Abstract
Global burden of acute viral gastroenteritis remains high, particularly in developing countries including Bangladesh. Sewage water (SW) is an important node to monitor enteric pathogens both in the environment and among the population. Analysis of SW in Dhaka city deems crucially important because a large number of urban-city dwellers live in Dhaka city, the capital of Bangladesh, under a constant threat of precarious sewerage system. In this study, we collected raw SW from five locations of Dhaka city every month from June 2016 to May 2017. It was concentrated with polyethylene glycol (PEG) and investigated for three major enteric viruses, rotavirus A (RVA), norovirus GII (NoV GII) and adenovirus (AdV) using polymerase chain reaction (PCR). Most of these SW samples collected from both hospitals and non-hospital areas yielded enteric viruses: 76% samples were positive for AdV, followed by 53% NoV GII and 38% RVA. Viral load was determined as much as 1 × 107 copies/ml for RVA and 3.5 × 103 copies/ml for NoV GII. Importantly, NoV GII and AdV that can affect people of all ages were predominated during monsoon also when SW overflows and spreads over a wide and crowded area. Genotypes G1, G2, G3, G8, and G9 for RVA, GII.4 for NoV, and type 41 for AdV were detected representing the current profile of circulating genotypes in the population. This study provides the first evidence of distribution of major diarrheal viruses in SW in Dhaka city which is alarming showing grave risk of impending outbreaks through exposure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute gastroenteritis (AGE) remains a major public health problem worldwide causing significant morbidity and mortality leading to serious economic burden both in developing and developed countries (Chow et al. 2010). Viruses are the leading cause of AGE, particularly in the children, accounting for 70% of total AGE in the children (Maria and Hrishikesh 2018; Chow et al. 2010). Several groups of viruses, including rotavirus (RV), norovirus (NoV), adenovirus (AdV), astrovirus, and sapovirus etc. are responsible for viral AGE (Thongprachum et al. 2016). RV infection mainly affects young children under 5 years of age. The number of RV death in children under five was 215,000 per year in 2013 of which 3% deaths occurred in Bangladesh (Pecenka et al. 2017). Among eight RV groups (A–H), RV group A (RVA) in 5 G-types: G1, G2, G3, G4, and G9 remains clinically important causing more than 90% of RV infections in humans (Hoque et al. 2018).
Unlike RV, NoV affects all age-groups of people and is considered as the most common cause of AGE worldwide accounting for 685 million cases every year including 200 million children < 5 years estimating 50,000 child deaths annually that almost all occur in developing countries (CDC 2016). Among six genogroups (GI–GVI) of NoV, all GI, majorities of GII and a few GIV genotypes infect humans, while a single GII genotype, GII.4, remains predominant over the last few decades causing over 70% of NoV outbreaks in human (Jin et al. 2016).
AdV, a double standard DNA virus, can cause a wide range of illness including AGE (Matsushima et al. 2012). Among many serotypes, AdV type 40 and 41 are mainly related to AGE (Matsushima et al. 2012). Globally, AdV is thought to be responsible for approximately 1.5–5.4% of the diarrhea cases in children under 2 years old (Oude Munnink and van der Hoek 2016).
In Bangladesh, prevalence of viral diarrhea remains 32.8% for RV (WHO), 25% for NoV (Rahman et al. 2016), and 10.7% for AdV (Afrad et al. 2018). These fecal-orally transmitted infections are mostly associated with consumption of contaminated water and food (Motayo et al. 2016; Rahman et al. 2010). Sewage is a major source of disseminating fecal-orally transmitted enteric viruses in the environment those are excreted in high concentrations (105–1013/g) in the feces (Dey et al. 2011). These viruses have low infectious doses and being resistant to adverse conditions they can persist in the environment for a long time (Victoria et al. 2014). Several diarrhea outbreaks caused by surface water contaminated with sewage water (SW) have been reported, particularly in developing countries, where the sewerage system usually remains inadequate (He et al. 2011).
In Bangladesh, untreated SW is often drained off right into the river or canal (Editorial 2017). This contaminated surface water is frequently used for various purposes: in irrigation, washing, bathing, or swimming etc. Again, overflow of SW remains a quite common scenario here during the rainy season (Sharmin 2016). Analyses of SW for pathogenic human enteric viruses are therefore indispensable to assess the risk of possible outbreaks through exposure (Thongprachum et al. 2018), and to understand the status of circulating strains in the population (Tort et al. 2015). SW has been investigated for enteric viruses in Japan (Thongprachum et al. 2018), China (He et al. 2011), France (Bisseux et al. 2018), Italy (Iaconelli et al. 2017), Uruguay (Tort et al. 2015), and Nigeria (Motayo et al. 2016) etc., but little is known from Bangladesh. Although it is certain that SW contains diarrheal viruses, evidence-based data on the distribution of diarrheal viruses in the SW in Bangladesh remained unavailable.
In this study, we conducted a year-long surveillance on the distribution of three major diarrheal viruses, RVA, NoV GII, and AdV, in the SW in Dhaka city, Bangladesh. The intent of this study was to provide information on the season-specific distribution of these three major diarrheal viruses in the SW in Dhaka city which is useful prudently for risk assessment of probable outbreaks due to exposure.
