Abstract
Population size and structure, as well as individual growth rates, condition, and reproductive output, respond to environmental factors, particularly in short-lived and fast-growing squid species. We need to understand the mechanisms through which populations respond to environmental conditions, to predict when or if established relationships, used as management tools to forecast recruitment strength, might break down completely. Identifying characteristics of successful recruits who have grown under different environmental scenarios may improve our understanding of the mechanistic connections between environmental conditions and the temporal variation in life history characteristics that ultimately affect recruitment. This 5-year study sought to determine the association between key life history characteristics of southern calamary Sepioteuthis australis (growth rate, body size, and patterns of repro-somatic energy allocation) and the environmental conditions experienced by individuals on the east coast of Tasmania, Australia. Among years, all population and individual parameters examined were highly variable, despite the environmental regime during the study not encompassing the extremes that may occur in this dynamic region. Temperature was not clearly associated with any of the individual or population differences observed. Populations of apparently similar abundance were composed of individuals with strikingly different biological characteristics, therefore seeking relationships between abundance and environmental parameters at gross levels did not shed light on the mechanisms responsible for population size. Importantly, inter-annual differences in squid size, condition, reproductive investment, and possibly growth rate, were sex-specific, indicating that males and females responded differently to similar factors. Among years differences in body size were extreme, both among the male component of the population and between genders. The relative importance of many size-based processes that contribute to population size and structure (e.g. predation, starvation, competition, and reproductive success) will therefore vary inter-annually.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a consequence of their fast growth and short life cycle, the size of cephalopod populations can fluctuate over several orders of magnitude between years (Boyle 1990). This potentially limits more directed exploitation (Bellido et al. 2001) and creates difficulties in assessing stock size for fisheries management. However, recent studies suggest that like fish (Cury et al. 1995), strong environment-recruitment relationships may exist for a number of squid stocks (e.g. Loligo forbesi, Robin and Denis 1999; L. gahi, Agnew et al. 2000, 2002; Illex argentinus, Waluda et al. 2001). The temperature dependence of squid growth has been suggested as an explanatory factor for environment-recruitment relationships (Robin and Denis 1999; Agnew et al. 2002). However, growth and reproductive processes are tightly linked (Mangold et al. 1993), and as such any impact of temperature or other factors on growth will also impact on the timing and level of energy allocated to reproductive effort. There is considerable variation in the reproductive strategies of individuals within some cephalopod populations, particularly age and size at maturation (Boyle et al. 1995; Moltschaniwskyj 1995; Arkhipkin et al. 2000). Once mature, many cephalopods show a high degree of individual variation in the level of anatomical investment in reproductive structures, particularly females (Jackson 1993; Pecl 2001). The allocation of energy to reproduction will also directly affect subsequent population size as this determines the fertility and survival schedule (Brommer 2000).
Although causal links for relationships between stock abundance and environmental parameters have been suggested, there are still no rigorous working hypotheses explaining observed fluctuations in squid abundance. The progression of our understanding in this area is severely hindered by the lack of comprehensive multi-year studies examining changes in population structure and plasticity in a suite of individual life history characteristics of wild populations. This is a critical knowledge gap as the ultimate effects of environment on stock abundance are transduced through individual organisms into population-level phenomena, with the population response arising as the summation of individual responses (Dunham and Overall 1994). There is also a continuous interplay between population-level and individual-level processes (Brommer 2000), although for cephalopods the role that density-dependence may play in regulating population size and structure has not been examined. Without understanding the mechanisms through which population responses to environmental conditions are mediated, we cannot predict when or if established relationships might break down completely (Agnew et al. 2002). Identifying the growth, condition and reproductive characteristics of recruits who have grown under different environmental scenarios may lead to an improved understanding of the mechanistic connections between environmental conditions and the temporal variation in life history characteristics that might ultimately affect recruitment. Establishing the characteristics of successful recruits may lead to improved forecasts of annual population size.
Southern calamary (Sepioteuthis australis) are a fast-growing, short-lived (<1 year) and multiple-spawning inshore species (Pecl 2001). All life history characteristics of southern calamary examined to date are highly variable, including, egg size and embryo mortality (Steer et al. 2002), annual egg production (Moltschaniwskyj and Pecl 2003), hatchling size (Steer et al. 2003), level of reproductive investment (Pecl 2001), and growth (Pecl, in press). Although low levels of spawning take place throughout the year, there is a distinct observable peak in the austral spring and summer (Oct–Jan) when southern calamary aggregate over shallow (<10 m) inshore spawning grounds. The individual and population characteristics of southern calamary during the spawning season are variable at daily (Moltschaniwskyj et al. 2003a), weekly (Jackson and Pecl 2003), and monthly (Moltschaniwskyj and Pecl 2003) time scales.
