Abstract
Temperature is one of the most important variables that influence aquatic organisms. This study aimed to test physiological and molecular differences in the thermal tolerance of two morphologically similar Marsupenaeus japonicus. The physiological parameter was the upper thermal limit, tested via the critical thermal maximum, and the molecular parameter was the relative messenger RNA expression of heat shock proteins (HSP40, HSP60, HSP70 and HSP90), quantified via real-time polymerase chain reaction. Oxygen consumption of shrimp was measured with a respirometer. M. japonicus variety II showed a higher critical thermal maxima and survival rate and lower oxygen consumption rate than variety I, suggesting that it had higher thermal tolerance and acclimatory plasticity. M. japonicus variety I showed significantly higher production of HSP70 and HSP90, suggesting that these shrimp may have experienced more thermal damage. By comparing the differences in the stress response between two morphologically similar M. japonicus, this study provides useful information for the aquaculture of M. japonicus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Kuruma prawn Marsupenaeus japonicus is distributed from Japan and Southeast Asia to East Africa and the Red Sea (Holthuis 1980). It is one of the most promising marine invertebrates for aquaculture in Asia, Europe and Australia. In China, the estimated annual production of M. japonicus was 55,885 tons in 2016 (Ministry of Agriculture and Rural Affairs of the People’s Republic of China 2017). However, pond temperatures throughout southeast China are always maintained at > 30°C in summer, which is higher than the optimal temperature for the growth of M. japonicus (23–30 °C) (Chiu and Chien 1994), resulting in considerable economic losses for the M. japonicus industry. High temperatures might affect the M. japonicus industry in several ways. First, M. japonicus is generally less tolerant of high temperatures than other penaeid shrimp. Previous studies have also reported that high water temperatures (> 30 °C) resulted in high mortality of kuruma shrimp. Second, an increase in acclimation temperature is accompanied by increased oxygen consumption and a decreased level of dissolved oxygen, which directly contribute to the hypoxia seen in shrimp at high seasonal temperatures (Palacios and Ross 2010; González et al. 2010). High mortality resulting from exposure to high temperatures hinders the economically sustainable development of M. japonicus aquaculture. A previous study (Tsoi et al. 2005) suggested that two morphologically similar varieties of M. japonicus, I and II, from the South China Sea, have different heat tolerances, which are probably related to their different color banding patterns on the carapace, different geographical distributions and significant genetic divergence. Thus, in the present study, we mainly investigated the heat tolerance differences between these two varieties of M. japonicus, with regard to their critical thermal maxima (CTMax), acclimation response ratio (ARR), heat shock protein (HSP) expression, oxygen consumption, survival and growth rate.
Materials and methods
Shrimp
F1 progeny of two morphologically similar varieties, I and II, of M. japonicus were established in April 2011. Parent prawns were caught from the Huilai Sea in Guangdong Province, China. When the mean body length reached 4–5 cm, progeny of M. japonicus varieties I and II were injected with a visible implant elastomer tag and then placed in a 5-m2 cement tank at 28 ± 1 °C for 7-day recovery culture. Before the experiment commenced, the average wet weights of M. japonicus I and II progeny were 3.038 ± 0.663 g and 2.386 ± 0.521 g, respectively. Some of the experimental individuals were used to compare survival, growth and HSP expression before and after high temperature stress. The rest were divided into three subgroups with different acclimation temperatures (24 °C, 28 °C, 32 °C), each of which comprised 50 shrimp of both varieties. When the acclimation temperature reached the experimental temperature, at a rate of 2 °C day−1, it was maintained at this temperature for 7 days, then the CTMax and oxygen consumption of the shrimp varieties were measured at three specific temperatures. All these procedures were approved by the Institutional Animal Care Committee of Xiamen University.
Measurement of survival and growth
A total of 1200 shrimp (variety I, 600; variety II, 600) initially placed in 5-m2 cement tank at 28 ± 1.0 °C were used to compare survival and growth before and after high temperature stress. The experimental shrimp were divided into a high temperature group (32 ± 1 °C) and a control group (28 ± 1 °C). Each group consisted of three replicates, and each replicate comprised 100 shrimp of each variety. The initial wet weights of the shrimp were recorded at the beginning of the experiment. After the 60-day experimental period, all live shrimp were harvested and counted to obtain survival rate; the final wet weights of the shrimp were also recorded. The specific growth rate (SGR) was calculated with the following equation: SGR = (ln Wt – lnW0) × 100/t, where W0 and Wt are the respective initial and final average wet weights of the shrimp and t is the sampling interval.