Materials and Methods
SW Collection
Raw SW samples were collected from open drains from five road-side locations in central Dhaka: three from hospital sewage lines and two from non-hospital community-based sites. All these five locations were selected considering its location from over-crowded places, having a possibility to overflow on the roads, or that drained off finally into rivers without any treatment. Samples were collected from each location once every month from June 2016 to May 2017. Nearly 500 ml of raw SW was collected in a clean water pet bottle and brought to the laboratory within 2 h of collection for further processing.
Sample Concentration
Raw SW was first passed through a cotton cloth to remove floating particles. About 100 ml of raw SW was mixed with 8 g of PEG-6000 and 2.3 g of NaCl in a beaker and stirred at room temperature for 4 h. The suspension was then centrifuged at 10,000 g for 30 min and the supernatant was discarded without disturbing the pellet. Afterward, the pellet was suspended in 1 ml of supernatant water, aliquoted, and stored at − 80 °C until further use.
Extraction of Viral RNA and Reverse Transcription
Viral RNA or DNA was extracted from 200 µl of concentrated samples using QIAamp Viral RNA mini kit (QIAGEN, Hilden, Germany) following manufacturer’s instructions on spinning procedures. This kit purifies both the RNA and DNA simultaneously once present in any sample. Therefore, the same extracted materials were suitable for detection of viral RNA or DNA as shown in earlier studies (Thongprachum et al. 2018). The extracted nucleic acids were stored at − 80 °C until used further. For cDNA synthesis from viral RNA, 5 µl of extracted nucleic acid solution was denatured at 95 °C for 5 min in the presence of 0.5 µl of 50% DMSO, followed by chilling immediately on ice. The reverse transcription (RT) was performed using Superscript reverse transcriptase III (Invitrogen, Carlsbad, CA, USA) and random primers (Takara, Shiga, Japan) through a single thermal cycle of 30 °C for 10 min, followed by 50 °C for 40 min, 95 °C for 5 min and rapid cooling on ice.
Detection and Quantification of Viruses
For polymerase chain reaction (PCR), 1.5 µl of cDNA/DNA solution was mixed with 5 × Taq DNA polymerase buffer (Promega, Madison, WI, USA), 1 µl of 2.5 mM dNTPs (Roche, Mannheim, Germany), 0.25 µl of virus-specific each primer (20 µM), and 0.05 µl of Taq DNA polymerase (Promega, Madison, WI, USA). The RNase-free distilled water was added to make up a total volume of 12.5 µl. The specific primers for RVA were sBeg9 and VP7-1′, for NoV GII were G2SKF and G2SKR, and for AdV were Ad1 and Ad2, as shown previously (Thongprachum et al. 2017). First PCR products (2 µl) were re-amplified using the same specific primers to increase the intensity of the detection band. A sample was considered negative if target band was not detected even after second PCR. The condition of the PCR was maintained as 1 cycle with 94 °C for 3 min, followed by 35 cycles with 94 °C, 42 °C, and 72 °C each for 1 min and finally 1 cycle at 72 °C for 10 min.
The copy number of RVA and NoV GII was determined by real-time PCR in raw sewage samples that yielded strong target band without any nonspecific band. For RVA quantification, forward primer JVKF, reverse primer JVKR and TaqMan® probe JVKP were used to target NSP3 gene as described earlier (Jothikumar et al. 2009). Sample RNA was subjected to denaturation at 95 °C for 5 min followed by incubation in ice for 2 min to separate the rotaviral dsRNA prior to addition to master mix. One-step real-time RT-PCR amplifications were performed using the TaqMan Fast Virus 1-step Master Mix (Perkin Elmer-Applied Biosystems, Inc., Foster City, CA, USA) with the following conditions: reverse transcriptase reaction for 30 min at 50 °C, followed by denaturation at 95 °C for 20 s, and 45 cycles of denaturation at 94 °C for 10 s, annealing at 55 °C for 30 s, and extension at 72 °C for 20 s. For NoV GII quantification, in brief, extracted RNA was first treated with RQ1 DNase (Promega) and then cDNA was produced as described previously (Kageyama et al. 2003). A primer set, G2FB and G2SKR with RING2-TP fluorogenic probe were used to amplify the ORF1-ORF2 junction as described elsewhere (Kageyama et al. 2003).
For RVA-vaccine strain differentiation, the real-time RT-PCR designed for vaccine specific targets in the genomes of Rotarix® (NSP2) and RotaTeq® (VP6) were used (Gautam et al. 2014).
G Genotyping of RVA by Semi-Nested Multiplex PCR
cDNAs from strongly positive RVA samples that yielded strong band at second PCR during detection were further analyzed by semi-nested multiplex PCR for G genotyping. Partial VP7 region was targeted and amplified in first PCR using primers sBeg9 (Thongprachum et al. 2017) and End9(s) (Khamrin et al. 2011). In the second PCR, a mixture of all six genotype-specific primers, aBT1, aCT2, aET3, aDT4, aAT8, and aFT9 were used as shown previously (Gouvea et al. 1990) with the common primer End9(s). The PCR condition was maintained as described above. If any sample yielded one or more bands of sizes 628, 531, 253, 462, 764, and 185 bps were specific for G1, G2, G3, G4, G8, and G9 genotypes, respectively.