Much of the spawning activity on the east coast of Tasmania is concentrated in Great Oyster Bay (Moltschaniwskyj and Pecl 2003), which is an area of convergence between warm, nutrient-poor East Australian Current (EAC) water of sub-tropical origin and cool, nutrient-rich water of sub-antarctic origin. The latitudinal position of the convergence varies both between seasons and years and is determined by zonal westerly wind stress and large-scale oceanographic circulation associated with El Niño/Southern Oscillation events (Harris et al. 1987). Periodic intrusions of EAC water onto the shelf south of Great Oyster Bay, results in stratification, and a large decrease in productivity due to reduced input and recycling of nutrients (Harris et al. 1991). Under such conditions the dominant large zooplankter, krill (Nyctiphanes australis), and pelagic fish, jack mackerel (Trachurus declivis) disappear from the shelf (Young et al. 1993). In contrast, when nutrient-rich water dominates the shelf, primary and secondary productivity are higher (Harris et al. 1987; Young et al. 1993) and numbers of predators increase (Young et al. 1996). The oceanographic variability evident within Great Oyster Bay makes this region an ideal area to study environmentally induced individual and population variability in loliginid squid.
The aim of this 5-year study was to determine the association between key life history characteristics of southern calamary (growth rate, body size, and patterns of repro-somatic energy allocation) and oceanographic conditions experienced by the individuals. The extended temporal scope of the study allowed a description of the variation in individual and population characteristics under a range of environmental conditions. An added dimension of this study was the approach of utilizing population- and individual-based information, with the aim of linking population-level information with individual processes of energy allocation to somatic and reproductive growth. Understanding the inter-annual dynamics of this abundant, short-lived, and highly plastic species may help generate mechanism-based environment-recruitment hypotheses worth exploring further in other coastal squid species.
Materials and methods
Sepioteuthis australis individuals were collected using hand jigs, and to a lesser extent purse-seine, from the fishery and targeted research sampling area from Great Oyster Bay on the east coast of Tasmania, Australia (42°07′34″S, 148°17′51″E). Squid were collected during the austral summer over five spawning seasons; early 1996 (January), 1996/1997, 1999/2000, 2000/2001 and 2001/2002 (all November, December and January).
A total of 1,211 squid were examined throughout the study, with weight information recorded for 837 squid and a sub-sample of 484 individuals aged via increments in the statolith. Most individuals from January 1996 and 1996/1997 were refrigerated or placed on ice within a few hours of capture and measured within 12 h. All other squid were stored frozen until processed. Squid were assigned a maturity stage according to the relative size and colour of reproductive organs, following the six-stage maturity scale of Lipinski (1979), as per Pecl (2001). Under this scheme, stages IV and V are mature and are hence part of the spawning stock. Dorsal mantle length (ML) was measured to the nearest millimetre, and gonad (ovary or testis) and total body weight to the nearest 0.01 g.
To examine the relationship between somatic condition and level of reproductive investment by females, residuals were generated for the ovary-ML and body weight-ML relationships from model II regressions using log data. Residuals were standardised by dividing each residual by the SD of the predicted values. Residuals of the weight-length relationship provide a size-independent measure of the somatic condition of an individual at the whole animal level (Moltschaniwskyj and Semmens 2000). An individual that is lighter for its length than predicted from the regression equation (negative residual), is suggested to be in poorer somatic condition than an individual who is heavier for its length than predicted from the regression equation (with a positive residual). Residuals from the ovary-ML relationship provide an indicator of the level of reproductive investment.
S. australis statolith increments have been validated as daily in squid of known age up to 102 days of age (Pecl, in press). Statoliths from southern calamary collected during January 1996, 1996/1997, and part of 1999/2000 were stored, treated, and processed as detailed in Pecl (in press). Work in 2000 revealed that the statolith microstructure was clearer towards the dorsal margins when statoliths were ground along the dorsoventral axis (as per Dawe and Beck 1993). Examination of 20 adult statoliths and five juvenile statoliths of known age (from Pecl, in press) prepared using both methods revealed no statistical difference between the methods (paired t -test, t =−0.774, df =24, P =0.446). Statoliths from the remainder of the study were thus prepared using the method of Dawe and Beck (1993). All statoliths were read twice, most by two different readers, with one reader common to all statoliths. Statoliths from January 1996 and 1996/1997 combined were read in a random order, as were statoliths from the last years of the study (i.e. not all statoliths from 1 year were read and then all from the next year). Statoliths were classified as unreadable if the natal ring was unclear, if two consecutive counts differed by >10%, or >10% of the rings were interpolated. From each sample 4–7% of statoliths were discarded as unreadable due to statolith microstructure.