Measurement of thermal tolerance
The methods used for measuring thermal tolerance followed González et al. (2010). Ten shrimp of each variety at the same acclimation temperature were transferred to 5-l aquaria and starved for 24 h before the experiments. To determine the CTMax, the organisms were subjected to water bath heating with a constant rate of water temperature increase (1 ± 0.2 °C h−1) and observed continuously until they reached the end point. The end point for CTMax was loss of righting response (LRR) when shrimp were on their backs and could not recover their upright posture, or remained in a reclining position at 90° (Nelson and Hooper 1982). When shrimp reached this point they were returned to their acclimation salinity and temperature conditions. Individual shrimp were used only once and the data for the animals which did not recover after returning them to their acclimation salinity and temperature after LRR were discarded. Each group consisted of five replicates, and two shrimp of each variety from each replicate were used for the measurement of CTMax.
We also determined ARR as defined by Claussen (1977) as ΔCTMax/ΔT, where ΔCTMax is the difference in temperature tolerance of shrimp at different acclimation temperatures and ΔT is the acclimation temperature difference.
Measurement of oxygen consumption
Oxygen consumption of shrimp was measured with a respirometer, as described in Re et al. (2009). Shrimp were acclimated for 30 days at 24 °C, 28 °C and 32 °C and starved for 24 h before initiating the measurements of oxygen consumption. One shrimp of each variety from each acclimation temperature (24 °C, 28 °C and 32 °C) was placed in one of three 5-l flasks used as respiratory chambers, with the exception of one flask that was used as a control without shrimp for the correction of oxygen consumption due to microorganisms present in the system. Before closing the chambers, we took a water sample to quantify the initial concentration of oxygen; another sample was taken after 1.5 h to measure the final concentration of dissolved oxygen. This procedure was carried out twice at an interval of 2 h for each condition. The initial and final concentration of dissolved oxygen was measured by iodometry and spectrophotometry. The wet weights of shrimp were also recorded. The oxygen consumption of the shrimp was calculated from the difference between the initial and final concentrations of oxygen consumed by them at each acclimation temperature, expressed as volume of oxygen consumed (VO2) (milligrams O2 per kilogram per hour wet weight).
The thermal coefficient for the metabolic rate of shrimp (Q10), which represents an organism’s sensitivity to temperature variation, was calculated for each acclimation temperature by Eq. 1, proposed by Schmidt-Nielsen (1997):
where, OR1 and OR2 are the metabolic rates at temperatures 1 (Temp1) and 2 (Temp2), respectively.
HSP extraction and quantification
Ten shrimp of each variety were chosen for real-time polymerase chain reaction (PCR). Five shrimp of each variety were placed in a water bath at 36 °C for 1 h for the heat-induced production of HSP transcripts. The reaction was stopped by immersion of shrimp in water at ambient temperature (27 °C). For tissue distribution analysis, the gill and hepatopancreas were collected from ten shrimp and immediately frozen in liquid nitrogen (− 196 °C). The other five shrimp out of ten of each variety comprised the control, and were not exposed to the heat shock treatment.
The total RNA of gill and hepatopancreas tissue was extracted using RNAiso Plus (TaKaRa) according to the manufacturer’s protocol. The HSP complementary DNA (cDNA) fragment was synthesized using a specific primer produced from total RNA. The synthesized products were used as the templates for quantitative real-time-PCR analysis of HSP expression using SYBR Premix Ex TaqTM (TaKaRa). Real-time PCR of HSP40, HSP60, HSP70, HSP90 and β-actin (the internal reference gene) was performed individually in triplicate in a 20-μl reaction containing 2 × 10 μl SYBR Premix Ex Taq TM II, 10 μM forward primer and reverse primer (0.8 μl each), 2 μl template cDNA, and 6.4 μl distilled deionized water. PCR primers used for validation are given in Table 1. Amplification reactions were carried out using a PCR profile of 95 °C for 30 s followed by 40 cycles of denaturation at 95 °C for 5 s, annealing at 60 °C for 31 s, and extension at 40 °C for 30 s. The relative mRNA expression level was calculated using the 2−ΔΔCt method, where Ct is cycle threshold, with the Ct values normalized by using β-actin as the internal control (Livak and Schmittgen 2002; Schmittgen and Livak 2008).
