, g granulosa cell layer, t thecal cell layer. Scale bars 100 μm
Ovarian maturity stages
Evynnis tumifrons has an asynchronous ovary containing oocytes at various stages of development (Fig. 3). Based on the appearance of the most advanced oocytes, post-ovulatory follicles and atretic oocytes, the ovaries were classified into six ovarian maturity stages: immature (I), maturing (II), mature (III), spawned (IV), spent (V) and resting (VI). The development of oocytes at each stage is shown in Table 1.
Size at sexual maturity
The size at sexual maturity was estimated from the 177 females collected in the spawning months from November 2013 to May 2014. The minimum size of the mature females was 161 mm FL. The estimated logistic function was as follows:
FL50 was estimated to be 179 mm FL (Fig. 4).
Annual reproductive cycle
According to the results shown in Fig. 5a, the GSI had two yearly peaks, a higher one in November to December (late autumn–winter 2012, 2013), and a lower one around April (spring 2012, 2013, 2014). The monthly occurrences of the six ovarian maturity stages from April 2012 to June 2014 are shown in Fig. 5b. Though E. tumifrons had an asynchronous ovary, which suggested multiple spawning during its reproductive season (Fig. 3), we noticed a general trend in which females tended to be mostly immature from June to September (early summer–early autumn 2012, 2013, 2014), and mature or spawned from November to May (late autumn–late spring 2012–2013, 2013–2014), though in March 2013 mature or spawned females could not be detected within our samples.
Monthly changes in the gonadosomatic index (a) and percentage occurrence of the six ovarian maturity stages (b) of female E. tumifrons off the southwestern coast of Kyushu, Japan. Samples could not be collected in August 2012. Fish smaller than the minimum size of mature females (< 161 mm) were excluded from this analysis. Numbers of females examined shown above the bars
Annual condition factor cycle
The condition factor of fish tended to be high from June to October (summer–autumn 2012, 2013), decreased in November (late autumn 2012, 2013) and was lowest in December or January (winter 2012, 2014) (Fig. 6). However, there seemed to be variation in the increase after the periods of lowest condition factor until May (spring 2013, 2014) within the 2 years of study. From January 2013 to May 2013, the increase was sharp compared to that from February 2014 to May 2014.
Discussion
Ovarian maturity stages of female E. tumifrons were classified into six categories based on the appearance of the most advanced oocytes, post-ovulatory follicle and atretic oocytes. Stage III was regarded as mature because a migratory nucleus or hydrated oocytes occurred in the ovary. In the present study, the time course of the maturation process was not clarified, but in the related species P. major and D. hypselosomus, nucleus migration starts at around 24 h before spawning, and the subsequent hydration of the oocyte starts at around 12 h before spawning (Matsuyama et al. 1988; Yoda and Yoneda 2009). Stage IV (spawned) could be determined from the occurrence of post-ovulatory follicles (evacuated follicles) in the ovary. Post-ovulatory follicles occurring at ovulation are believed to be reabsorbed within 24 h (Matsuyama et al. 1988; Yoda and Yoneda 2009). Thus, stage III (mature) and stage IV (spawned) E. tumifrons were assumed to appear within a few days of spawning. It is also important to note that the time course of the maturation process is temperature dependent (Kurita et al. 2011). However, according to Hunter and Macewicz (1985), a migratory nucleus and hydrated oocytes indicate imminent spawning, while post-ovulatory follicles are histological evidence of very recent spawning (Hunter et al. 1992).
Size at sexual maturity is an important reference point for decision-making in fisheries management (Zhu et al. 2011). However, some previous related research (e.g. Mio 1962; Toriyama et al. 1975; Yamahora 1983) did not estimate this vital biological parameter. Anzawa et al. (1987), however, did estimate the minimum size of mature female E. tumifrons, i.e. 170 mm FL, from its GSI. In the present study, we estimated the minimum size of mature females as 161 mm FL and their size at FL50 as 179 mm FL (Fig. 4), based on the histological technique used. Thus, the minimum size of mature females differed slightly between the current study and Anzawa et al.’s (1987). According to DeMartini et al. (2000) and Alejo-Plata et al. (2011), a histological approach has been proven to have greater precision for the estimation of size at maturity, so the difference in minimum size estimated in our and Anzawa et al.’s (1987) might be due to the different methodologies used. On the other hand, the locality and date on which these two studies were conducted were very different; this may be another factor contributing to the different estimated minimum size of females at sexual maturity. In future studies, histological techniques should be used to examine samples collected at the same time from different localities.