Estimates of abundance
Independent fishery estimates of biomass over the entire duration of the study are unavailable for southern calamary; however, as population parameters are affected by biomass, catch per unit effort (CPUE) has been used as a proxy of relative abundance. Scuba surveys directly quantifying southern calamary egg production within Great Oyster Bay over a 4-year period have shown a good relationship between measured egg production and commercial CPUE on a monthly basis, suggesting that CPUE is a good proxy for biomass (Moltschaniwsky et al. 2003b). Southern calamary are harvested commercially using squid jigs, hand-spear, dip net, and purse-seine, with squid jigs the primary method accounting for 61% of the total Tasmanian catch and 86% of the Great Oyster Bay catch in 2000/2001. Therefore, for the CPUE analysis only catch reported by the jig fishery in Great Oyster Bay was used and was calculated as the geometric mean of kilograms per jig hour (Lyle and Hodgson 2001). Catch and effort data are reported on a daily basis, via monthly returns of fishers. CPUE estimates were derived for each spawning season for the months of November, December and January combined. As the average weight of individuals varied substantially between years, CPUE values were also adjusted using the average weight of squid in each spawning season to determine the relative abundance as number of individuals per jig hour.
Oceanographic data
Sea surface temperature (SST) data were derived for an area encompassing the central third of the east coast of Tasmania to provide an indicator of temperature variations for that broad region. Reynolds SST data were sourced from the NOAA-CIRES Climate Diagnostics Centre, Boulder, Colorado, from their web site at http://www.cdc.noaa.gov/. The optimum interpolated SST analysis was produced weekly on a 1° grid (i.e. an area much larger than Great Oyster Bay). Southern calamary is an inshore coastal species that is not commonly caught in offshore Tasmanian waters (Jordan 1997), therefore, the temperature data are derived from an area encompassing the region that calamary are likely to have hatched and subsequently grown in. Vertical temperature profiling of the east coast of Tasmania has shown that the thermocline, if present, is generally deeper than 30 m, with the upper layers well mixed (Jordan et al. 1995). As the vast majority of Great Oyster Bay is <20 m deep, with only small sections at the mouth of the bay approaching 40 m in depth, estimates of SST used in this study are likely to reflect the general thermal conditions of the bay. Weekly SST data were used to generate a sine function [temperature=mean location+amplitude×sin (t -phase/cycle)] to formally characterise the temperature regime in each year in terms of mean location, amplitude, phase and cycle. The best fit was decided by minimising the sums of squares residuals.
Results
Individual size and population sex ratios
In each year of sampling, individuals of both sexes were obtained over a wide size and age range (Table 1). Average sizes of males and females were significantly different, but the nature of the difference was dependent upon year (F sex×year =5.85, df =9, 811, P <0.001). Males were between 31–88% larger on average in each year compared with females, except in January 1996 and 1999/2000 when there was no difference (Fig. 1). The difference in size between the sexes was particularly dramatic in the later years of the study (Fig. 1). Combined ANOVA regression revealed a significant positive linear relationship between average male size and year (F =402.31, df =1, 3, P <0.001); the rate of increase in weight through time was 167.36 g (±17.88 SE) per year. Female size varied over the duration of the study, but this was not as dramatic as the changes in average size of the males, with males in the later 2 years twice the size of males in the earlier years.
In each year, 72–100% of the squid sampled were mature and in spawning condition. However, in January 1996, 23 juveniles and immature females were caught, and eight immature females were also caught in 1996/1997. Between 1999/2000 and 2001/2002, only three immature squid were caught, and these were two stage-two males and one juvenile caught in 1999/2000. There was a significant difference in the sex ratios between the years (χ2=27, df =4, P <0.001). In January 1996 there was a similar number of males and females sampled (51.5% females); however, in all other samples the sex ratios consistently favoured males (Table 1). The percentage of females decreased from 41.3% in late 1996 to 20.4% in 2000/2001, with an increase in 2001/2002 to 35.3%.
A comparison of the average size of jig- vs. seine-caught individuals from 1996/1997 (from all of November–January combined) and 1999/2000 (seine and jig sample from the one commercial fisher, same location and day) show no evidence of differential selectivity between the gear types (F <1, df =1, 271, P =0.526; F <1, df =1,68, P =0.984, respectively). The sex ratios in the jig vs. seine samples were also not significantly different in either 1996/1997 (χ2=0.52, df =1, P =0.473) or 1999/2000 (χ2=0.87, df =1, P =0.768), with the percentage of females ranging between 37–44% for each combination of gear type and year.
Age
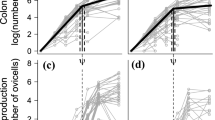
Differences in the range of ages and sizes across the years, and the unbalanced sample sizes made comparisons between the years difficult (Table 2). However, examination of the weight-at-age graph (Fig. 2) shows that squid from January 1996 and 1996/1997 did not appear to have grown either comparatively very quickly or very slowly. In contrast, squid from 1999/2000 display a high degree of variability in size-at-age and are found above and below the main group. This indicates that in this year, in addition to squid with “average” growth rates, there was a component of the population that had grown very fast, and a component that had grown very slowly. Likewise, a component of the 2000/2001 individuals also grew very fast, but slower growing squid were absent. Using only age data over a range common to all samples (140–220 days), size-at-age was determined to be dependent upon sex (F =12, df =1, 386, P <0.001) and year (F =6.5, df =3, 386, P <0.001). Males were on average 21% heavier at a given age compared with females, indicating that they had grown faster for some duration of their life. On average, squid caught in 1996/1997 had grown considerably slower than individuals caught in 1999/2000 and 2000/2001 (Fig. 3). Although the sex×year term in the model was not significant (F =2.46, df =3, 386, P =0.06), examination of the estimated marginal means show that the difference in size-at-age between males and females was relatively small in squid from January 1996 and 1996/1997, compared to the later years where males appeared to be growing much faster than females. The non-significance of the analysis of covariance (ANCOVA) may be due to the unbalanced model and the low numbers of females aged in 2000/2001.