DNA Sequencing and Phylogenetic Analysis
The specific PCR products of strongly positive samples were purified first using QIAquick Gel Extraction Kit (Qiagen, Hilden, Germany) and sequenced by using the BigDye Terminator Cycle Sequencing kit (Perkin Elmer-Applied Biosystems, Inc.) on an automated DNA sequencer (ABI 3100; Perkin Elmer-Applied Biosystems, Inc.). The accession numbers for 6 NoV GII and 16 AdV sequences were MH791995-MH792000 and MH793327-MH793342, respectively. A phylogenetic tree was constructed according to the neighbor-joining method using MEGA version 6 where aligning was done with the ClustalW program.
Statistical Analysis
The mean of two groups were compared employing two-tailed t test using GraphPad Software (Quick Calcs Online Calculators for Scientists, Graphpad Software Inc., La Jolla, CA).
Results
Detection of Diarrheal Virus in SW
Raw SW, collected from five road-side locations (three hospitals and two non-hospital community-based sewage lines), were concentrated and investigated for the presence of RVA, NoV and AdV by (RT-) PCR.
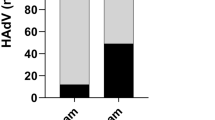
Among 60 samples, 56 (93%) were positive for these viruses: RVA was 23 (38%), NoV GII was 32 (53%), and AdV was 46 (76%) (Table 1). Out of 36 SW samples from hospital areas, RVA, NoV, and AdV were detected in 14 (39%), 20 (56%) and 27 (75%) samples, respectively (Fig. 1a). Again, of 24 SW samples from non-hospital areas, 9 (38%), 12 (50%), and 19 (79%) samples were positive RVA, NoV, and AdV, respectively (Fig. 1a). These data suggest that most of the SW samples contained enteric viruses: the detection frequencies of RVA (P = 0.9), NoV (P = 0.7) and AdV (P = 0.6) did not differ significantly among hospital and non-hospital sewages samples indicating that both hospital and non-hospital sewages were similarly positive for these viruses.
Seasonal Distributions
Although Bangladesh is a land of six seasons, three seasons are clearly distinguishable: the pre-monsoon/hot humid summer (March through May), the rainy monsoon (June through October), and a cool dry winter (November through February) (Banglapedia 2014). RVA was detected more frequently in summer (67%) than in winter (50%) being less frequent in the rainy season (12%) (Fig. 1b). The average detection of RVA per month in the rainy season remained significantly lower than that of in summer (P = 0.009) and/or in winter (P = 0.02) season(s). In contrast, NoV GII was detected moderately (50–60%) in all three seasons. AdV was detected more regularly than RVA and NoV GII, being highest in the summer (93%), followed by 75% in winter and 68% in the rainy season.
Genotype Distribution, Quantification of RVA in Raw Sewage
Ten RVA-PCR-positive samples were further investigated for six important genotypes (G1, G2, G3, G4, G8, and G9) by semi-nested multiplex PCR. Mainly a mixture of different genotypes, G1, G2, G3, G8, and G9, were detected in the samples as shown in Table 1. The most common genotype was G3 (70%), followed by G1 (40%), G9 (40%), G2 (30%), and G8 (30%).
Total five RVA positive samples including both hospital and non-hospital sewage samples were subjected to quantifying virus copy numbers. As shown in Table 1, RVA ranging from 1 × 107 to 1.9 × 105 copies/ml were detected in the non-hospital SW while in hospital SW contained 1.5 × 106–2 × 105 copies/ml, indicating no significant differences (P = 0.3) in virus copy numbers between the hospital and non-hospital sewage samples.
These five RVA positive samples were also investigated for RVA-vaccine strains for both Rotarix® and RotaTeq® by real-time RT-PCR, but no vaccine strain was detected (Table 1) representing the poor RVA-vaccine coverage status in Bangladesh.
Genetic Diversity and Quantification of NoV in Raw Sewage
The capsid region of six NoV GII positive samples were sequenced and analyzed for genotyping. All six samples were of GII.4 genotype. Phylogenetic analysis of these strains is shown in Fig. 2. The strains detected in this study were found closely related to the most predominant GII.4 variant 2012, Sydney_2012 (JX459908.1 Norovirus Hu/GII.4/Sydney/NSW0514/2012/AU).
Phylogenetic tree of partial capsid sequences (344 bp) of NoV GII.4 strains detected in SW. The tree was constructed using the neighbor-joining method whereby strains detected in this study (boldface; H: Hospital, NH: Non-hospital originated) were compared with reference sequences and other sequences of NoV GII.4 strains detected in Bangladesh or in the environment
Virus copy number was quantified in three NoV GII.4 positive samples. Around 5.7 × 102 and 3.9 × 102 copies/ml were detected in two hospital sewage samples, while the copy number remained 3.5 × 103 copies/ml in a non-hospital SW, conveying that both hospital and non-hospital sewage samples are similarly hazardous with NoV GII.