Statistical analyses
The data were analyzed using one-way ANOVA followed by post hoc least significant difference and are presented as the mean ± SD; p < 0.05 was considered significant. Data were analyzed using SPSS version 17.
Results
At the pre-culture stage, there was no significant difference between the initial weights of juvenile shrimp of varieties I and II (I, 0.013 ± 0.002 vs. II, 0.012 ± 0.005; p > 0.05; Table 2); however, significant differences in SGR were seen after 105 days of culture (I, 2.88 ± 0.012 vs. II, 2.26 ± 0.014; p < 0.05). At the experimental culture stage, although there were clear differences in initial weight between varieties I and II (p < 0.05), no significant differences were seen in SGR between them under a normal or high temperature (p > 0.05). Therefore, it could be reasonably speculated that shrimp weight did not have a major effect on their SGR.
In the temperature experiments, the SGR of both varieties of M. japonicus showed a declining trend. Compared with the group maintained at the normal temperature (28 ± 1 °C), shrimp cultured at high temperature (32 ± 1 °C) presented a more significant decline in SGR, indicating that high temperature exposure exerted a negative effect on this parameter. Furthermore, the SGR decline was greater in variety I shrimp than in variety II at high temperature, suggesting that the latter are better adapted to and tolerant of a higher temperature environment.
There was a noticeable decline in SR in shrimp cultured at a normal temperature with an extension of the culture time, but there was no significant SR difference between the two varieties of shrimp according to weight, implying that shrimp weight is not closely related to SR. Compared with the normal temperature group, shrimp cultured at high temperature showed a clearer decline in SR, suggesting that high temperatures have a negative effect on the survival of these shrimp. Although a high culture temperature caused a clear decline in survival, the SR of variety II shrimp was still significantly higher than that of variety I (p < 0.01), indicating that the former survive high temperatures better.
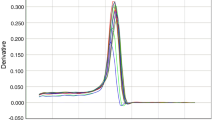
The CTMax of the shrimp increased with acclimation temperature (24–32 °C), and the thermal tolerance of M. japonicus varieties I and II increased by 1 °C and 2.5 °C, respectively, indicating that acclimation temperature had an effect on their CTMax (Fig. 1). In particular, at the three specific temperature points of the experiment (24 °C, 28 °C, 32 °C), M. japonicus variety II had significantly higher CTMax than variety I (p < 0.05), suggesting that the former had a higher thermal tolerance.
The ARR of M. japonicus varieties I and II exposed to different combinations of temperature had a range of 0.13–0.29, 0.29–0.35, respectively. In line with the CTMax results, variety II shrimp showed a higher ARR than variety I.
The rate of oxygen consumption increased for both varieties of M. japonicus shrimp as the acclimation temperature increased from 24 to 32 °C (Table 3). However, the oxygen consumption rate of variety I was significantly higher than that of variety II at the three observed temperatures (p < 0.05), i.e., a maximum of 1.809 ± 0.070 mg O2 kg−1 h−1 wet weight and 1.218 ± 0.111 mg O2 kg−1 h−1 wet weight, respectively. The temperature coefficient (Q10) of variety I was highest, at 3.747, between 28 and 32 °C and lowest, at 2.985, between 24 and 28 °C; however, the Q10 of variety II shrimp showed an opposite trend: its lowest Q10 was 3.340 between 24 and 28 °C and highest Q10 3.520 between 28 and 32 °C (Table 4).
The production of each HSP in the gill and hepatopancreas was altered by increasing the temperature (Fig. 2). HSP70 levels in the gill and hepatopancreas of M. japonicus variety I were significantly increased after heat shock (p < 0.05), and the HSP90 levels in the hepatopancreas of variety I were also markedly increased (p < 0.05).
Discussion
CTMax is a measure of the thermal tolerance of aquatic organisms (Beitinger et al. 2000). The CTMax value increased for both M. japonicus varieties I and II when the temperature rose from 24 to 32 °C, indicating that the thermal tolerance of M. japonicus was enhanced with increasing temperature. A similar relationship between the CTMax and acclimation temperature has been demonstrated in other crustacean species (Wortham et al. 2014; Tung et al. 2014). Variety II shrimp had significantly higher CTMax value than variety I at the three experimental temperature points (p < 0.05), and the difference in CTMax between the two varieties increased with acclimation temperature, implying that the thermal tolerance of variety II was markedly greater than that of variety I, and that it increased further with acclimation temperature.