Based on age, the back-calculated hatch date for each individual indicated significantly different hatching periods for each of the years. Squid caught in January 1996 were hatched mostly in July, August and September, whilst those caught in 1996/1997 were estimated to have hatched in May, June and July. In contrast, squid caught in 1999/2000 and 2000/2001 appeared to have similar hatch peaks, mostly hatching in April, May and June (Fig. 4).
Reproduction and condition
For both males and females there were differences in the length-weight relationship of squid on the spawning grounds in each of the years (males F =7.18, df =9, 524, P <0.001; females F =7.28, df =9, 251, P <0.001). Comparison of the slopes of the length-weight relationship showed that this was because female squid caught in January 1996 were in better somatic condition than those caught in 2000/2001 (Fig. 5). Male squid caught in January 1996 and 2000/2001 were heavier at a given length than males caught in 2001/2002 (Fig. 5).
In each year males showed a very weak relationship between somatic and testis mass (r 2 =0.26–0.36). An ANCOVA showed that testis weight, adjusted for body weight, varied significantly as a function of year (F =2.331, df =9, 489, P =0.014). Males caught in 1996/1997 had an adjusted testis mass greater than males from 1999/2000 and 2001/2002. In contrast to the males, females caught in each year had a very strong relationship between somatic and gonad mass (r 2 =0.83–0.97). Ovary mass, adjusted for body weight, varied as a function of year (F =2.52, df =9, 235, P =0.042). Females caught in 1996/1997 had an adjusted ovary mass greater than females from all other years, and females caught in January 1996 had greater ovary weights than those caught in 1999/2000 and 2001/2002 (Fig. 6).
Across all years, female squid in better somatic condition also tended to have a higher level of reproductive investment, as shown by the correlation between body weight-ML residuals and ovary-ML residuals (r =0.407, n =242, P <0.0001). The ovary-ML residuals were compared among years, using the body weight-ML residuals as a covariate. Reproductive investment by females was dependent upon year and body weight-ML residuals (F =2.42, df =9, 232, P =0.04). Generally, females from January 1996 and 1996/1997 had higher levels of reproductive investment for their size at any given level of somatic condition compared with females from other years (Fig. 7).
Residual values for each female from the body weight-dorsal mantle length (body weight-ML) and ovary weight-body weight relationships. Individuals with higher body weight-ML residuals are heavier than predicted from the regression equation, and individuals with higher ovary weight-body weight residuals have greater ovary weights than predicted from the regression equation
Abundance
The highest relative abundance of squid in Great Oyster Bay, based on CPUE (kg h-1), occurred during 2001/2002 followed closely by that of 1999/2000 (Fig. 8). The abundance of squid during these 2 years also appeared very similar using CPUE adjusted by the average size of individuals caught during each respective spawning season. The lowest abundance of squid during the study period occurred in 1996/1997 and 2000/2001 using both abundance indices (kg h-1 or individuals h-1). During the more recent years of the study, abundance estimates based on kg h-1 and individuals h-1 were very similar. However, in the first 2 years where squid were on average much smaller, the two abundance indices differed, particularly for 1995/1996. During 1999 and 2001 the fishery was closed to commercial and recreational fishing for 4 weeks (two, 2-week closures) and in 2000 it was closed for 6 weeks (three, 2-week closures). There is, however, no evidence that densities of squid increase during fishing closures (Moltschaniwskyj et al. 2003a).
Oceanographic conditions along the east coast of Tasmania 1995–2001
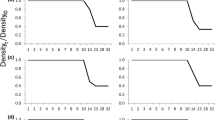
SSTs in the Great Oyster Bay region showed a distinct seasonal cycle, with temperatures highest between January and March and lowest in September (Fig. 9). SST maximums varied by up to 2 months between years reaching a peak in mid-January in 1999 and mid-March in 2000. There was also evidence of considerable variations in the maximum and minimum SSTs between years with 1999, 2000 and 2001 being consistently warmer than 1995 and 1996 (Table 2). The presence of EAC water is characterised by the 17°C isotherm indicating that its presence in the Great Oyster Bay region varied greatly among years. The EAC was present between January and late March in 1999 and 2001 and February and late April in 2000, and absent in 1995 and 1996.