Genetic Diversity of AdV in Raw Sewages
AdV was detected more frequently than NoV and RVA in raw SW. The nucleotide sequence of partial hexon gene of 16 samples were analyzed and shown in phylogenetic tree (Fig. 3).
Phylogenetic tree of partial hexon gene (482 bp) of adenovirus (AdV) strains detected in SW. The tree was constructed using the neighbor-joining method whereby strains detected in this study (boldface; H: Hospital, NH: Non-hospital originated) were compared with reference sequences and other sequences AdV strains detected in Bangladesh earlier
Among 16 strains isolated from sewage samples, 6 were clustered together with human AdV (HAdV) group D, three were identified into HAdV group F and related with AdV41 isolate (GU564475 and AB330122), and seven strains were found closely related to Pigeon AdV strain (FN824512), i.e., nearly 50% of AdV detected in raw sewage are of non-human origin indicating that raw SW remains threatening to livestock also.
Discussion
Dhaka is the most crowded city in the world (Forum). Inadequate sewerage and sanitation system of Dhaka poses huge environmental and health threat to 17.5 million of people living here (Editorial 2017). Currently, Dhaka Water Supply and Sewerage Authority (DWASA) operates a single sewage treatment plant that treats only 20% of generated sewage of the city, while the remaining 80% is passed through open shallow-surface drains that mostly are not connected to storm sewer and ultimately disposed off right into the rivers without any treatment (Editorial 2017). Though some people use under-ground septic tanks for dumping toilet waste, while clearing the pit they often discard the waste indiscriminately in open water bodies (Sharmin 2016). Again, most of the open drains are overflowed during monsoon submerging surrounding roads and low lying areas with wastewater (Sharmin 2016). We conducted this study since analysis of SW is important to understand the risk of possible outbreaks among city dwellers those are exposed to SW continuously.
SW has been investigated for enteric viruses like RVA, NoV, AdV, astrovirus, Aichi virus, parechovirus, hepatitis A virus, and hepatitis E virus etc. in many other countries (Thongprachum et al. 2018; Hellmer et al. 2014; van den Berg et al. 2005). Detection of these viruses in SW often serves as early warnings of incipient outbreaks of these viruses (Hellmer et al. 2014). In this study, we investigated RVA, NoV, and AdV in SW since these viruses are more predominant than other gastroenteric viruses in Bangladeshi patients (Rahman et al. 2016; Afrad et al. 2018). We collected SW from road-side open shallow-surface drains, which are located in crowded places, flow directly into the river, and/or submerge the roads frequently due to overflow in the rainy season. As much as 93% of SW samples were positive for RVA, NoV GII, and/or AdV. Although RVA remains more prevalent in Bangladeshi patients followed by NoV GII and AdV, we found totally opposite trend in year-long SW surveillance. Namely, RVA was less common (38%) than NoV (53%) and AdV (76%) in SW samples. It is because RVA infection occurs mainly among young children and more during winter and spring seasons whereas NoV GII and AdV infections are found round the year in all age-groups of people (Thongprachum et al. 2016). NoVs were detected in more than 50% of SW samples and in greater abundance than RVA in many other countries where NoVs were considered as an important causative agent for gastroenteritis (La Rosa et al. 2007; Rodriguez-Diaz et al. 2009; Victoria et al. 2014; Kamel et al. 2010). Again, AdV has been detected more consistent in the environment and even more frequent than NoVs in several other studies (Charles et al. 2009; Wyn-Jones et al. 2011). Seasonal variation in detection of enteric viruses in SW has been repeatedly reported for RVA (Motayo et al. 2016; Kargar et al. 2013) and NoV (Farkas et al. 2018) but not for AdV (Wieczorek et al. 2015) which remain consistent with our findings as well (Fig. 1b). The average detection level of these three enteric viruses in SW was maximum in summer (71%), followed by winter (62%) and monsoon (43%). Importantly, NoV GII and AdV were often detected in the samples of the rainy season when these sewages are overflowed.
Another explanation of getting AdVs in greater abundance is that the DNA of AdV is thought to be more stable in aquatic environments than RNA viruses (Sun et al. 2016), and it has wider host range causing various types of illness like AGE, respiratory illness, and conjunctivitis etc. (Thongprachum et al. 2016). In this study, nearly 50% of AdV samples were from non-human origin related to pigeon AdV while 35% were clustered into human AdV group D strain, and 19% matched with human enteric pathogen AdV group F type 41 (Fig. 3). Notably, huge genetic diversity in AdVs including clinically important, non important and/or animal strains in waste water samples have been reported in many other studies (Iaconelli et al. 2017; Kuo et al. 2009; Kokkinos et al. 2017). AdV serotype 41 remains the most common identified AdV type in the waste water because of environmental stability (Hokajarvi et al. 2013). Furthermore, our data remain consistent with the clinical data of Bangladesh which have also highlighted lots of genetic diversity in human AdVs including both enteric- and non-enteric AdVs where human AdV type 41 predominated (Afrad et al. 2018).
