Abstract
As poikilotherms, fish health is compromised by exposure to elevated temperatures (e.g. climate change-related warming, anthropogenic thermal pollution, and/or hatchery processes). While fish thermotolerance has been demonstrated to be plastic, the downstream impacts of early life-stage high temperature exposure are not known. In the present study, we investigated the thermotolerance of rainbow trout (Oncorhynchus mykiss) fry 2 months after being exposed to elevated temperature (22°C) for 96 h. Exposed fry demonstrated a reduced critical thermal maxima (CTmax) in comparison to control fish. Using the RNase H-dependent quantitative PCR method, expression of rainbow trout hsp70 isoforms was determined immediately after the acute thermal stress and immediately following the thermotolerance trials. The lowered CTmax was associated with a reduced ability to upregulate the hsp70b gene during the thermotolerance trials, whereas no changes in hsp70a were observed. Overall, these results indicate that exposure to thermal stress in early life-stages of rainbow trout can have negative effects on future physiological function.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Given the current global temperature increases, there is a growing concern regarding the potential effects on aquatic environments. (Schindler, 1997; Mohseni et al., 2003; McCullough et al., 2009). Stream and river ecosystems, and the biota therein, are considered particularly at risk, with the effects of a warming climate likely to be exacerbated by low water flows, longitudinal fragmentation, urbanization, and destruction of the riparian buffer zones (see below; Kaushal et al., 2010). As poikilotherms, stenothermal cold water fish such as the rainbow trout, Oncorhynchus mykiss (Walbaum, 1792), are likely to be especially susceptible to elevated environmental temperatures (Buisson et al., 2008). This widely distributed salmonid has significant socioeconomic value as both a sportfish and an aquaculture species, but has a low and narrow thermal optimal range (Schurmann et al., 1991). For example, spawning and successful egg maturation in rainbow trout occurs from 4 to 13°C (Billard, 1983; Morrison and Smith, 1986; Pankhurst et al., 1996), and thus even small changes in stream temperatures could have a significant impact on the sustainability of this species.
The ability to cope with warming water temperature will depend on the thermotolerance of a fish, a parameter that can be measured by metrics such as the critical thermal maximum (CTmax) calculated at the point the animal experiences a loss of equilibrium (LOE) and is unable to right itself (Becker & Genoway, 1979). Critically, studies using CTmax have shown that thermotolerance in fish is plastic (Lee & Rinne, 1980; Currie et al., 1998), suggesting that the capacity of a fish to cope with elevated water temperature will depend on its exposure history.
Most studies of thermotolerance use juvenile or adult fish, and consequently, less is known regarding the thermal requirements and tolerance of larval fish. Salmonid larvae and fry routinely utilize the marginal or lateral regions of rivers and stream channels, which can be up to 1.5°C higher than the main channels (Symons & Heland, 1978; Moore & Gregory, 1988) and presumably prone to more variations in temperature. It is also important to note that climate change modelling scenarios predict both a gradually warming environment and an increase in the frequency and intensity of rare extreme weather events (e.g. floods and droughts; IPCC, 2007). Thus, larval fish may be exposed to longer than normal periods of high temperatures and prolonged thermal stress during drought conditions or sequential early season warm days. Experimentally, some authors have argued that larval fish are more susceptible to temperature increases than adult fish (Rombough, 1997; Brett, 1970; Blaxter, 1991), while other studies have demonstrated that, relative to adults, juvenile fish possess significantly higher levels of heat shock proteins (HSP) in their tissues following acute heat shock (Krone et al., 1997; Fowler et al., 2009), which may be indicative of greater temperature tolerance.
It is well established that inducible HSP protect against cellular damage resulting from stressors, including temperature, anoxia, and chemical toxicity in rainbow trout (Currie and Tufts, 1997; Currie, 2000; Rendell et al., 2006). The Hsp70 family of proteins has been extensively studied in this species, and well characterized in both cultured cells and whole animal experiments (Currie & Tufts, 1997; Ojima et al., 2005a, b; Yamashita et al., 2010). Two distinct paralogues, hsp70a and hsp70b have been identified, and their inducibility confirmed by the demonstration of transcription increases following 30 min of thermal stress at 25°C (Ojima et al., 2005a). In immortalized rainbow trout gonadal fibroblasts (RTG-2 cells), there was an increase in mRNA expression of hsp70a and hsp70b following heat-shock. However, due to the high sequence similarity, the authors were unable to clearly differentiate between the isoforms, and expression changes were simply ascribed to “hsp70” (i.e. the combined values of both isoforms; Ojima et al., 2005b). It was clearly demonstrated, however, that hsp70 expression was increased relative to the other hsp gene families following thermal stress (Ojima et al., 2005b). Additionally, following moderate repeated thermal stress events, induction of hsp70 expression by lake whitefish (Coregonus clupeaformis Mitchell, 1818) embryos was observed at several stages of development, but this was temporally dependent as some stages could mount a response while others could not (Whitehouse et al., 2017).
In addition to the threat of a warming climate, other sources of thermal exposure may place salmonids at risk. Deforestation by clear cut logging has been shown to increase summer stream average temperatures by 7.8°C (Brown & Krygier, 1970). With increasing urbanization and paved surfaces, rainfall events have been shown to cause transient increases in average stream temperatures by a maximum of 6.8°C in streams with low flow rates (Herb et al., 2008). Heated effluent discharges, such as those used for cooling in electrical power plants, may also alter the thermal regimes of streams (Webb, 1996; Parker, 1974; Langford, 1990). Temperature exposure is also a vital component of rearing fish in aquaculture and hatchery environments, with heat shock early in development used to induce polyploidy (Thorgaard et al., 1981).