Discussion
All aspects of Sepioteuthis australis life history and population dynamics are highly plastic, a combination of phenotypic response to biotic (predators and prey) and abiotic factors, and possibly genetic differences. Across this 5-year study changes in squid size, condition and possibly growth rate were sex-specific indicating that males and females respond differently to similar factors. Importantly, populations of apparently similar abundance (e.g. 1996/1997 and 2000/2001, 1999/2000 and 2001/2002) had very different population structures and were composed of individuals with strikingly different life history characteristics (see Table 2 for summary). The population dynamics of cephalopods generally appear to be driven primarily by phenotypic plasticity in response to environmental variability (Boyle and Boletzky 1996). However, the relative roles of phenotypic plasticity vs. genetic diversity, and the extent to which density-dependent processes may operate in shaping the observed life histories remain unknown.
Inter-annual variation in the influence of warm nutrient-poor EAC and cool nutrient-rich sub-antarctic water masses cause strong variations in SST, productivity, and trophic structure of eastern Tasmanian shelf waters. Variability in the influence of the EAC was evident in this study, with differences of ca. 2.5°C in SSTs among years. Overall, 1995 and 1996 were cooler than 1999, 2000, and 2001. There is also evidence that SSTs peaked up to a month later in 2000 than in 1999 or 2001 reflecting the persistence of cooler water of sub-antarctic origin on the shelf during January 2000. With no direct estimates of primary or secondary productivity in this study, patterns of SST provide a proxy measure of productivity (Harris et al. 1991). Overall, the cool years of 1995 and 1996 can be defined as more productive across all trophic levels than the warmer years of 1999, 2000 and 2001. Southern calamary are known to eat fish, octopus and small crustaceans (Jackson and Pecl 2003). An increase in the influence of water of sub-antarctic origin in eastern Tasmania is associated with an increase of 2 orders of magnitude in abundance of the dominant crustacean, Nyctiphanes australis (Young et al. 1993), and an order of magnitude increase in overall micronekton biomass (i.e. animals between 2 and 20 cm) (Young et al. 1996). Given these inter-annual variations in abundance of higher trophic levels, prey availability for southern calamary is likely to have been higher during 1995 and 1996, particularly during and after the hatching period for these adults (March–July) when EAC water influence is often more pronounced (Harris et al. 1987). Individual life history responses to environmental stress often involve significant changes in resource acquisition, which may be as important as the abiotic changes themselves (Glazier 1999).
This study identified two groups of years of similar abundance (e.g. 1996/1997 and 2000/2001, and 1999/2000 and 2001/2002), but with very different population structure and characteristics. If we were generating environment-recruitment relationships, these two populations would be considered equivalent. However, the factors responsible for generating a population consisting of smaller, slower growing squid with little sexual dimorphism and high levels of reproductive investment are going to be very different to those factors resulting in a population of larger, faster growing squid with extreme sexual dimorphism in body size and low levels of reproductive investment. In the search for environment-recruitment relationships for squid, the “spawning stock” is considered as a single uniform unit. However, seeking a relationship between abundance and environmental parameters at such a gross level will not shed light on the mechanisms that lead to any given level of abundance. Therefore, use of environmental-recruitment relationships as predictive management tools may be limited.
Indices of stock abundance have relationships of varying strength with a number of environmental characteristics; sea surface temperatures preceding recruitment (Loligo forbesi, Robin and Dennis 1999; L. gahi, Agnew et al. 2002), proportion of hatching areas occupied by frontal waters in the previous year (Illex argentinus, Waluda et al. 2001), and water mass movement and river runoff (I. argentinus, Chang 2001 in Agnew et al. 2002). For short-lived species with highly plastic life history characteristics and where the standing stock is entirely replaced each year (Boyle and Boletzky 1996), environmental predictors of recruitment may be useful in highly variable environments (Agnew et al. 2002). Environmental predictors of recruitment are, however, going to be more useful and much more rigorous if we work towards incorporating life history processes and their plasticity into the models. This will involve accumulating longer time-series of life history data, in addition to recruitment data.
Southern calamary exhibited sex-specific life history plasticity. The males varied more in size and growth, whilst females varied more in somatic condition and level of reproductive investment. Male squid have a relatively smaller anatomical investment in reproduction and therefore may have a larger energetic scope to respond rapidly to environmental changes (Jackson and Domeier 2003). This study suggests that the level of reproductive investment of female S. australis may be either directly or indirectly related to temperature, with females from cooler (and possible more productive) years having a higher level of reproductive investment. Given the relationship between SST and productivity on the east coast of Tasmania, we are unable to identify the relative importance of temperature vs. food, although as female southern calamary derive their energy for reproduction primarily from food and not body stores (Ho 2000), food availability is likely to have the larger influence.