Here, we detected NoVs genotype GII.4 in the SW that belonged to clinically important Sydney_2012 variant (Fig. 2). Indeed, different variants of NoV GII.4 genotype remain predominating worldwide for more than two decades while Sydney_2012 variant is predominating since 2012 (Sdiri-Loulizi et al. 2010). NoV GII.4 is also thought to be the most prevalent (42%) genotype in Bangladeshi patients, followed by GII.3 (21%), GII.6 (7%), GII.7 (6%), and GII.21 (6%) (Rahman et al. 2016). Like AdV type 41, NoV GII.4 is also shed in higher amounts and remains more stable in the environment than other NoV genotypes, and thus remains common in environmental samples as shown in prior studies also (Sdiri-Loulizi et al. 2010; Campos et al. 2008).
In this study, G3 genotype of RVA was detected most frequently in the SW followed by G1, G9, G2, and G8 (Table 1). According to earlier reports, the most common RVA genotype in Bangladeshi patients was G2 (43.3%), followed by G4 (19.5%), G9 (13.7%), G1 (12.7%), and G3 (2.6%) during 2004–2005 (Dey et al. 2009). However, a disparate profile of RVA genotypes was found during 2006–2012, where G1P[8] (22.4%) predominated mostly, followed by G9P[8] (20.8%), G2P[4] (16.9%) and G12P[8] (10.4%) (Afrad et al. 2013). Our findings on SW revealed that this genotype profile of RVA has been altered further and G3 has been re-emerged in Bangladesh after a long-term interval. Although G3 is one of the most prevalent genotypes worldwide, it was reported less frequently in Bangladesh since 1993 (Dey et al. 2009). In line of our findings, a very recent report on hospital-based RVA surveillance also has documented the presence of G3P[8] in children < 5 years of age during 2012–2017 (Satter et al. 2018). Greater abundance of G3P[8] in the SW was also reported earlier in Tunisia between 2003 and 2007 was correlated with circulating strains in their pediatric population (Sdiri-Loulizi et al. 2010). In addition, we could not detect RVA-vaccine strains in any of five SW samples that we investigated (Table 1), which is quite reasonable if low RVA-vaccine coverage rate is considered in Bangladesh. This study, thus, provides important information on circulating strains of RVA, NoV GII and AdV in the population. In the current SW samples, RVA copies number were detected as high as 1 × 107 copies/ml, which is comparable with the copies number (9 × 106 copies/ml) detected by our group in recent years in the raw SW of Japan (Thongprachum et al. 2018).
Notably, the hospitals selected here for sample collection were neither child nor diarrhea specialized, yet high copy number of diarrheal viruses, particularly RVA, were detected in both hospital and non-hospital originated SW samples (Table 1) presenting a scenario of circulating viruses among general population having either asymptomatic or non-severe infections.
Moreover, in Bangladesh, bacterial diarrhea caused by Vibrio cholerae, some toxin-producing Eschericia coli, Salmonella spp., Shigella spp., and Camphylobacter spp. etc. remains more prevalent and causing more severe diarrhea than that caused by enteric viruses (Ahmed 2014). It is possible that enormous pathogenic diarrheal bacteria are also present in the SW multiplying faster in the suitable weather of Bangladesh. Thus, an alarming situation of possible outbreaks of these viruses and bacteria has been expected here.
One major limitation of this study is that we investigated genotypes of RVA, NoV GII, and AdV from strongly positive samples only. As a result samples giving weak bands in PCR may contain less prevalent genotypes that were not subjected for genotyping. It may have caused the detection of dominating strains only in the samples. In addition, we had limited facilities for quantification. Therefore, limited numbers of samples that gave strongly positive bands in PCR detection assays were selected for quantification. However, we often did not get specific target bands or good sequences for typing. Hence, it was not possible to demonstrate viral concentration and genotyping always together in Table 1. Another limitation is that we did not use any virus control mixed with the samples to investigate the presence of PCR inhibiting factors in the SW samples. However, viruses were detected in most samples (93%), which have minimized the chance of the presence of PCR inhibiting factors in the samples.
Conclusion
Our findings evidence that: (1) SW in Dhaka city contains enteric viruses and often in high quantity, (2) both hospital and non-hospital sewages remain grossly contaminated, and (3) SW in the rainy season that often overflows out of sewerage lines also contains NoV GII and AdV. The potential release of the pathogenic viruses from wastewater to the environment possesses a grievous risk to public health. This study also has brought out important information to understand the season-specific distribution of pathogenic enteric viruses in the environment allowing potential insights that the policymakers may capitalize to pay serious emphasis and immediate consideration in adopting a sustainably impacted well-planned safe and cost-effective approach of improved wastewater management system in Dhaka city to prevent impending health and environmental disasters in the near future.
References
Afrad, M. H., Avzun, T., Haque, J., Haque, W., Hossain, M. E., Rahman, A. R., et al. (2018). Detection of enteric- and non-enteric adenoviruses in gastroenteritis patients, Bangladesh, 2012–2015. Journal of Medical Virology, 90(4), 677–684. https://doi.org/10.1002/jmv.25008.