The simultaneous occurrence of a high GSI and mature or spawned maturity stages, which were considered to show the spawning season, lasted from November to May (7 months). The onset of the spawning season was clearly marked by the sharp increase in GSI and the occurrence of the mature or spawned stage in November. The preceding ovarian maturity stage II (maturing), which also occurred during the spawning season, may subsequently shift to ovarian stages III (mature) and IV (spawned), so that most fish were considered to undergo spawning judging from the transition of the six ovarian maturity stages (Fig. 5b). The end of the spawning season around May and June was clearly marked by the subsequent precipitous decline in the monthly change in GSI and the occurrence of fish with spent ovaries at stage V. This spent stage indicates that the cessation of spawning is imminent (Hunter and Macewicz 1985; Yoneda et al. 1998; Granada et al. 2004). However, in 2013 (Fig. 5b), the spent stage occurred in February and in the subsequent month, no mature or spawned females were detected. This suggests that there was an intermission in spawning in March 2013. In some fish species, skipped spawning is observed, which is mostly attributed to a deficient diet and poor nutritional condition (Rideout et al. 2005; Rideout and Tomkiewicz 2011). However, in the present study, the mean condition factors of the individuals collected in February and March 2013 were higher than those of individuals collected in February and March 2014 (Fig. 6). Figure 7 shows the monthly size composition during the spawning season (November–May) of the two study years. Small individuals were collected in April and May 2013 and May 2014, but not in March 2013. Thus the intermission in spawning in March 2013 may have not been due to individual status such as poor body condition and small body size. Figure 8 shows the water temperature from April 2012 to June 2014 off Ichikikushikino City, which is located near the study area. A similar trend in temperature fluctuations occurred throughout each year, but it should be noted that the minimum temperature in February 2013 was only 15.4 °C and in the following month (March) spawning was interrupted. On the other hand, in January 2014 the minimum temperature was 1 °C higher (16.4 °C), and this might have made conditions favourable for continuous spawning until May of that year. Although these temperature data were measured at a different depths to those of the species’ distribution, it should be noted that 5 m is below the water surface. Moreover, these temperatures were measured at a consistent depth throughout the study. Hence, the monthly temperature change observed should be sufficient to explain the change in spawning behaviour. Furthermore, the co-occurrence of sexually mature and immature individuals in April and May 2013 (Fig. 5b) following the intermission in spawning in March 2013 is an interesting phenomenon. According to Lowerre-Barbieri et al. (2011), larger, older individuals exhibited an extended spawning season compared to younger, mature individuals. However, our investigation revealed that nearly half or all larger individuals were immature during this extended spawning season (Fig. 9a). There was no significant difference (P > 0.05) in the condition factors of the immature and sexually mature individuals either (Fig. 9b). Therefore, we could not determine the reason for the co-occurrence of sexually mature and immature individuals in April and May 2013.
Monthly changes in mean water temperature off the southwestern coast of Kyushu, Japan. Data were recorded daily off Ichikikushikino City near the study area by a temperature sensor at 5 m below the water surface attached to the hull of a ferry. Data are cited from the database of Kagoshima Prefectural Fisheries Technology and Development Centre
Percentage occurrence (a) and condition factor (b) at 20-mm FL intervals of immature and mature female E. tumifrons collected in April and May 2013 off the southwestern coast of Kyushu, Japan. Fish smaller than the minimum size of mature females (< 161 mm) were excluded from this analysis. Non-shaded bars indicate immature females, shaded bars indicate mature females. Numbers of females shown above the bars
The spawning season of E. tumifrons in the coastal waters of Japan varied according to locality (see Table 2; localities at the top of the table are located further north). In northern Japan (Yamagata and Niigata Prefectures), it tended to occur from mid-summer to mid-autumn, whereas in southern Japan (Fukuoka, Kochi and Miyazaki Prefectures), it occurred from early autumn to early winter. In the present study, which was conducted off the southwestern coast of Kyushu (the southernmost limit of E. tumifrons’ distribution in Japanese coastal waters), it occurs from late autumn to late spring. This trend shows that in the north E. tumifrons spawns during the warmer months of the year, but shifts its spawning season closer to the colder months in southern areas, while at its southernmost limit, it spawns throughout the cold season. In addition, there seems to be a trend in the duration of spawning according to locality: at the southernmost location of E. tumifrons (present study), the spawning season lasts longer than at higher latitudes. This phenomenon was also observed in Plectropomus areolatus and Epinephelus fuscoguttatus, in which spawning season appeared longer nearer to the equator in the northern hemisphere (Sanchez-Cardenas and Arregiun-Sanchez 2012).