Investigation of the effects of thermal exposures at all stages of development are critical for a better understanding of the thermotolerance of salmonid species. In the current study, the long-term effects of an acute thermal exposure (e.g. mimicking a rare climate event, or point source pollution) on the future thermotolerance of larval rainbow trout was investigated. We compared the thermal tolerances (CTmax) between two groups of rainbow trout fry (control and temperature treatment) 2 months after the treatment group underwent a 96-h acute temperature exposure (22°C). Additionally, we utilized a recently developed quantitative real-time PCR method to definitively distinguish the expression profiles of the hsp70a and hsp70b isoforms. We initially confirmed these isoforms were heat inducible by exposing juvenile trout to 1-h heat shock and measuring their expression in excised brain tissue. Thereafter, expression of hsp70a and hsp70b were measured immediately following the 96-h exposure and after the CTmax test. This the first reported account of definitive differentiation of gene expression between these two isoforms in a salmonid. There are commercially available antibodies against rainbow trout Hsp70 protein, however, these antibodies are unable to differentiate between Hsp70a and Hsp70b, hence the current study utilized mRNA expression analysis to delineate the role of HSP isoforms in thermotolerance. We hypothesized that, consistent with previous findings, exposure to elevated sub-lethal temperatures would increase the tolerance of heat exposed fish, relative to those kept under control conditions.
Methods
Animals
Fertilized diploid rainbow trout embryos (from six females crossed with ten males, Mount Lassen strain) were obtained from Raven Brood Trout Station (Caroline, Alberta) and reared in a Heath tray incubator system supplied with 800 l of recirculating UV-sterilized dechlorinated Edmonton city tap water (pH 7.5, alkalinity as calcium carbonate 138 mg/l) at the University of Alberta. Water was chilled to 10°C by an attached chiller unit and constantly aerated with immersed air stones. Following hatch, yolk-sac larvae (alevins) were transferred from Heath trays to a 60-l tank supplied by the same recirculating system. Juvenile trout (9.60 ± 0.41 g, n = 12) used solely to confirm the inducibility of HSP by temperature exposure were obtained from Sam Livingston Fish Hatchery (Calgary, Alberta) and held at 4°C. All animal experiments were conducted under the Animal Use Protocol #072 as approved by the Biological Sciences Animal Policy and Welfare Committee at the University of Alberta and per the Canadian Council for Animal Care.
Determination of HSP inducibility
For the sole purpose of confirming the inducibility of the hsp gene isoforms, juvenile trout (n = 12) were placed in individual 4 l tanks supplied with flow-through aerated Edmonton city tap water at 4°C. After 4 days of acclimation, six of the tanks were then supplied with heated flow-through water resulting in a rapid increase in tank temperature to 20°C, and this temperature was maintained for 1 h. Following this period, both control and heat-shocked fish were sacrificed by lethal overdose of MS-222 (200 mg/l buffered with 400 mg/l NaHCO3). Brain tissue was then excised and immediately frozen in liquid nitrogen for hsp70a and hsp70b gene expression analysis.
Experimental protocol
During the period of yolk-sac absorption, 18 days’ post-hatch (dph) trout larvae (n = 15) were placed in 400-ml beakers (4 replicates per treatment) containing 250 mL of aerated Edmonton city tap water. Exposure beakers were partly immersed in a wet table supplied with flowing water at rearing temperature (10°C), to maintain constant temperature. Following a 24-h acclimation period, and a complete water change, control animals continued to receive 10°C water, but the treatment animals were placed into a separate water flow supplied with 22°C water. Over the course of the next 96 h, a 75% water change with temperature-specific water was performed every 24 h for both the control and treatment animals to avoid build-up of nitrogenous waste products.
At the end of the 96-h exposure, 5 larvae from each replicate were euthanized by concussive blow and snap-frozen in liquid nitrogen for later gene expression analysis, while the remaining larvae (~ 10) were transferred to rearing tanks. Each rearing tank was supplied with flow-through dechlorinated Edmonton city tap water at 10°C and aerated with an immersed air stone. Each tank consisted of divided chambers, with both control and treated larvae randomly assigned to individual chambers, to avoid tank or position bias. Chambers were separated by mesh barriers allowing water and oxygen flow but prohibiting mixing of larvae between groups. Trout larvae were reared under these conditions for 2 months (78 dph). Upon complete yolk sac resorption, fry were fed trout pellets ground to a powdered form (Trout Grower-Finisher Ration Pellets, Nu-Way, Okotoks, AB). Fish health was checked daily, and any mortality was recorded.
Critical thermal maxima
Following the two-month growth period, trout fry from both control and heat treatments underwent a critical thermal maximum (CTM) test to determine their individual thermal tolerance. This is a widely used method for estimating the maximum temperature a fish can tolerate (Cowles and Bogert, 1944; Becker and Genoway, 1979). In brief, 10°C water was re-circulated between the experimental tank, and an adjacent tank that contained a submersible water pump (RYOBI, Markham, ON) and a TA200 aquarium heater (AQUEON, Franklin, WI). The tanks contained 6 L, and were bubbled constantly and consistently with an air stone. This system facilitated water oxygen and temperature uniformity. Fish were placed in the experimental tank and then subjected to a constant linear temperature increase (0.331 ± 0.010°C/min) until they demonstrated a LOE. This exact endpoint was determined by two separate observing researchers, one of whom was blind to treatment status, and was defined as the point at which the fish was unable to right itself for five consecutive seconds. Control (n = 21) and elevated temperature (n = 17) groups were tested in random order throughout the CTM trials to avoid time of day as a confounding factor. Trials took about 1 h to complete. A 100% water change was performed between each trial to re-establish the ambient acclimation temperature and to rid the system of carryover waste/stress hormones from the previous trial. Water temperature was monitored and recorded with a Loligo Witrox system temperature probe and software (Loligo Systems, Denmark). Following the test, fish were weighed, euthanized by concussive blow, and snap-frozen for later gene expression analysis.