Afrad, M. H., Hassan, Z., Farjana, S., Moni, S., Barua, S., Das, S. K., et al. (2013). Changing profile of rotavirus genotypes in Bangladesh, 2006–2012. BMC Infectious Diseases, 13, 320. https://doi.org/10.1186/1471-2334-13-320.
Ahmed, Z. U. (2014). Diarrhoeal diseases. http://en.banglapedia.org/index.php?title=Diarrhoeal_Diseases. Accessed 18 Nov 2018.
Banglapedia (2014). Season. http://en.banglapedia.org/index.php?title=Season. Accessed 18 Nov 2018.
Bisseux, M., Colombet, J., Mirand, A., Roque-Afonso, A. M., Abravanel, F., Izopet, J., et al. (2018). Monitoring human enteric viruses in wastewater and relevance to infections encountered in the clinical setting: A one-year experiment in central France, 2014 to 2015. Eurosurveillance. https://doi.org/10.2807/1560-7917.ES.2018.23.7.17-00237.
Campos, G. S., Moreau, V. H., Bandeira, A., Barberino, G., Almeida, P. F., Amador, D. M., et al. (2008). Molecular detection and genetic diversity of norovirus in hospitalized young adults with acute gastroenteritis in Bahia, Brazil. Archives of Virology, 153(6), 1125–1129. https://doi.org/10.1007/s00705-008-0078-x.
CDC (2016). Norovirus worldwide. https://www.cdc.gov/norovirus/worldwide.html. Accessed 18 Nov 2018.
Charles, K. J., Shore, J., Sellwood, J., Laverick, M., Hart, A., & Pedley, S. (2009). Assessment of the stability of human viruses and coliphage in groundwater by PCR and infectivity methods. Journal of Applied Microbiology, 106(6), 1827–1837. https://doi.org/10.1111/j.1365-2672.2009.04150.x.
Chow, C. M., Leung, A. K., & Hon, K. L. (2010). Acute gastroenteritis: From guidelines to real life. Clinical and Experimental Gastroenterology, 3, 97–112.
Daily Star Editorial (2017). Most of city’s human waste untreated. https://www.thedailystar.net/The Daily Star Editorial/most-citys-human-waste-untreated-1489462. Accessed 11 June 2018.
Dey, S. K., Hayakawa, Y., Rahman, M., Islam, R., Mizuguchi, M., Okitsu, S., et al. (2009). G2 strain of rotavirus among infants and children, Bangladesh. Emerging Infectious Diseases, 15(1), 91–94. https://doi.org/10.3201/eid1501.080883.
Dey, S. K., Phan, T. G., Mizuguchi, M., Okitsu, S., & Ushijima, H. (2011). Genetic diversity and emergence of norovirus GII/4-2006b in Japan during 2006–2007. Clinical Laboratory, 57(3–4), 193–199.
Farkas, K., Marshall, M., Cooper, D., McDonald, J. E., Malham, S. K., Peters, D. E., et al. (2018). Seasonal and diurnal surveillance of treated and untreated wastewater for human enteric viruses. Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-018-3261-y10.1007/s11356-018-3261-y
Forum, W. E. These are the world’s most crowded cities. https://www.weforum.org/agenda/2017/05/these-are-the-world-s-most-crowded-cities/. Accessed 18 Nov 2018.
Gautam, R., Esona, M. D., Mijatovic-Rustempasic, S., Ian Tam, K., Gentsch, J. R., & Bowen, M. D. (2014). Real-time RT-PCR assays to differentiate wild-type group A rotavirus strains from Rotarix((R)) and RotaTeq((R)) vaccine strains in stool samples. Human Vaccines & Immunotherapeutics, 10(3), 767–777.
Gouvea, V., Glass, R. I., Woods, P., Taniguchi, K., Clark, H. F., Forrester, B., et al. (1990). Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. Journal of Clinical Microbiology, 28(2), 276–282.
He, X. Q., Cheng, L., Zhang, D. Y., Xie, X. M., Wang, D. H., & Wang, Z. (2011). One-year monthly survey of rotavirus, astrovirus and norovirus in three sewage treatment plants in Beijing, China and associated health risk assessment. Water Science and Technology, 63(1), 191–198. https://doi.org/10.2166/wst.2011.032.
Hellmer, M., Paxeus, N., Magnius, L., Enache, L., Arnholm, B., Johansson, A., et al. (2014). Detection of pathogenic viruses in sewage provided early warnings of hepatitis A virus and norovirus outbreaks. Applied and Environmental Microbiology, 80(21), 6771–6781.
Hokajarvi, A. M., Pitkanen, T., Siljanen, H. M., Nakari, U. M., Torvinen, E., Siitonen, A., et al. (2013). Occurrence of thermotolerant Campylobacter spp. and adenoviruses in Finnish bathing waters and purified sewage effluents. Journal of Water and Health, 11(1), 120–134. https://doi.org/10.2166/wh.2012.192.
Hoque, S. A., Kobayashi, M., Takanashi, S., Anwar, K. S., Watanabe, T., Khamrin, P., et al. (2018). Role of rotavirus vaccination on an emerging G8P[8] rotavirus strain causing an outbreak in central Japan. Vaccine, 36(1), 43–49. https://doi.org/10.1016/j.vaccine.2017.11.056.