To elucidate why the spawning season varied depending on latitude, three localities were selected to investigate the effect of water temperature and day length on spawning season because these two factors are generally thought to be the most important triggers in the timing of gametogenesis and termination of spawning in temperate fish (Lam 1983; Hanyu 1991; Tominaga et al. 2005). In Fig. 10 we illustrate the mean water temperatures during E. tumifrons’ spawning season in Niigata (northern location) and Fukuoka (southern location), which had similar high water temperatures at spawning that overlapped. On the other hand, off the southwestern coast of Kyushu (southernmost location, present study), the trigger seemed to change to colder rather than warmer water; spawning in this species probably also has a lower threshold of water temperature beyond which the spawning process may stop.

(http://www.jodc.gojp/jodcweb/). Water temperature data off the southwestern coast of Kyushu are pooled mean data from Fig. 8. Day length data are records for 1987 in Niigata, 1962 in Fukuoka and April 2012–June 2014 off the southwestern coast of Kyushu, and are cited from the database of the National Oceanic and Atmospheric Administration (https://www.esrl.noaa.gov/gmd/grad/solcalc/)
Mean water temperature and day length when spawning occurred: a Niigata Prefecture, b Fukuoka Prefecture and c southwestern coast of Kyushu. Solid circles indicate spawning months and open circles indicate non-spawning months of E. tumifrons. Water temperature data in Niigata and Fukuoka Prefectures were obtained from serial station data between 1906 and 2003 at 10-m depth and are cited from the database of the Japan Oceanographic Data Centre
Regarding the effect of day length (Fig. 10), spawning in northern Niigata was favoured when conditions combined warmer water with longer days, while to the south in Fukuoka a combination of warmer water conditions but with shorter days was preferred; finally in the southernmost location off the southwestern coast of Kyushu (present study), day length did not seem to have much effect. Thus, the range as well as the transition of water temperature and day length during the spawning season differed between these three localities, which might have influenced the timing of gametogenesis and termination of spawning in different ways. Moreover, the following factors should be considered: firstly, each population of E. tumifrons distributed in the coastal waters of Japan may have evolved an intrinsical spawning behaviour to ensure a match between emergent larvae and their food source; secondly, each population might have evolved to suit the characteristics of the environment they are distributed in. Because the coastal waters of Niigata (northern Japan) are part of the Sea of Japan, those of Fukuoka (southern Japan) are part of the Tsushima Strait, and those off the southwestern coast of Kyushu (further south) are part of the East China Sea, they differ greatly from one another. Hence, E. tumifrons may have evolved over time to contend with the conditions of the habitat they are distributed in. To elucidate this assertion, future experimental studies on each population are needed, as this will help to achieve an understanding of their behavioural characteristics, especially the degree at which genetic variation and/or thermal or light plasticity contribute towards the variation in their spawning seasons.
The present study was conducted to promote an understanding of the reproductive biology of E. tumifrons off the southwestern coast of Kyushu, Japan, and is the first to provide detailed information on this species’ ovarian maturation using an histological technique. The minimum size of mature females was estimated to be 161 mm FL and the FL50 179 mm FL. The spawning season was estimated to last from November to May. These findings and those of previous studies evidence regional differences in the reproductive biology of E. tumifrons that may have important implications for the appropriate management of this species. It is therefore paramount that current management measures targeting this species are scrutinized and updated so that the biological variability of each geographical population is taken into account and incorporated into future management policies.