M. japonicus variety I is confined to the East China Sea and the northern South China Sea (temperate region), whereas variety II is distributed in the South China Sea, Australia and the western Indian Ocean (tropical and subtropical regions). It has been reported that subtropical and tropical shrimp species show higher ARR than shrimp species inhabiting temperate and cold regions (Pérez et al. 2003). Because the former experience their greatest thermal extremes over short periods of time they should have a broad range of tolerance to survive relatively rapid changes in water temperature without an acclimation period in which to adjust their tolerance (Johnson and Kelsch 1998). This agrees with our results, in that variety II showed a higher ARR and better thermal tolerance and adaptation to its thermal environment. This appears to indicate that the capacity for thermal tolerance in shrimp is closely related to distribution latitude.
Oxygen requirements and oxygen consumption rates (VO2) are closely related to a wide range of biotic and abiotic factors including activity, environmental temperature, salinity, body weight, and diet. However, temperature is one of the most important abiotic factors affecting oxygen consumption in aquatic organisms (Wolken et al. 2010). In this study, the VO2 of the two varieties of shrimp increased with acclimation temperature, in line with previous studies (Song et al. 2009; Wen 2003). The conflict between an increasing oxygen requirement and insufficient dissolved oxygen places limitations on the survival of aquatic organisms under high temperatures (Fangue et al. 2006). In the present study, the VO2 was significantly higher in variety I than in variety II, suggesting that variety I is more challenged by hypoxia and requires more energy for the maintenance of normal physiological functions under these conditions. In other words, variety II shrimp has a better ability to adapt to high temperatures.
HSPs are important components of the heat shock response (Ojima et al. 2005) and play a role in cell functioning and protection during both normal and stressful conditions (Currie et al. 1999; Kregel 2002). This may explain why HSP production in the gill and hepatopancreas changes with increasing temperature. HSP70 is highly sensitive to temperature (Iwama et al. 1998; Feder and Hofmann 1999) and has been linked to thermal tolerance in animal cells (Bahrndorff et al. 2009). In the present study, M. japonicus variety I produced significantly more HSP70 in the gill and hepatopancreas (p < 0.05) in response to heat stress, suggesting that it may be more vulnerable to thermal damage than variety II.
Like HSP70, HSP90 is a molecular chaperone which has been conserved in all living organisms to protect cells against stress. In the present study, HSP 90 levels in the gill and hepatopancreas were higher in variety II shrimp than in variety I, but the results were inverted after heat stress, suggesting that variety I shown a greater response to thermal stress.
In this study, a large decrease in the SR of shrimp was observed after thermal stress, indicating that it exerts a negative effect on their SR. However, the SR of variety II shrimp was significantly higher than that of variety I, which can probably be attributed to greater thermal tolerance of the former, as indicated in the present study by their with greater CTMax values. The SGR before thermal stress was markedly higher in variety I shrimp than in variety II; after thermal stress, a decrease in SGR in variety I shrimp was observed. However, this was not statistical different from the SGR of variety II shrimp, which indicates that the growth of the former was affected by thermal stress, consistent with the results of the HSPs.
In conclusion, this study provides evidence that M. japonicus variety I is more sensitive to thermal stress due to its limited acclimatory plasticity, while variety II shrimp showa a stronger thermal tolerance indicated by a higher survival rate. These findings demonstrate that the two morphologically similar M. japonicus varieties, I and II, have different stress response mechanisms, causing differences in their thermal tolerance and survival rate. The present study provides useful guidance for the selection of locations suitable for the intensive aquaculture of M. japonicus, as well as for suitable acclimation temperatures and the effective control of the growth and survival rate of M. japonicus. Crossbreeding the sister varieties of M. japonicus examined here to develop an improved variety with high growth, a high survival rate and strong thermal tolerance would be beneficial for the aquaculture of this species.