Iaconelli, M., Valdazo-Gonzalez, B., Equestre, M., Ciccaglione, A. R., Marcantonio, C., Della Libera, S., et al. (2017). Molecular characterization of human adenoviruses in urban wastewaters using next generation and Sanger sequencing. Water Research, 121, 240–247.
Jin, M., Zhou, Y. K., Xie, H. P., Fu, J. G., He, Y. Q., Zhang, S., et al. (2016). Characterization of the new GII.17 norovirus variant that emerged recently as the predominant strain in China. Journal of General Virology, 97(10), 2620–2632. https://doi.org/10.1099/jgv.0.000582.
Jothikumar, N., Kang, G., & Hill, V. R. (2009). Broadly reactive TaqMan assay for real-time RT-PCR detection of rotavirus in clinical and environmental samples. JIN2@cdc.gov. Journal of Virological Methods, 155(2), 126–131. https://doi.org/10.1016/j.jviromet.2008.09.025.
Kageyama, T., Kojima, S., Shinohara, M., Uchida, K., Fukushi, S., Hoshino, F. B., et al. (2003). Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. Journal of Clinical Microbiology, 41(4), 1548–1557.
Kamel, A. H., Ali, M. A., El-Nady, H. G., Aho, S., Pothier, P., & Belliot, G. (2010). Evidence of the co-circulation of enteric viruses in sewage and in the population of Greater Cairo. Journal of Applied Microbiology, 108(5), 1620–1629, https://doi.org/10.1111/j.1365-2672.2009.04562.x.
Kargar, M., Javdani, N., Najafi, A., & Tahamtan, Y. (2013). First molecular detection of group A rotavirus in urban and hospital sewage systems by nested-RT PCR in Shiraz, Iran. Journal of Environmental Health Science and Engineering. 11(1), 4. https://doi.org/10.1186/2052-336X-11-4.
Khamrin, P., Okame, M., Thongprachum, A., Nantachit, N., Nishimura, S., Okitsu, S., et al. (2011). A single-tube multiplex PCR for rapid detection in feces of 10 viruses causing diarrhea. Journal of Virological Methods, 173(2), 390–393.
Kokkinos, P., Kozyra, I., Lazic, S., Soderberg, K., Vasickova, P., Bouwknegt, M., et al. (2017). Virological quality of irrigation water in leafy green vegetables and berry fruits production chains. Food and Environmental Virology, 9(1), 72–78. https://doi.org/10.1007/s12560-016-9264-210.1007/s12560-016-9264-2.
Kuo, D. H., Simmons, F., & Xagoraraki, I. (2009). A new set of PCR assays for the identification of multiple human adenovirus species in environmental samples. Journal of Applied Microbiology, 107(4), 1219–1229.
La Rosa, G., Fontana, S., Di Grazia, A., Iaconelli, M., Pourshaban, M., & Muscillo, M. (2007). Molecular identification and genetic analysis of Norovirus genogroups I and II in water environments: Comparative analysis of different reverse transcription-PCR assays. Applied and Environmental Microbiology, 73(13), 4152–4161.
Maria, C., & Hrishikesh, S. (2018). Diarrhea, Viral: StatPearls Publishing.
Matsushima, Y., Shimizu, H., Kano, A., Nakajima, E., Ishimaru, Y., Dey, S. K., et al. (2012). Novel human adenovirus strain, Bangladesh. Emerging Infectious Diseases, 18(5), 846–848. https://doi.org/10.3201/eid1805.111584.
Motayo, B., Adeniji, A., & Faneye, A. (2016). First molecular detection and VP7 (G) genotyping of group a rotavirus by semi-nested RT-PCR from sewage in nigeria. Journal of the São Paulo Institute of Tropical Medicine. https://doi.org/10.1590/S1678-9946201658074.
Motayo, B. O., Adeniji, A. J., & Faneye, A. O. (2016). First molecular detection and Vp7 (G) genotyping of group a rotavirus by semi-nested Rt-Pcr from Sewage in Nigeria. Journal of the São Paulo Institute of Tropical Medicine, 58, 74.
Oude Munnink, B. B., & van der Hoek, L. (2016). Viruses causing gastroenteritis: The known, the new and those beyond. Viruses, 8(2), v8020042.
Pecenka, C., Parashar, U., Tate, J. E., Khan, J. A. M., Groman, D., Chacko, S., et al. (2017). Impact and cost-effectiveness of rotavirus vaccination in Bangladesh. Vaccine. 35(32), 3982–3987.
Rahman, M., Hassan, Z., Nahar, Z., Faruque, A. S., Van Ranst, M., Rahman, S. R., et al. (2010). Molecular detection of noroviruses in hospitalized patients in Bangladesh. European Journal of Clinical Microbiology & Infectious Diseases, 29(8), 937–945. https://doi.org/10.1007/s10096-010-0948-5.
Rahman, M., Rahman, R., Nahar, S., Hossain, S., Ahmed, S., Golam Faruque, A. S., et al. (2016). Norovirus diarrhea in Bangladesh, 2010–2014: Prevalence, clinical features, and genotypes. Journal of Medical Virology, 88(10), 1742–1750. https://doi.org/10.1002/jmv.24530.