Quantitative gene expression
Juvenile trout brain tissue and whole trout larvae samples were ground with mortar and pestle in liquid nitrogen and total RNA was isolated using column purification with a NucleoSpin RNA Plus Kit (Machery-Nagel, Germany) per the manufacturer’s protocol. RNA was quantified and checked for purity via spectrophotometry (NanoDrop, ND-1000; Thermo Fisher Scientific, USA), and further validated via gel electrophoresis to visualize the 28S and 18S bands. For each individual sample, complementary DNA (cDNA) was synthesized from the RNA template (1.5 µg) using Superscript III Reverse Transcriptase (Invitrogen) with a mix of oligo(dT) (125 ng) and random primers (62.5 ng). Using the RNase H-Dependent qPCR technique (Integrated DNA Technologies, Coralville, IA, USA; Dobosy et al., 2011; Blair et al., 2016), gene-specific rhqPCR primers were designed for rainbow trout hsp70a (AB176854.1) and hsp70b (AB176855.1). These primers, which contain an integrated RNA nucleotide base, are blocked and cleavable by the addition of RNase H2 enzyme to the reaction, ensuring single product specificity when measuring expression of closely related isoforms. This was verified here by dissociation curve analysis displaying a single peak (Fig. 1). Gene specific primers were also designed for the endogenous control house-keeping gene elongation factor ef1 alpha (NM_001124339.1). All primers that were utilized are listed in Table 1. Quantitative real-time PCR (qPCR) was performed in a light cycling PCR machine (ABI Prism 7500 sequence detection system). Individual samples were run in triplicate using 2X SYBR green PCR mastermix (Thermo Fischer Scientific, Rockford, IL, USA), with a total reaction volume of 10 µl including 300 nmol of each primer, 1:20 dilution of cDNA template, and 0.5 µl of RNase H2 enzyme buffer mixture. The PCR reaction cycle was as follows: initial denaturation at 95°C for 2 min, 40 thermal cycles of melt for 15 s at 95°C followed by annealing and extension for 1 min at 60°C. Primer efficiency curves were run with a random sample of larval trout cDNA across a fivefold dilution series to evaluate replication efficiency. Sample values were calculated per a delta Ct quantification with reaction efficiency correction, normalized to ef1a, and expressed relative to the hsp70a control treatment (Pfaffl, 2001; Boyle et al., 2016).
Statistical analysis
Analysis was performed using GraphPad Prism 7 software (GraphPad Software, Inc. v. 7). Data are reported throughout as mean ± SE. All data were tested for normality (Shapiro–Wilk test). Significant differences (P < 0.05) in CTmax values and growth parameters between treatments were determined by Student’s t tests. Data were also analyzed for tank effects by comparing the mean CTmax values of each individual tank, irrespective of treatment, using one-way ANOVA with Tukey’s multiple comparison post hoc test (P < 0.05, as significant). Differences in gene expression were analyzed by two-way ANOVA with Holm–Sidak’s multiple comparison post hoc test (P < 0.05, as significant).
Results
Juvenile brain tissue hsp70 expression
Heat-shocked juvenile trout displayed higher levels of hsp70a and hsp70b isoform gene expression compared to control fish that did not receive a heat shock. While this increase in expression for hsp 70b was statistically significant, hsp70a expression was not (Fig. 2; one-way ANOVA, n = 6).
Relative gene expression of hsp70a and hsp70b in brain tissue of juvenile trout exposed to 1-h heat shock (20°C). In addition to the central experiment, to confirm hsp70a and hsp70b mRNA was heat inducible, six juvenile rainbow trout underwent a 1-h heat shock at 20°C and were compared to those held at 4°C. Relative gene expression was normalized to ef1a, and compared relative to the hsp70a control. Data are represented as mean ± SE (n = 6). Bars sharing letters are not significantly different as determined by two-way ANOVA with Holm–Sidak post hoc analysis (P < 0.05)
Mortality and growth
The initial 96-h exposure resulted in zero deaths for the control temperature (10 °C) group (0% mortality), while trout larvae exposed to elevated temperature (22 °C) experienced 15% mortality. From the beginning of the acute 96-h exposure to the day of thermal tolerance testing, the control group (10°C) experienced a significantly lower mortality rate (15%) over the entire course of the experiment (> 2 months) with no deaths during the 96-h exposure period, compared to the elevated temperature treatment group (22°C), which experienced 45% mortality, including six individuals that died during the initial high temperature exposure (P = 0.024, t test). At the conclusion of thermal maxima assessment there was no difference (P = 0.19, t test) between the mass of control 10°C-exposed trout (0.195 ± 0.017 g, n = 21) and the 22°C exposed fish (0.165 ± 0.015 g, n = 17).
Thermal tolerance
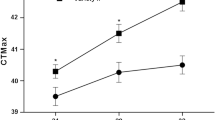
Control fish previously exposed for 96-h at 10°C displayed a significantly higher CTmax (28.22 ± 0.08°C, n = 21) compared to the elevated temperature exposed fish (CTmax = 27.57 ± 0.21°C, n = 17, P = 0.0037, t test) (Fig. 3). Analysis of the mean CTmax values originating from individual tanks revealed no significant differences between treatment groups due to tank distribution (P > 0.05, one-way ANOVA), ruling out the holding conditions as a factor contributing to the difference in CTmax values between treatments. The weight of trout fry was not a significant contributing factor to CTmax, as there was no relationship between CTmax values and mass (linear curve fit R2 = 0.039).
Rainbow trout fry critical thermal maxima (CTmax). As larvae, fish were exposed for 96 h to either control treatment (10°C) or elevated temperature treatment (22°C), and following a two-month recovery period, underwent the thermotolerance challenge. Control trout fry (n = 21) and elevated temperature (n = 17) exposed fry CTmax were significantly different (*) as determined via Student’s t test
Hsp70 expression
The acute exposure of larval trout to 22°C for 96 h resulted in significantly higher levels of hsp70b expression, relative to those of hsp70a (P < 0.05, n = 4, two-way ANOVA) (Fig. 4). However, there were no measurable differences in the relative expression levels of either hsp70a or hsp70b between the elevated temperature-exposed and control animals maintained at 10°C when analyzed at the 96-h post-acute exposure time point (P > 0.05, two-way ANOVA, n = 4 per treatment).
