Abstract
Human enteroviruses (HEVs) occur in high concentrations in wastewater and can contaminate receiving environmental waters, constituting a major cause of acute waterborne disease worldwide. In this study, we investigated the relative abundance, occurrence, and seasonal distribution of polio and other enteroviruses at three wastewater treatment plants (WWTPs) in Naples, Southern Italy, from January 2010 to December 2014. Influent and effluent samples from the three WWTPs were collected monthly. One hundred and sixty-one of the 731 wastewater samples collected (22.0%) before and after water treatment were CPE positive on RD cells; while no samples were positive on L20B cells from any WWTPs. Among the 140 non-polio enterovirus isolated from inlet sewage, 69.3% were Coxsackieviruses type B and 30.7% were Echoviruses. Among these, CVB3 and CVB5 were most prevalent, followed by CVB4 and Echo6. The twenty-one samples tested after treatment contained 6 CVB4, 5 CVB3, 3 Echo11, and 2 Echo6; while other serotypes were isolated less frequently. Data on viral detection in treated effluents of WWTPs confirmed the potential environmental contamination by HEVs and could be useful to establish standards for policies on wastewater management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The human enteroviruses (HEVs) are responsible of a wide variety of infections that affect humans (Griffin et al. 2003; Hewitt et al. 2011; Lin and Ganesh 2013; Adeniji and Faleye 2014). Their normal site of replication is the gastrointestinal tract, and the infection can be subclinical or cause only mild illness (Pellegrinelli et al. 2013; van der Linden et al. 2015). In some cases, the virus spreads to other organs, causing severe disease, including acute meningitis, encephalitis, paralysis, Bornholm disease, pericarditis, acute hemorrhagic conjunctivitis, and hand, foot, and mouth diseases; recent studies showed that HEV infection could be implicated in diabetes mellitus type 1 and myocarditis (La Rosa et al. 2012; Peci et al. 2014; Kaas et al. 2016). HEVs are transmitted from person to person by direct contact with virus excreted from the gastrointestinal or upper respiratory tract (fecal–oral route) (Sdiri-Loulizi et al. 2010; Simmons and Xagoraraki 2011): children represent the most infected of the population (Wikswo et al. 2009; Richter et al. 2011; Seppälä et al. 2016). The Enterovirus circulation occurs mainly during the summer and early fall in temperate climates (Wyn-Jones and Sellwood 2001; Wieczorek et al. 2015; Cordey et al. 2017).
The HEVs are members of the genus Enterovirus within the family Picornaviridae, order Picornavirales, consisting of four species: EV-A, EV-B, EV-C, and EV-D. Till date, HEVs comprise more than 100 serotypes, which are the most common pathogens infecting humans, especially children, worldwide, including the poliovirus, coxsackievirus, enterocytopathic human orphan (ECHO) virus, and enterovirus 68–71 (Rhoades et al. 2001; Laxmivandana et al. 2013; Qiu et al. 2015, Tian et al. 2017).
HEVs may survive on environmental surfaces for long periods (Fong and Lipp 2005; Prevost et al. 2015) and may be present naturally in aquatic environments or, more commonly, are introduced through human activities, such as leaking sewage and septic systems, urban runoff, agricultural runoff, and, in the case of estuarine and marine waters, sewage outfall and vessel wastewater discharges (Fong and Lipp 2005; Iaconelli et al. 2017). These viruses can be transported in the environment through groundwater, estuarine water, seawater, rivers, aerosols emitted from sewage treatment plants, insufficiently treated water, drinking water, and private wells that receive treated or untreated wastewater either directly or indirectly (Sano et al. 2016; Staggemeier et al. 2017). HEVs are shed in extremely high numbers by infected individuals feces, typically between 105 and 1011 virus particles per gram of stool (Farthing 1989; Qiu et al. 2015; Steyer et al. 2015; Sano et al. 2016). Therefore, HEVs are present at high levels in treated wastewater (Petrinca et al., 2009; Donia et al. 2010; Prado et al. 2011; Osuolale and Okoh 2017). To date, no regulations have been implemented to monitor viral concentrations in wastewater before it is discharged into a water body, as it is done currently for bacteria. HEVs are currently considered by the United States Environmental Protection Agency Contaminant Candidate List (USEPA CCL) as emerging contaminants (Qiu et al. 2015; Iaconelli et al. 2017; Osuolale and Okoh 2017).