References
Akazaki M (1984) Sparidae. In: Masuda H et al (eds) The fishes of Japanese archipelago, English edition. Tokai University Press, Tokyo, pp 170–171
Akazaki M (1962) Studies on the spariform fishes. Anatomy, phylogeny, ecology and taxonomy, vol 1, pp 1–368. Misaki Mar Biol Inst, Kyoto Univ Spec Rep (in Japanese with English abstract)
Alejo-Plata C, Díaz-Jaimes P, Salgado-Ugarte IH (2011) Sex ratios, size at sexual maturity and spawning seasonality of dolphinfish Coryphaena hippurus captured in the gulf of Tehuantepec, Mexico. Fish Res 110:207–216
Anzawa W, Tomioka N, Yamabuki K (1987) Report on the stock enhancement of sea bream Evynnis japonica. Fisheries Experimental Station of Niigata Prefecture, Japan, pp 1–31 (in Japanese)
Blazer VS (2002) Histopathological assessment of gonadal tissue in wild fishes. Fish Physiol Biochem 26:85–101
DeMartini EE, Uchiyama JH, Williams HA (2000) Sexual maturity, sex ratio, and size composition of swordfish Xiphias gladius caught by the Hawaii-based pelagic longline fishery. Fish Bull 98:489–506
Farley JH, Davis TLO, Bravington MV, Andamari R, Davies CR (2015) Spawning dynamics and size related trends in reproductive parameters of southern bluefin tuna Thunnus maccoyii. PLOS ONE 10(5):e0125744. https://doi.org/10.1371/journal.pone.0125744. Accessed 15 Dec 2018
Granada PV, Masuda Y, Matsuoka T (2004) Annual reproductive cycle and spawning frequency of the female yellowbelly threadfin bream Nemipterus bathybius in Kagoshima Bay, southern Japan. Suisanzoshoku 52:329–340
Hanyu I (1991) Reproductive cycle. In: Itazawa Y et al (eds) Fish physiology. Koseiya-Koseikaku, Tokyo, pp 287–325
Hayashi M (2002) Sparidae. In: Nakabo T (ed) Fishes of Japan with pictorial keys to the species. Tokai University Press, Tokyo, p 858
Hunter JR, Macewicz BJ (1985) Measurement of spawning frequency in multiple spawning fishes. In: Lasker R (ed) An egg production method for estimating spawning biomass of pelagic fish: application to the northern anchovy, Engraulis mordax. NOAA technical report NMFS 36. US Departement of Commerce, VA, pp 79–94
Hunter JR, Macewicz BJ, Lo NC, Kimbrell CA (1992) Fecundity, spawning and maturity of female dover sole Microstomus pacificus with an evaluation of assumptions and precision. Fish Bull 90:101–128
Iwatsuki Y, Akazaki M, Taniguchi N (2007) Review of the species of the genus Dentex (Perciformes: Sparidae) in the western Pacific defined as the D. hypselosomus complex with the description of a new species, Dentex abei and a redescription of Evynnis tumifrons. Bull Natl Mus Nat Sci Ser A 1:22–49
Iwatsuki Y, Russell B, Pollard D, Mann BQ, Carpenter KE, Buxton CD, Shao K, Liao W (2014) Evynnis tumifrons. The IUCN Red List of threatened species 2014: e.T1702212A1294201. http://dx.doi.org/10.2305/IUCN.UK.2014-3.RLTS.T170212A1294201.en. Accessed 25 Apr 2016
King M (2007) Fisheries biology, assessment and management. Wiley, Oxford
Kudoh T, Yamaoka K (2004) Territorial behavior in juvenile red sea bream Pagrus major and crimson sea bream Evynnis japonica. Fish Sci 70:241–246
Kurita Y, Fujinami Y, Amano M (2011) The effect of temperature on the duration of spawning markers-migratory-nucleus and hydrated oocytes and postovulatory follicles-in the multiple-batch spawner Japanese flounder (Paralichthys olivaceus). Fish Bull 109:79–89
Lam TJ (1983) Environmental influences on the gonadal activity in fish. In: Hoar WS et al (eds) Fish physiology. Academic Press, New York, pp 65–166
Lowerre-Barbieri SK, Ganias K, Saborido-Rey F, Murua H, Hunter JR (2011) Reproductive timing in marine fishes: variability, temporal scales, and methods. Mar Coast Fish 3:71–91
Matsuyama M, Adachi S, Nagahama Y, Matsuura S (1988) Diurnal rhythm of oocyte development and plasma steroid hormone levels in the female red sea bream Pagrus major during the spawning season. Aquaculture 73:357–372
Mio S (1961) Age and growth of red sea bream, Evynnis japonica Tanaka. Rec Oceanogr Works Jpn 5:95–101