Relative gene expression of hsp70a and hsp70b in trout larvae post 96-h exposure to control (10°C) or elevated temperature (22°C). Relative gene expression was normalized to ef1a, and compared relative to the hsp70a control. Data are represented as mean ± SE (n = 4). Bars sharing letters are not significantly different as determined by two-way ANOVA with Holm–Sidak post hoc analysis
Following the CTmax trial, there was no difference in relative expression of hsp70a between control and elevated temperature treatments (1.00 ± 0.37 and 0.72 ± 0.13, respectively). However, there was a significant reduction in expression of hsp70b for the elevated temperature treated fish (15.26 ± 1.75), compared to the control fish (26.54 ± 5.57) (P = 0.0364, two-way ANOVA). Similar to the pattern observed in larval fish, the relative expression of the hsp70b isoform was significantly higher than hsp70a in trout fry, irrespective of treatment conditions (P < 0.05, n = 4, two-way ANOVA) (Fig. 5).
Relative gene expression of hsp70a and hsp70b post CTM trial of trout fry previously exposed to control (10°C) or elevated temperature (22°C). Relative gene expression was normalized to ef1a, and expressed relative to the hsp70a control. Data are represented as mean ± SE (n = 4). Bars sharing letters are not significantly different, as determined by two-way ANOVA with Holm–Sidak post hoc analysis
Discussion
While the current study confirms that HSPs are upregulated to deal with thermal stress, our data indicates that early exposure to high temperature reduces the thermotolerance of young fish, in contrast to previous work demonstrating that fish can achieve a higher level of thermotolerance after prior acclimation to an elevated temperature (Beitinger et al., 2000; Yamashita et al., 2010). Spatial and temporal variation in environmental temperature is a challenging abiotic stressor, particularly for ectothermic animals with narrow thermal optima such as rainbow trout. The ability of rainbow trout to cope with a temperature stress is likely to depend on their exposure history. The proximate mechanism underlying reduced tolerance is hypothesized to be a reduced capacity for HSP induction. These results reveal populations of young salmonids may be more vulnerable to thermal stress related to extreme climate events and thermal pollution discharge.
Critical thermal maxima
The average CTmax values for both control (28.23°C) and elevated temperature exposed (27.57°C) fish fell within the reported range of other thermotolerance studies using rainbow trout. For example, there are several reported CTmax values calculated with LOE as an endpoint for rainbow trout: 28.45 ± 0.28°C (Lee & Rinne, 1980), 27.6 ± 0.13°C (Myrick & Cech, 2000), 28.0 ± 0.36°C (Currie et al., 1998). Grande & Andersen (1991) reported an LT50 (temperature giving 50% mortality) of 26.2°C for 2–3-month-old rainbow trout larvae exposed to a temperature rate increase of 1°C/day. Variations in the CTmax among the same species are commonly attributed to acclimation temperature, heating rate, or significant differences in fish mass (Becker & Genoway, 1979). Notably, intraspecies variation in thermotolerance can also occur across the geographical range of a population, suggesting a genetically mediated physiological adaptation. This has been shown for both killifish (Fangue et al., 2006) and rainbow trout populations (Verhille et al., 2016). In the current study, we observed a significant decrease in the CTmax of rainbow trout fry exposed to a thermal stress occurring during development, compared to control fry. The two-month growth period between the thermal stress and the thermotolerance trial makes it difficult to accurately compare these results to previous work as most studies measure thermotolerance directly following, or soon after, the exposure period.
However, there have been studies that have examined the latency of chemical exposures on upper thermal tolerances. Claireaux et al. (2013), reported significant decreases in thermal tolerance between exposed and control European sea bass (Dicentrarchus labrax Linnaeus, 1758) 7 days after a 48-h exposure to crude oil; however, after 1-month recovery no differences in thermotolerance existed. For rainbow trout, a 48-h exposure to crude oil resulted in an increased CTmax following a 7-day recovery, while European sea bass in the same study demonstrated no significant differences in CTmax compared to controls following a six-month recovery period (Anttila et al., 2017). Furthermore, it was reported that when challenged with CTmax tests 1 month and 10 months after exposure to crude oil, juvenile sea bass displayed no differences in thermal tolerance (Mauduit et al., 2016). In light of these findings, and the presence of long-term thermotolerance changes in response to acute temperature shock in the current study, it appears that the persistence of stressor impacts on thermal maxima are stressor-dependent. This would likely rule out generalized mechanisms of stressor impact as an explanation for the reduced performance in temperature-exposed trout in the current study. For example, it has been noted that diverse stressors with distinct modes of action, all impair energy metabolism, resulting in this parameter being proposed as a common currency facilitating the development of multi-stressor risk assessment (Segner et al., 2014). On this basis it might be hypothesized that the elevated metabolic rate and costs of enacting defense mechanisms in heat exposed larval rainbow trout, led to a long-term deficit in energy resources, which subsequently reduced the capacity to respond to a subsequent stress (i.e. the CTmax assessment). However, if this were the case, then changes in energy status would likely be reflected in changes in body mass, which were not observed. Together the lack of evidence for altered energy metabolism and the distinct persistence of effects to stressor exposure over time, suggests that the differences between the current study (temperature as the stressor) and previous work (chemicals as the stressor) are due to the nature of the stressor. It is, however, worth highlighting that in the current study fish were exposed to the stressor at a much earlier life-stage than in the work cited above, which may also explain the distinct outcomes.