Egg production by individuals that produce multiple batches of eggs can vary in response to food, growth, temperature, population size, and age structure (Lowerre-Barbieri et al. 1998). Given that squid with indeterminate and non-asymptotic growth allocate energy to growth and reproduction across all ages and sizes, we could not assess how the populations differed in longevity and total reproductive output. How the life history traits of the spawning stock affects subsequent recruitment would depend on the strength and nature of the stock-recruitment relationship. Agnew et al. (2000) found evidence of a negative density-dependent effect in the stock-recruitment relationship for Loligo gahi, with high spawning stock sizes leading to below-average recruitment. Perhaps this is evident in our study with the high spawning stock of 1999 resulting in an apparent low abundance of 2000.
Between years, differences in body size were extreme, both among the male component of the population and between genders. Size can influence food gathering, size-selective predation or starvation, and reproductive success (Cargnelli and Gross 1996). Small individuals may survive and reproduce better when food is limited because they need less food to sustain themselves; however, larger individuals may survive better when there is no food at all (Nylin and Gotthard 1998). The mortality schedules of southern calamary populations with large fast-growing males and small females with low reproductive output will differ from populations of smaller males and females of similar size. The influence of size-dependent behaviours such as male-male competition (Hanlon and Messenger 1996), mate selection (Jantzen and Havenhand 2003) and cannibalism (Jackson and Pecl 2003) may also differ among years. Gender-specific responses of growth rate to environmental factors such as photoperiod, temperature, diet and mortality risk occurs in insects (Nylin and Gotthard 1998). The sexual dimorphism in the size of southern calamary may be unrelated to temperature as the sexes were of equal size in 1999/2000, and yet the populations of 2000/2001 and 2001/2002, under a similar temperature regime, displayed very large differences in size between the sexes.
During the 2 warmer years size-at-age relationships were weaker than those observed during the 2 cooler years. This trend has also been seen in both laboratory-reared and wild caught Loligo pealeii (Hatfield et al. 2001, Hatfield and Cadrin 2002). Additionally, potential variability in growth rate increases with temperature in many fish (Houde 1989). We found evidence of fast- and slow-growing squid in 1 warmer year (1999/2000); however, only “average” and faster growing individuals were present in the other warmer year (2000/2001). Several explanations are possible for this observation. The total annual catch and CPUE values for 1998 were the highest on record (Lyle and Hodgson 2001), and perhaps the large population of 1998 resulted in a high diversity in the life history traits of subsequent populations. Alternatively, total catch, CPUE and egg production (Moltschaniwskyj and Pecl 2003) in 1999/2000 were also very high, and 1999/2000 may have therefore been a very good year for survival. Individuals from 2000/2001 may have been exposed to higher levels of size- (or growth rate)-dependent predation, removing the smaller, slower growing individuals from the population. For larval fish, as food abundance increases the mean and variance in growth rates increase; however, as predator abundance increases, the variance in growth rates for any given mean decreases via culling of slow-growing larvae (Pepin 1989). It is important to note that factors other than temperature are playing a major role affecting the observed growth rates in adult southern calamary.
Embryonic duration, hatchling size (Bouchaud 1991) and juvenile growth rates (Forsythe 1993) are all influenced by temperature in cephalopods. With recruits of many squid species hatching over at least 4 months of the year (e.g. Sepioteuthis australis, this study, Loligo gahi Hatfield 1991), a “spawning stock” will be composed of individuals who have experienced very different environmental conditions during the critical early periods of their life-cycle. Partitioning the variance in cephalopod recruitment into its component sources and establishing the basis behind environment-recruitment relationships is therefore going to be a challenging task. The mechanistic connections between environmental variation and population dynamics are complex and indirect, operating at the level of individual organisms (Dunham and Overall 1994). Progress towards understanding recruitment processes will be accelerated by a systematic and in-depth consideration of individual variability and the attributes of survivors (Rice et al. 1993). This study was by design exploratory in nature; however, these findings provide a strong basis for the direction of future studies.