Rodriguez-Diaz, J., Querales, L., Caraballo, L., Vizzi, E., Liprandi, F., Takiff, H., et al. (2009). Detection and characterization of waterborne gastroenteritis viruses in urban sewage and sewage-polluted river waters in Caracas, Venezuela. Applied and Environmental Microbiology, 75(2), 387–394.
Satter, S. M., Aliabadi, N., Gastanaduy, P. A., Haque, W., Mamun, A., Flora, M. S., et al. (2018). An update from hospital-based surveillance for rotavirus gastroenteritis among young children in Bangladesh, July 2012 to June 2017. Vaccine. https://doi.org/10.1016/j.vaccine.2018.05.032.
Sdiri-Loulizi, K., Hassine, M., Aouni, Z., Gharbi-Khelifi, H., Chouchane, S., Sakly, N., et al. (2010). Detection and molecular characterization of enteric viruses in environmental samples in Monastir, Tunisia between January 2003 and April 2007. Journal of Applied Microbiology, 109(3), 1093–1104.
Sharmin, A. (2016). Water and wastewater in Bangladesh, current status and design of a decentralized solution. Lund: Lund University
Sun, S., Shi, Y., Tong, H. I., Kang, W., Wang, Z., Allmann, E., et al. (2016). Effective concentration, recovery, and detection of infectious adenoviruses from environmental waters. Journal of Virological Methods, 229, 78–85.
Thongprachum, A., Fujimoto, T., Takanashi, S., Saito, H., Okitsu, S., Shimizu, H., et al. (2018). Detection of nineteen enteric viruses in raw sewage in Japan. Infection, Genetics and Evolution. https://doi.org/10.1016/j.meegid.2018.05.006.
Thongprachum, A., Khamrin, P., Maneekarn, N., Hayakawa, S., & Ushijima, H. (2016). Epidemiology of gastroenteritis viruses in Japan: Prevalence, seasonality, and outbreak. Journal of Medical Virology, 88(4), 551–570. https://doi.org/10.1002/jmv.24387.
Thongprachum, A., Khamrin, P., Pham, N. T., Takanashi, S., Okitsu, S., Shimizu, H., et al. (2017). Multiplex RT-PCR for rapid detection of viruses commonly causing diarrhea in pediatric patients. Journal of Medical Virology, 89(5), 818–824. https://doi.org/10.1002/jmv.24711.
Tort, L. F., Victoria, M., Lizasoain, A., Garcia, M., Berois, M., Cristina, J., et al. (2015). Detection of common, emerging and uncommon VP4, and VP7 human group a rotavirus genotypes from urban sewage samples in Uruguay. Food and Environmental Virology, 7(4), 342–353. https://doi.org/10.1007/s12560-015-9213-510.1007/s12560-015-9213-5.
van den Berg, H., Lodder, W., van der Poel, W., Vennema, H., & de R. Husman, A. M (2005). Genetic diversity of noroviruses in raw and treated sewage water. Research in Microbiology, 156(4), 532–540. https://doi.org/10.1016/j.resmic.2005.01.008.
Victoria, M., Tort, L. F., Garcia, M., Lizasoain, A., Maya, L., Leite, J. P., et al. (2014). Assessment of gastroenteric viruses from wastewater directly discharged into Uruguay River, Uruguay. Food and Environmental Virology, 6(2), 116–124. https://doi.org/10.1007/s12560-014-9143-7.
WHO Rotavirus deaths by country. (2000–2013). http://www.who.int/immunization/monitoring_surveillance/rotavirus_deaths_by_country_2000-2013.xlsx?ua=1. Accessed 11 June 2018.
Wieczorek, M., Krzysztoszek, A., & Witek, A. (2015). Species-specific identification of human adenoviruses in sewage. Polish Journal of Microbiology, 64(1), 23–28.
Wyn-Jones, A. P., Carducci, A., Cook, N., D’Agostino, M., Divizia, M., Fleischer, J., et al. (2011). Surveillance of adenoviruses and noroviruses in European recreational waters. Water Research, 45(3), 1025–1038. https://doi.org/10.1016/j.watres.2010.10.015.
Acknowledgements
This work was supported by the Ministry of Science and Technology, Bangladesh (Grant Number: 39.00.0000.09.02.069.16-17/BS-127/141), Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research (Grant Number: 24390266, 16H05360), Public Foundation of the Vaccination Research Center (Grant Number: 2015-34, 2018-38) and Kurozumi Medical Foundation. We are thankful to Professor Dr. Golam Mohammed Bhuiyan, Director, Centre for Advanced Research in Sciences (CARS), University of Dhaka, Bangladesh for allowing this collaborative study between University of Dhaka, Bangladesh and Nihon University School of Medicine, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We do not have any association either directly or indirectly that might pose a conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hoque, S.A., Thongprachum, A., Takanashi, S. et al. Alarming Situation of Spreading Enteric Viruses Through Sewage Water in Dhaka City: Molecular Epidemiological Evidences. Food Environ Virol 11, 65–75 (2019). https://doi.org/10.1007/s12560-018-09363-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12560-018-09363-z