The current study utilized a single 96-h constant temperature exposure, characteristic of conditions found during point-source pollution events or temperature control malfunctions in a hatchery setting, as the initial thermal stress incurred by the rainbow trout larvae. In this regard the exposure mimicked standard assay protocols for the determination of medial lethal concentrations (i.e. LC50) of chemical stressors to fish. However, a diel temperature regime, more representative of natural environmental conditions in a river, has been shown to produce different results, and methodologically these experiments and outcomes are distinct. For example, Hokanson et al. (1977) demonstrated that under fluctuating temperature conditions, rainbow trout could increase their upper thermal tolerance limits, whereas those exposed to constant temperature could not. In contrast, while juvenile Atlantic salmon demonstrated an increased induction of Hsp70, there were no significant differences between the CTmax of fish exposed to a one-day diel temperature heat shock and those exposed to control temperature (Gallant et al., 2017). The high exposure temperature of 22°C utilized in the current work was chosen based on historical data (Hokanson et al., 1977), in addition to preliminary results that indicated if exposure temperature exceeded 22°C a higher mortality rate would occur. Indeed, the magnitude of the chosen temperature was stressful for the fish given the reported mortalities, and downstream tolerance effects. Studies showing an increase in thermotolerance resulting from a previous acclimation (exposure to an elevated temperature beyond ambient) utilize temperatures that are well below the upper thermotolerance threshold, whereas the current exposure conditions were much closer to the upper temperature limit for the species. Compared to ambient (i.e. 10°C), acclimation to elevated temperatures resulting in increased thermotolerance for rainbow trout are seldom conducted at temperatures above 20°C (Lee and Rinne, 1980; Currie et al., 1998; Beitinger et al., 2000). Certainly, experiments employing heat shock as a treatment for fish utilize higher temperatures (i.e. 25°C), however, the time of heat shock is relatively short, routinely only for 2 h or less (Currie, 2000; Basu et al., 2001; Sung et al., 2014).
Our original hypothesis, based on previous work on thermal tolerance in rainbow trout, was that fish exposed to high temperature would demonstrate a higher thermotolerance than controls, a hypothesis that was refuted by the current results. The magnitude and time of exposure are likely to account for this unanticipated experimental outcome. Given the high mortality (45%) in the elevated temperature group, 22°C is certainly near the chronic thermal capacity of these larval trout. Our exposure regime was chosen to fit the aims of our study, which was to examine the downstream thermotolerance effects of a thermal stress during development. Because larval fish are not easily amenable to thermotolerance studies, the two-month recovery period was chosen to allow the fish to grow to the fry stage, a more suitable life-stage at which to conduct a critical thermal maxima trial. The timing of thermal exposure in this work is ecologically-relevant given rainbow trout in Northern Hemisphere climates spawn in the spring, thus exposing young trout fry to the waters at their warmest (July and August, ~ 2 months post-hatch). This will be exacerbated by the utilization by young trout of the lateral regions of streams vulnerable to higher temperatures (Moore and Gregory, 1988; Behnke, 2010). However, future work should examine a range of temperature exposure regimes (e.g. one that follows a diel temperature cycle), which would better facilitate interpretation of effects related to climate change. The effect of temperature on hatching time has been well documented (Blaxter, 1991; Weber et al., 2016), however, the future physiological and thermotolerance effects of high temperature exposure during embryogenesis has only rarely been performed (e.g. in lake whitefish; Whitehouse et al., 2017), and thus further studies investigating this phenomenon in rainbow trout are warranted. Nevertheless, our data indicate that if a thermal stress is endured during early trout development, future thermal tolerance of young fry may be hindered.
Expression of Hsp70 isoforms
In the current work, we confirmed that hsp70a and hsp70b mRNA expression was heat inducible, with hsp70b displaying a significant increase compared to hsp70a in the brain tissue of heat-shocked juvenile rainbow trout. In concert with constitutive proteins such as Hsc70 (a member of the HSP family), the induction of HSP is a vital physiological mechanism protecting fish from thermal stressors (see reviews: Iwama et al., 1998; Basu et al., 2002; Yamashita et al., 2010). In rainbow trout, hsp70a and hsp70b were previously identified and shown to be induced by heat stress in fish (Kothary et al., 1984; Ojima et al., 2005a, b). In the current study, quantitative RT-PCR indicated larval mRNA expression of both isoforms of hsp70 following the 96-h exposure and post-CTmax sampling points. The RNase H-Dependent qPCR technique used allows for detection of single-nucleotide polymorphisms (SNPs) and subsequent differentiation between highly conserved sequences (Dobosy et al., 2011). This approach was critical for effectively distinguishing between hsp70a and hsp70b, which share a 93.1% nucleotide sequence identity. In many cases, primers can be designed to target genes with high specificity and with low primer dimer probabilities. However, when dealing with salmonids and other species that have undergone whole genome duplication events (Berthelot et al., 2014), developing gene primers that produce a single product is challenging, as genes tend to have nearly identical isoforms that differ in biological function (Richards et al., 2003). Utilizing the RNase H-Dependent qPCR technique, this is the first reported account of positive differentiation of gene expression between these two isoforms in rainbow trout. This technique will, therefore, be useful in future investigations of isoform-specific gene/protein functions. Although protein expression measures of HSP would support the direct functional role of HSP, the aim of this study to delineate the roles of the two different hsp70 isoforms was only achievable with mRNA expression analysis due to the current unavailability of specific antibodies able to differentiate between the Hsp70 isoforms.
The 96-h exposure to elevated temperature resulted in slight increases in gene expression of both hsp70a and hsp70b. Differences between treatments (i.e. control versus heat-stressed fish) were statistically non-significant, although fish demonstrated higher expression of hsp70b than hsp70a irrespective of treatment. In general, alterations in mRNA expression from baseline occur within minutes to hours following stress and thereafter tend to decline once translation and protein synthesis occur (Urbina et al., 2013; Wan et al., 2014). As indicated in other studies, fish exposed to sub-lethal heat shock and immediately sampled demonstrate upregulation of hsp70 mRNA and protein expression (Currie, 2000; Ojima et al. 2005a; Sung et al., 2014). The fact that the 96-h exposure in the current study was longer than previous heat shock treatments (elevated temp for a short period of 1–2 h), could suggest that the peak of expression was missed, thus explaining why the gene expression differences between control and treatment were not more dramatic.
The CTmax trial induced a significant upregulation of hsp70b for both control fish and those subjected to a heat shock early in development, while hsp70a expression was not altered in either treatment. Furthermore, fish that were previously exposed to elevated temperature for 96-h 2 months prior to CTmax assessment had significantly lower levels of hsp70b than the control fish. Consequently, it is likely that the lower CTmax of the elevated temperature exposed fish may have resulted from their relative inability to upregulate hsp70b (~ 58% reduction compared to control fish). These data are supported by similar results of hsp mRNA level suppression observed in various stages of embryonic development of whitefish (Whitehouse et al., 2017) and in medaka (Oryzias latipes Temminck & Schlegel, 1846; Werner et al., 2001) following a thermal stress.