References
Agnew D, Hill S, Beddington JR (2000) Predicting the recruitment strength of an annual squid stock: Loligo gahi around the Falkland Islands. Can J Fish Aquat Sci 57:2479–2487
Agnew D, Beddington J, Hill S (2002) The potential use of environmental information to manage squid stocks. Can J Fish Aquat Sci 59:1851–1857
Arkhipkin A, Jereb P, Ragonese S (2000) Growth and maturation in two successive seasonal groups of the short-finned squid, Illex coindetii from the Strait of Sicily (central Mediterranean). ICES J Mar Sci 57:31–41
Bellido J, Pierce G, Wang J (2001) Modelling intra-annual variation in abundance of squid Loligo forbesi in Scottish waters using generalised additive models. Fish Res 52:23–39
Bouchaud O (1991) Energy consumption of the cuttlefish Sepia officinalis L. (Mollusca: Cephalopoda) during embryonic development, preliminary results. Bull Mar Sci 49:333–340
Boyle PR (1990) Cephalopod biology in the fisheries context. Fish Res 8:303–321
Boyle PR, Boletzky S (1996) Cephalopod populations: definition and dynamics. Phil Trans R Soc Lond B 351:985–1002
Boyle PR, Pierce GJ, Hastie LC (1995) Flexible reproductive strategies in the squid Loligo forbesi. Mar Biol 121:501–508
Brommer J (2000) The evolution of fitness in life history theory. Biol Rev 75:377–404
Cargnelli L, Gross M (1996) The temporal dimension in fish recruitment: birthdate, body size and size-dependent survival in a sunfish (bluegill: Lepomis macrochirus). Can J Fish Aquat Sci 53:360–367
Cury P, Roy C, Mendelssohn R, Bakun A, Husby D, Parrish R (1995) Moderate is better: exploring nonlinear climatic effects on the Californian northern anchovy (Engraulis mordax). In: Beamish R (ed) Climate change and northern fish populations. Can Spec Publ Fish Aquat Sci 121:417–424
Dawe EG, Beck PC (1993) Age, growth and population structure of short-finned squid (Illex illecebrosus). In: Okutani T, O’Dor RK, Kubodera T (eds) Recent advances in cephalopod fisheries biology. Tokai University Press, Tokyo, p 678
Dunham AE, Overall KL (1994) Population responses to environmental change: life history variation, individual based models, and the population dynamics of short-lived organisms. Am Zool 34:382–396
Forsythe JW (1993) A working hypothesis of how seasonal temperature change may impact the field growth of young cephalopods. In: Okutani T, O’Dor RK, Kubodera T (eds) Recent advances in cephalopod fisheries biology. Tokai University Press, Tokyo, pp 133–143
Glazier D (1999) Trade-offs between reproductive and somatic (storage) investments in animals: a comparative test of the Van Noordwijk and De Jong model. Evol Ecol 13:539–555
Hanlon RT, Messenger JB (1996) Cephalopod behaviour. Cambridge University Press, Cambridge
Harris G, Nilsson C, Clementson L, Thomas D (1987) The water masses of the east coast of Tasmania: seasonal and interannual variability and the influence on phytoplankton biomass and productivity. Aust J Mar Freshwater Res 38:569–590
Harris G, Griffiths F, Clementson L, Lyne V, Van der Doe H (1991) Seasonal and interannual variability in physical processes, nutrient cycling and the structure of the food chain in Tasmanian shelf waters. J Plankton Res 13:109–131
Hatfield EMC (1991) Post-recruit growth of the Patagonian squid Loligo gahi (D’Orbigny). Bull Mar Sci 49:349–361
Ho J (2000) Structural and biochemical changes during growth and reproduction of the southern colonary Sepioteuthis australis. Hons thesis, University of Tasmania, p 73
Hatfield EMC, Cadrin SX (2002) Geographic and temporal patterns in size and maturity of the longfin inshore squid (Loligo pealeii) off the northeastern United States. Fish Bull 100:200–213
Hatfield E, Hanlon R, Forsythe J, Grist E (2001) Laboratory testing of a growth hypothesis for juvenile squid Loligo pealeii (Cephalopoda: Loliginidae). Can J Fish Aquat Sci 58:845–857
Houde ED (1989) Comparative growth, mortality, and energetics of marine fish larvae: temperature and implied latitudinal effects. Fish Bull 87:471–494
Jackson GD (1993) Seasonal variation in reproductive investment in the tropical loliginid squid Loligo chinensis and the small tropical sepioid Idiosepius pygmaeus. Fish Bull 91:260–270
Jackson GD, Domeier M (2003) The effects of an extraordinary El Niño/La Niña event on the size and growth of the squid Loligo opalescens off Southern California. Mar Biol 142:925–935
Jackson GD, Pecl GT (2003) The dynamics of the summer spawning populations of the loliginid squid Sepioteuthis australis in Tasmania, Australia—a conveyor belt of cohorts. ICES J Mar Sci 60:290–296
Jantzen TM, Havenhand JN (2003) Reproductive behaviour in the squid Sepioteuthis australis from south australia: interactions on the spawning grounds. Biol Bull 204:305–317
Jordan AR (1997) Demersal trawl surveys of the continental shelf of southern and eastern Tasmania 1993–1995. Technical report no. 50. Marine Laboratories Marine Research Division Tasmania, Tasmania
Jordan AR, Pullen G, Marshall JA, Williams H (1995) Temporal and spatial patterns of spawning in jack mackerel, Trachurus declivis (Pisces: Carangidae), during 1988–1991 in eastern Tasmanian waters. Aust J Mar Freshwater Res 46:831–42