References
Bahrndorff S, Maiean JL, Volker EJ (2009) Dynamics of heat-induced thermal stress resistance and HSP70 expression in the springtail, Orchesella cincta. Funct Ecol 23:233–239
Beitinger TL, Bennett WA, Mccauley RW (2000) Temperature tolerances of north American freshwater fishes exposed to dynamic changes in temperature. Environ Biol Fish 58:237–275
Chiu Li, Chien YH (1994) Culture of kuruma prawn (Penaeus japonicus) in Asia
Claussen DL (1977) Thermal acclimation in ambystomatid salamanders. Comp Biochem Phys C 58:333-340
Currie S, Tufts BL, Moyes CD (1999) Influence of bioenergetic stress on heat shock protein gene expression in nucleated red blood cells of fish. Am J Physiol 276:990–996
Fangue NA, Myriam H, Schulte PM (2006) Intraspecific variation in thermal tolerance and heat shock protein gene expression in common killifish, Fundulus heteroclitus. Exp Biol 209:2859–2872
Feder M, Hofmann G (1999) Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol 61:243–282
González RA, Díaz F, Licea A, Re AD, Sánchez LN, García-Esquivel Z (2010) Thermal preference, tolerance and oxygen consumption of adult white shrimp Litopenaeus vannamei (Boone) exposed to different acclimation temperatures. J Therm Biol 35:218–224
Holthuis LB (1980) FAO species catalogue, vol 1. Shrimps and prawns of the world: an annotated catalogue of species of interest to fisheries. J Endocrinol 75:801–809
Iwama GK, Thomas PT, Forsyth RB, Vijayan MM (1998) Heat shock protein expression in fish. Rev Fish Biol Fisher 8:35–56
Johnson JA, Kelsch SW (1998) Effects of evolutionary thermal environment on temperature-preference relationships in fishes. Environ Biol Fish 53:447–458
Kregel KC (2002) Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol 192:2177–2186
Livak KJ, Schmittgen TD (2002) Analysis of relative gene expression data using real-time quantitative PCR. Methods 25:402–408
Ministry of Agriculture and Rural Affairs of the People’s Republic of China (2017) China fishery statistical yearbook. China Agriculture Press
Nelson DH, Hooper DK (1982) Thermal tolerance and preference of the freshwater shrimp Palaemonetes kadiakensis. J Therm Biol 7:183–187
Ojima N, Yamashita M, Watabe S (2005) Comparative expression analysis of two paralogous HSP70s in rainbow trout cells exposed to heat stress. BBA Gen Subj 1681:99–106
Palacios CAM, Ross LG (2010) The effects of temperature, body weight and hypoxia on the oxygen consumption of the Mexican mojarra, Cichlasoma urophthalmus (Günther). Aquac Res 17:243–248
Pérez E, Diaz F, Espina S (2003) Thermoregulatory behavior and critical thermal limits of the angelfish Pterophyllum scalare (Lichtenstein) (Pisces: Cichlidae)
Re AD, Díaz F, Valdez G, Flores M, López M (2009) Physiological energetics of blue shrimp Penaeus stylirostris (Stimpson) juveniles acclimated to different salinities. Open Zool J 2:102–108
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108
Song XF, Liu P, Chang-Zi GE (2009) Interactive effects of temperature and salinity on oxygen consumption, ammonia-nitrogen excretion and phosphate excretion in Litopenaeus vannamei. Fish Model
Tsoi KH, Wang ZY, Chu KH (2005) Genetic divergence between two morphologically similar varieties of the kuruma shrimp Penaeus japonicus. Mar Biol 147:367–379
Tung CP, Lee T-Y, Huang J-C, Perng P-W, Kao S-J, Liao L-Y (2014) The development of stream temperature model in a mountainous river of Taiwan. Environ Monit Assess 186:7489–7503
Wen XK (2003) Effects of temperature, body weight and feeding on metabolismon of Procambrus clarkii. J Huazhong Agric 22:152–156
Wolken JJ, Mellon AD, Greenblatt CL (2010) Environmental factors affecting growth and chlorophyll synthesis in euglena. I. Physical and chemical. II. The effectiveness of the spectrum for chlorophyll synthesis. J Eukaryot Microbiol 2:89–96
Wortham JL, Vanmaurik LN, Price WW (2014) Setal morphology of the grooming appendages of Macrobrachium rosenbergii (Crustacea: Decapoda: Caridea: Palaemonidae) and review of decapod setal classification. J Morphol 275:634–649
Funding
This study was financed by the China Agriculture Research System (CARS-48) and the Natural Science Foundation of Guangdong Province (2017A030313147).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not describe any studies on animals performed by any of the authors which were not approved by the Institutional Animal Care Committee of Xiamen University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dong, H., Mao, Y., Duan, Y. et al. Physiological and molecular differences in the thermal tolerance of two varieties of kuruma prawn Marsupenaeus japonicus: critical thermal maximum and heat shock protein 70. Fish Sci 86, 163–169 (2020). https://doi.org/10.1007/s12562-019-01383-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-019-01383-3