In the current study, the initial 96-h elevated temperature exposure negatively affected the developing larvae, resulting in a reduced expression of hsp70b and a lower thermotolerance. This suggests that the decreased hsp induction in elevated temperature-exposed trout results in a reduced thermotolerance by impairing the capacity of the fish to protect protein integrity. The effect of early heat shock on subsequent hsp induction explains the contrast between this study and previous work that has demonstrated increased CTmax in response to previous exposure to elevated temperature (Lee & Rinne, 1980; Currie et al., 1998; Beitinger et al., 2000). Previous studies have shown that hsp70 expression is associated with negative outcomes in thermally exposed fish. For example, in green sturgeon (Acipenser medirostris Ayres, 1854), heat treatment of 26°C resulted in an increase in hsp70 expression and larval spinal deformities. Furthermore, the deformed individuals demonstrated the highest levels of hsp70 expression, suggesting deformities and increased hsp levels were associated with thermal damage (Werner et al., 2007). Additionally, an overexpressing Hsp70 transgenic zebrafish (Danio rerio Hamilton, 1822) line was characterized by high apoptosis in embryos, and surviving fish developed morphological deformities including jaw defects, smaller eyes, and tail fin abnormalities (Yamashita & Hojo, 2004). The role of HSP during development is complex with various members showing differential spatiotemporal expression. Coordination of HSP expression is a highly regulated process and interruptions such as a thermal stress may result in elevated or overexpression of HSP gene and protein levels. The downstream effects of this expression have been shown to result in morphological deformities and, as our current study results indicate, possible inhibition of future physiological functions including thermotolerance. Of these two hsp70 isoforms, we suggest hsp70b rather than hsp70a, plays the more important role in thermoregulatory strategies for rainbow trout. However, a comprehensive tissue expression analysis would be necessary to fully assess the physiological impact of each hsp70 isoform.
Conclusion
Two months after rainbow trout fry were exposed to 22°C for 96 h during development, a significantly lower CTmax, paralleled by a reduced expression of hsp70b, were discerned. This indicates that exposure to thermal stress in early life-stages of rainbow trout may have negative effects on future physiological function. In concordance with Bevelhimer & Bennett (2000), our study clearly demonstrates that once a certain temperature or time threshold is surpassed, the benefits of acclimation are lost and physiological stress caused by exposure to sub-lethal elevated temperatures will ultimately result in decreased thermotolerance. This study, therefore, provides novel insight into possible long-term effects associated with temperature exposures in hatcheries and the aquaculture industry, and in natural environments subjected to a warmer climate, continued deforestation, and industrial expansion resulting in thermal pollution discharge.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Anttila, K., F. Mauduit, S. Le Floch, G. Claireaux & M. Nikinmaa, 2017. Influence of crude oil exposure on cardiac function and thermal tolerance of juvenile rainbow trout and European sea bass. Environmental Science and Pollution Research 24: 19624–19634.
Basu, N., T. Nakano, E. G. Grau & G. K. Iwama, 2001. The effects of cortisol on Heat Shock Protein 70 levels in two fish species. General and Comparative Endocrinology 124: 97–105.
Basu, N., A. E. Todgham, P. A. Ackerman, M. R. Bibeau, K. Nakano, P. M. Schulte & G. K. Iwama, 2002. Heat shock protein genes and their functional significance in fish. Gene 295: 173–183.
Becker, C. D. & R. G. Genoway, 1979. Evaluation of the critical thermal maximum for determining thermal tolerance of freshwater fish. Environmental Biology of Fishes 4: 245–256.
Behnke, R., 2010. Trout and Salmon of North America. Simon and Schuster, Inc., New York.
Beitinger, T. L., W. A. Bennett & R. W. McCauley, 2000. Temperature tolerances of North American freshwater fishes exposed to dynamic changes in temperature. Environmental Biology of Fishes 58: 237–275.
Berthelot, C., F. Brunet, D. Chalopin, A. Juanchich, M. Bernard, B. Noël, P. Bento, C. Da Silva, K. Labadie & A. Alberti, 2014. The rainbow trout genome provides novel insights into evolution after whole-genome duplication in vertebrates. Nature Communications 5: 3657.
Bevelhimer, M. & W. Bennett, 2000. Assessing cumulative thermal stress in fish during chronic intermittent exposure to high temperatures. Environmental Science Policy 3: 211–216.
Billard, R., 1983. Environmental factors in salmonid culture and the control of reproduction. In International Symposium on Salmonid Reproduction, Bellevue, Washington, USA, 31 October–2 November 1983. Washington Sea Grant Program. University of Washington, Washington DC.
Blair, S. D., D. Matheson, Y. He & G. G. Goss, 2016. Reduced salinity tolerance in the Arctic grayling (Thymallus arcticus) is associated with rapid development of a gill interlamellar cell mass: implications of high-saline spills on native freshwater salmonids. Conservation Physiology 4: cow010.
Blaxter, J. H. S., 1991. The effect of temperature on larval fishes. Netherlands Journal of Zoology 42: 336–357.
Boyle, D., S. D. Blair, D. Chamot & G. G. Goss, 2016. Characterization of developmental Na+ uptake in rainbow trout larvae supports a significant role for Nhe3b. Comparative Biochemistry and Physiology, Part A: Molecular & Integrative Physiology 201: 30–36.
Brett, J. R., 1970. Temperature. 3.3. Animals. 3.32. Fishes. In Kinne, O. (ed.), Marine Ecology. Wiley Interscience, London.
Brown, G. W. & J. T. Krygier, 1970. Effects of clear-cutting on stream temperature. Water Resources Research 6: 1133–1139.
Buisson, L., W. Thuiller, S. Lek, P. Lim & G. Grenouillet, 2008. Climate change hastens the turnover of stream fish assemblages. Global Change Biology 14: 2232–2248.