Mio S (1962) Maturity of red sea bream Evynnis japonica Tanaka. Rec Oceanogr Works Jpn 6:21–30
Oki D, Tabeta O (1998) Age, growth and reproductive characteristics of the yellow sea bream Dentex tumifrons in the East China Sea. Fish Sci 64:191–197
Okochi Y, Abe O, Tanaka S, Ishihara Y, Shimizu A (2016) Reproductive biology of female Pacific bluefin tuna Thunnus orientalis in the Sea of Japan. Fish Res 174:30–39
Rideout RM, Tomkiewicz J (2011) Skipped spawning in fishes: more common than you might think. Mar Coast Fish 3:176–189
Rideout RM, Rose GA, Burton MPM (2005) Skipped spawning in female iteroparous fishes. Fish Fish 6:50–72
Sanchez-Cardenas R, Arregiun-Sanchez F (2012) Latitudinal exploration of the temporalities of spawning for some tropical fish species (Epinephelidae: Plectropomus spp., Mycteroperca spp. and Epinephelus spp.). J Fish Aquat Sci 7:379–391
Shibuya K (1985) Annual report of sea farming centre of Akita Prefecture, Japan (in Japanese)
Sun C, Chang H, Liu T, Yeh S, Chang Y (2015) Reproductive biology of the black marlin Istiompax Indica off southwestern and eastern Taiwan. Fish Res 166:12–20
Tominaga O, Inoue M, Kamata M, Seikai T (2005) Reproductive cycle of yellow sea bream Dentex tumifrons in Wakasa Bay, the Sea of Japan off central Honshu. Fish Sci 71:1069–1077
Toriyama M, Kudo S (1976) Trend of demersal fish resources in Pacific region of southern Japan. Fisheries Agency of Japan (in Japanese)
Toriyama M, Kudo S, Asami T (1975) Evynnis tumifrons in southwestern sea region of Japan. Report on the demersal fish in west Japan at demersal fish meeting in fisheries resource conference. Fisheries Agency of Japan, pp 33–36 (in Japanese)
Tyler CR, Sumpter JP (1996) Oocyte growth and development in teleosts. Rev Fish Biol Fish 6:287–318
West G (1990) Methods of assessing ovarian development in fishes: a review. Aust J Mar Freshwater Res 41:199–222
Yamahora H (1983) Ecological comparison between Pagrus major and Evynnis tumifrons in the coastal sea regions of Yamagata Prefecture. Report on demersal fishes in northern Japan. Demersal Fish Meeting. Fish Resour Conf 1982:55–61 (in Japanese)
Yamamoto K (1956) Studies on the formation of fish eggs. 1. Annual cycle in the development of ovarian eggs in the flounder Liopsetta obscura. J Fac Sci Hokkaido Univ Ser VI Zool 12:362–374
Yoda M, Yoneda M (2009) Assessment of reproductive potential in multiple-spawning fish with indeterminate fecundity: a case study of Yellow Sea bream Dentex hypselosomus in the East China Sea. J Fish Biol 74:2338–2354
Yoneda M, Tokimura M, Fujita H, Takeshita N, Takeshita K, Matsuyama M, Matsuura S (1998) Ovarian structure and batch fecundity in Lophiomus Setigerus. J Fish Biol 52:94–106
Youn CH (2002) Fishes of Korea with pictorial key and systematic list. Ishisha, Seoul p 747
Zhu Y, Furukawa K, Aida K, Hanyu I (1989) Annual reproductive rhythm of the tobinumeri-dragonet Repromucenns beniteguri (Callionymidae) in Lake Hamana. Nippon Suisan Gakkaishi 55:591–599
Zhu GP, Dai XJ, Song LM, Xu LX (2011) Size at sexual maturity of bigeye tuna Thunnus obesus (Perciformes: scombridae) in the tropical waters: a comparative analysis. Turk J Fish Aquat Sci 11:149–156
Acknowledgements
We would like to acknowledge the tireless cooperation of the commercial fishers of the Eguchi Fishermen’s Cooperative and the students of the Fisheries Biology Lab, Faculty of Fisheries, Kagoshima University for data sampling and measurement. We would also like to express our sincere gratitude to Mr Shugo Matsuo and Ms Yuki Katsuha for their assistance in preparing the histological sections of the ovaries.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Havimana, L., Ohtomi, J., Masuda, Y. et al. The reproductive biology of female crimson sea bream Evynnis tumifrons off the southwestern coast of Kyushu, Japan. Fish Sci 86, 65–75 (2020). https://doi.org/10.1007/s12562-019-01380-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-019-01380-6