Lipinski MR (1979) Universal maturity scale for the commercially important squids. The results of maturity classification of the Illex illecebrosus population for the years 1973–1977. ICNAF research document 79/2/38, serial no. 5364. ICNAF
Lowerre-Barbieri SK, Lowerre JM, Barbieri LR (1998) Multiple spawning and the dynamics of fish populations: inferences from an individual-based simulation model. Can J Fish Aquat Sci 55:2244–2254
Lyle J, Hodgson K (2001) Tasmanian scalefish fishery assessment 2000. Technical report series no. 19. Tasmanian Aquaculture and Fisheries Institute, Tasmania 19
Mangold KM, Young RE, Nixon M (1993) Growth versus maturation in cephalopods. In: Okutani T, O’Dor RK, Kubodera T (eds) Recent advances in cephalopod fisheries biology. Tokai University Press, Tokyo, p 697–703
Moltschaniwskyj NA (1995) Multiple spawning in the tropical squid Photololigo sp.: what is the cost in somatic growth? Mar Biol 124:127–135
Moltschaniwskyj NA, Pecl G (2003) Small-scale spatial and temporal patterns of egg production by the temperate loliginid squid Sepioteuthis australis. Mar Biol 142:509–516
Moltschaniwskyj NA, Semmens JM (2000) Limited use of stored energy reserves for reproduction by the tropical loliginid squid Photololigo sp. J Zool Lond 251:307–313
Moltschaniwskyj NA, Pecl G, Lyle J (2003a) The effect of short temporal fishing closures to protect spawning southern calamary populations from fishing pressure in Tasmania, Australia. Bull Mar Sci 71:501–514
Moltschaniwskyj NA, Pecl G, Lyle J, Haddon M, Steer M (2003b) Population dynamics and reproductive ecology of the southern calamary (Sepioteuthis australis) in Tasmania. FRDC final report 2000/121. FRDC
Nylin S, Gotthard K (1998) Plasticity in life history traits. Annu Rev Entomol 43:63–83
Pecl GT (2001) Flexible reproductive strategies in tropical and temperate Sepioteuthis squids. Mar Biol 138:93–101
Pecl GT (in press) The in situ relationships between season of hatching, growth and condition of southern calamary, Sepioteuthis australis. Mar Freshwater Res
Pepin P (1989) Predation and starvation of larval fish: a numerical experiment of size- and growth-dependent survival. Biol Oceanogr 6:23–44
Rice J, Miller T, Rose K, Crowder L, Marschall E, Trebitz A, DeAngelis D (1993) Growth rate variation and larval survival: inferences from an individual-based size-dependent predation model. Can J Fish Aquat Sci 50:133–142
Robin J-P, Denis V (1999) Squid stock fluctuations and water temperature: temporal analysis of English Channel Loliginidae. J Appl Ecol 36:101–110
Steer M, Moltschaniwskyj NA, Gowland FC (2002) Temporal variability in embryonic development and mortality in the southern calamary Sepioteuthis australis: a field assessment. Mar Ecol Prog Ser 243:143–150
Steer MA, Pecl GT, Moltschaniwskyj NA (2003) Are bigger calamary Sepioteuthis australis hatchlings more likely to survive? A study based on statolith dimensions. Mar Ecol Prog Ser 261:175–182
Waluda C, Rodhouse PG, Podesta GP, Trathan PN, Pierce GJ (2001) Surface oceanography of the inferred hatching grounds of Illex argentinus (Cephalopoda: Ommastrephidae) and influences on recruitment variability. Mar Biol 139:671–679
Young JW, Jordan AR, Bobbi C, Johannes RE, Haskard K, Pullen G (1993) Seasonal and interannual variability in krill (Nyctiphanes australis) stocks and their relationship to the fishery for jack mackerel (Trachurus declivis) off eastern Tasmania, Australia. Mar Biol 116:9–18
Young JW, Bradford RW, Lamb TD, Lyne VD (1996) Biomass of zooplankton and micronekton in the southern bluefin tuna fishing grounds off eastern Tasmania, Australia. Mar Ecol Prog Ser 138:1–14
Acknowledgements
We are grateful to the large number of volunteers and Tasmanian Aquaculture and Fisheries Institute staff who contributed to the fieldwork involved in this research, in particular Mike Steer, Simon Wilcox, and Graeme Ewing. George Jackson provided support for the earlier years of study and aged some of the individuals. Thank you to Malcolm Haddon, Jeremy Lyle, Robert Wakeford and two anonymous reviewers for their constructive input, and to the Tasmanian calamary fishers for freely providing access to their catch. This study was funded by a number of organisations including the Fisheries Research Development Corporation (grant 2000/121 awarded to N. A. M.), Tasmanian Aquaculture and Fisheries Institute, Tasmanian Department of Primary Industries Water and Environment, and James Cook University of Queensland. The first 2 years of the study were conducted whilst G. P. was supported by an Australian Postgraduate Award.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00442-004-1619-y
Rights and permissions
About this article
Cite this article
Pecl, G.T., Moltschaniwskyj, N.A., Tracey, S.R. et al. Inter-annual plasticity of squid life history and population structure: ecological and management implications. Oecologia 139, 515–524 (2004). https://doi.org/10.1007/s00442-004-1537-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-004-1537-z