Claireaux, G., M. Théron, M. Prineau, M. Dussauze, F.-X. Merlin & S. Le Floch, 2013. Effects of oil exposure and dispersant use upon environmental adaptation performance and fitness in the European sea bass, Dicentrarchus labrax. Aquatic Toxicology 130–131: 160–170.
Cowles, R. B. & C. M. Bogert, 1944. A preliminary study of the thermal requirements of desert reptiles. Iguana 83: 53.
Currie, S., 2000. The effects of heat shock and acclimation temperature on hsp70 and hsp30 mRNA expression in rainbow trout: in vivo and in vitro comparisons. Journal of Fish Biology 56: 398–408.
Currie, S. & B. Tufts, 1997. Synthesis of stress protein 70 (Hsp70) in rainbow trout (Oncorhynchus mykiss) red blood cells. Journal of Experimental Biology 200: 607–614.
Currie, R. J., W. A. Bennett & T. L. Beitinger, 1998. Critical thermal minima and maxima of three freshwater game-fish species acclimated to constant temperatures. Environmental Biology of Fishes 51: 187–200.
Dobosy, J. R., S. D. Rose, K. R. Beltz, S. M. Rupp, K. M. Powers, M. A. Behlke & J. A. Walder, 2011. RNase H-dependent PCR (rhPCR): improved specificity and single nucleotide polymorphism detection using blocked cleavable primers. BMC Biotechnology 11: 80.
Fangue, N. A., M. Hofmeister & P. Schulte, 2006. Intraspecific variation in thermal tolerance and heat shock protein gene expression in common killifish, Fundulus heteroclitus. Journal of Experimental Biology 209: 2859–2872.
Fowler, S. L., D. Hamilton & S. Currie, 2009. A comparison of the heat shock response in juvenile and adult rainbow trout (Oncorhynchus mykiss)—implications for increased thermal sensitivity with age. Canadian Journal of Fisheries and Aquatic Sciences 66: 91–100.
Gallant, M. J., S. LeBlanc, T. J. MacCormack & S. Currie, 2017. Physiological responses to a short-term, environmentally realistic, acute heat stress in Atlantic salmon, Salmo salar. FACETS 2: 330–341.
Grande, M. & S. Andersen, 1991. Critical thermal maxima for young salmonids. Journal of Freshwater Ecology 6: 275–279.
Herb, W. R., B. Janke, O. Mohseni & H. G. Stefan, 2008. Thermal pollution of streams by runoff from paved surfaces. Hydrological Processes 22: 987–999.
Hokanson, K. E., C. F. Kleiner & T. W. Thorslund, 1977. Effects of constant temperatures and diel temperature fluctuations on specific growth and mortality rates and yield of juvenile rainbow trout, Salmo gairdneri. Journal of the Fisheries Research Board of Canada 34: 639–648.
IPCC, 2007. In Pachauri,R. K. & A. Reisenger (eds) Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, New York.
Iwama, G. K., P. T. Thomas, R. B. Forsyth & M. M. Vijayan, 1998. Heat shock protein expression in fish. Reviews in Fish Biology and Fisheries 8: 35–56.
Kaushal, S. S., G. E. Likens, N. A. Jaworski, M. L. Pace, A. M. Sides, D. Seekell, K. T. Belt, D. H. Secor & R. L. Wingate, 2010. Rising stream and river temperatures in the United States. Frontiers in Ecology and the Environment 8: 461–466.
Kothary, R. K., D. Jones & E. P. Candido, 1984. 70-Kilodalton heat shock polypeptides from rainbow trout: characterization of cDNA sequences. Molecular and Cellular Biology 4: 1785–1791.
Krone, P. H., J. B. Sass & Z. Lele, 1997. Heat shock protein gene expression during embryonic development of the zebrafish. Cellular and Molecular Life Sciences 53: 122–129.
Langford, T., 1990. Ecological Effects of Thermal Discharges. Elsevier, New York.
Lee, R. M. & J. N. Rinne, 1980. Critical thermal maxima of five trout species in the southwestern United States. Transactions of the American Fisheries Society 109: 632–635.
Mauduit, F., P. Domenici, A. P. Farrell, C. Lacroix, S. Le Floch, P. Lemaire, A. Nicolas-Kopec, M. Whittington, J. L. Zambonino-Infante & G. Claireaux, 2016. Assessing chronic fish health: An application to a case of an acute exposure to chemically treated crude oil. Aquatic Toxicology 178: 197–208.
McCullough, D. A., J. M. Bartholow, H. I. Jager, R. L. Beschta, E. F. Cheslak, M. L. Deas, J. L. Ebersole, J. S. Foott, S. L. Johnson, K. R. Marine, M. G. Mesa, J. H. Petersen, Y. Souchon, K. F. Tiffan & W. A. Wurtsbaugh, 2009. Research in thermal biology: burning questions for coldwater stream fishes. Reviews in Fisheries Science 17: 90–115.
Mohseni, O., H. G. Stefan & J. G. Eaton, 2003. Global warming and potential changes in fish habitat in US streams. Climatic Change 59: 389–409.
Moore, K. M. & S. V. Gregory, 1988. Summer habitat utilization and ecology of cutthroat trout fry (Salmo clarki) in Cascade Mountain streams. Canadian Journal of Fisheries and Aquatic Sciences 45: 1921–1930.
Morrison, J. K. & C. E. Smith, 1986. Altering the spawning cycle of rainbow trout by manipulating water temperature. The Progressive Fish-Culturist 48: 52–54.
Myrick, C. A. & J. J. Cech, 2000. Temperature influences on California rainbow trout physiological performance. Fish Physiology and Biochemistry 22: 245–254.
Ojima, N., M. Yamashita & S. Watabe, 2005a. Quantitative mRNA expression profiling of heat-shock protein families in rainbow trout cells. Biochemical and Biophysical Research Communications 329: 51–57.
Ojima, N., M. Yamashita & S. Watabe, 2005b. Comparative expression analysis of two paralogous Hsp70s in rainbow trout cells exposed to heat stress. Biochimica et Biophysica Acta – Gene Structure and Expression 1681: 99–106.
Pankhurst, N. W., G. J. Purser, G. Van Der Kraak, P. M. Thomas & G. N. R. Forteath, 1996. Effect of holding temperature on ovulation, egg fertility, plasma levels of reproductive hormones and in vitro ovarian steroidogenesis in the rainbow trout Oncorhynchus mykiss. Aquaculture 146: 277–290.
Parker, F. L., 1974. Thermal Pollution and the Environment. In Sax, N. I. (ed.), Industrial Pollution. Van Nostrand Rheinhold, New York.
Pfaffl, M. W., 2001. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Research 29: e45–e45.
Rendell, J. L., S. Fowler, A. Cockshutt & S. Currie, 2006. Development-dependent differences in intracellular localization of stress proteins (hsps) in rainbow trout, Oncorhynchus mykiss, following heat shock. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics 1: 238–252.
Richards, J. G., 2003. Na+/K+-ATPase-isoform switching in gills of rainbow trout (Oncorhynchus mykiss) during salinity transfer. Journal of Experimental Biology 206: 4475–4486.
Rombough, P. J., 1997. The effects of temperature on embryonic and larval development. In Wood, C. M. & D. G. McDonald (eds.), Global Warming: Implications for Freshwater and Marine Fish. Cambridge University Press, New York.
Schindler, D. W., 1997. Widespread effects of climatic warming on freshwater ecosystems in North America. Hydrological Processes 11: 1043–1067.
Schurmann, H., J. F. Steffensen & J. P. Lomholt, 1991. The influence of hypoxia on the preferred temperature of rainbow trout Oncorhynchus mykiss. Journal of Experimental Biology 157: 75–86.
Segner, H., M. Schmitt-Jansen & S. Sabater, 2014. Assessing the impact of multiple stressors on aquatic biota: the receptor’s side matters. Environmental Science & Technology 48: 7690–7696.
Sung, Y. Y., H. J. Liew, A. M. Ambok Bolong, M. E. Abdul Wahid & T. H. MacRae, 2014. The induction of Hsp70 synthesis by non-lethal heat shock confers thermotolerance and resistance to lethal ammonia stress in the common carp, Cyprinus carpio (Linn). Aquaculture Research 45: 1706–1712.
Symons, P. E. K. & M. Heland, 1978. Stream habitats and behavioral interactions of underyearling and yearling Atlantic salmon (Salmo salar). Journal of the Fisheries Research Board of Canada 35: 175–183.
Thorgaard, G. H., M. E. Jazwin & A. R. Stier, 1981. Polyploidy induced by heat shock in rainbow trout. Transactions of the American Fisheries Society 110: 546–550.
Urbina, M. A., P. M. Schulte, J. S. Bystriansky & C. N. Glover, 2013. Differential expression of Na+, K+-ATPase α-1 isoforms during seawater acclimation in the amphidromous galaxiid fish Galaxias maculatus. Journal of Comparative Physiology B 183: 345–357.
Verhille, C. E., K. K. English, D. E. Cocherell, A. P. Farrell & N. A. Fangue, 2016. High thermal tolerance of a rainbow trout population near its southern range limit suggest local thermal adjustment. Conservation Physiology 4: cow57.
Wan, J., X. Ge, B. Liu, J. Xie, S. Cui, M. Zhou, S. Xia & R. Chen, 2014. Effect of dietary vitamin C on non-specific immunity and mRNA expression of three heat shock proteins (HSPs) in juvenile Megalobrama amblycephala under pH stress. Aquaculture 434: 325–333.
Webb, B. W., 1996. Trends in stream and river temperature. Hydrological Processes 10: 205–226.
Weber, G. M., K. Martin, J. Kretzer, H. Ma & D. Dixon, 2016. Effects of incubation temperatures on embryonic larval survival in rainbow trout, Oncorhynchus mykiss. Journal of Applied Aquaculture 28: 285–297.
Werner, I., C. S. Koger, J. T. Hamm & D. E. Hinton, 2001. Ontogeny of the heat shock protein, hsp70 and hsp60, response and developmental effects of heat shock in the teleost, medaka (Oryzias latipes). Environmental Sciences 8: 13–29.
Werner, I., J. Linares-Casenave, J. P. Van Eenennaam & S. I. Doroshov, 2007. The effect of temperature stress on development and heat-shock protein expression in larval Green Sturgeon (Acipenser mirostris). Environmental Biology of Fishes 79: 191–200.
Whitehouse, L. M., C. S. McDougall, D. I. Stefanovic, D. R. Boreham, C. M. Somers, J. Y. Wilson & R. G. Manzon, 2017. Development of the embryonic heat shock response and the impact of repeated thermal stress in early stage lake whitefish (Coregonus clupeaformis) embryos. Journal of Thermal Biology 69: 294–301.
Yamashita, M. & M. Hojo, 2004. Generation of a transgenic zebrafish model overexpressing heat shock protein HSP70. Marine Biotechnology 6: S1–7.
Yamashita, M., T. Yabu & N. Ojima, 2010. Stress Protein HSP70 in Fish. Aqua-BioScience Monographs 3: 111–141.
Acknowledgments
The authors would like to thank Greg Goss for the use of laboratory space and animals. They are grateful for Brian Charles and Raven Brood Trout Station for providing rainbow trout embryos. Thanks to Tad Plesowicz and Erik Folkerts for assistance with fish husbandry. The authors would also like to thank Troy Locke and the staff at MBSU for their support and expertise. This research was supported by a Campus Alberta Innovates Program Research Chair to CNG.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Christian Sturmbauer
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Blair, S.D., Glover, C.N. Acute exposure of larval rainbow trout (Oncorhynchus mykiss) to elevated temperature limits hsp70b expression and influences future thermotolerance. Hydrobiologia 836, 155–167 (2019). https://doi.org/10.1007/s10750-019-3948-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-019-3948-1