Italy, particularly Southern Italy, for its geographical position, is at risk of importing wild poliovirus or neurovirulent Sabin-derived polioviruses (cVDPVs) following massive immigration flows from countries with endemic poliomyelitis or still using Sabin OPV vaccine. Our previous study evaluated the presence of HEVs in influent and effluent flow samples from wastewater treated plants (WWTPs) in Italy from July 2007 to July 2010 (Battistone et al. 2014a). In the present study, the presence of HEVs is evaluated in influent and effluent samples collected from three WWTPs located in Naples, between January 2010 and December 2014. The three distinct WWTPs investigated were “Cuma”, “San Giovanni Teduccio,” and “Napoli Est”, with WWTP-linked populations of 1,000,000, 700,000, and 500,000 inhabitants, respectively. This study confirmed an incomplete efficacy of some wastewater treatments to eliminate enteroviruses from sewage, highlighting the public health risks and the need for surveillance programs aimed to monitor and control the possible re-emergence of HEVs.
Our primary objective was to assess the efficiency of specific enterovirus removal of WWTP, collecting samples systematically before and after treatment using conventional methods indicated by WHO (2003) for detection of cultivatable enteroviruses (WHO 2004).
Materials and Methods
The three WWTPs (“Cuma”, “San Giovanni Teduccio,” and “Napoli Est”) monitored in Naples are conventional chemical–physical plants. The physical treatment common to all WWTPs included sand filtration and sedimentation, whereas sodium hypochlorite is used for chemical treatment.
Seven hundred and thirty-one wastewater samples were collected from three WWTPs in Naples, from January 2010 to December 2014. The samples collected from each plant were taken both before and after treatment, using 1-L sterile plastic bottles. All WWTPs monitored are conventional, activated sludge plants receiving waters from urban areas. The physical treatment common to all WWTPs included sand filtration and sedimentation, while sodium hypochlorite is used for chemical treatment. Sewage sampling started on different dates locally, causing different sample sizes between WWTPs. In each plant, wastewater samples were taken as single grabs during the peak usage, monthly, correlating their number to the population linked to the collector sewer (WHO 2003): three samples per month if the population collector was > 700,000 inhabitants (“Cuma”) and two samples per month for population size < 700,000 (“San Giovanni Teduccio” and “Napoli Est”). Samples were frozen and sent to Istituto Superiore di Sanità for concentration and virological analysis.
The recovery efficiency of the WHO two-phase separation method by Polyethylene glycol and Dextran (PEG-Dextran) (WHO 2003) used for virus concentration in this study was determined for six raw sewage samples (500 ml each). These samples were preliminarily autoclaved (121 °C for 30 min) to eliminate possible viruses present, and five samples were spiked with 1 ml of polio type 1 Sabin at a concentration of suspensions containing 2 × 105, 2 × 103, 20, 2, and 0.2 CCID50. The sixth sample was used as negative control. After concentration, samples were inoculated on RD cell cultures in 96-microwell plates (Di Lonardo et al. 2002), and virus titers were expressed as CCID50.
Details of the virus concentration method, cell cultures, HEV identification by PCR, and virus typing by seroneutralization are described in our previous paper (Battistone et al. 2014a). In brief, isolation of poliovirus and non-polio enteroviruses was performed according to WHO protocols (WHO 2004). To confirm the presence of enteroviruses, CPE-positive samples were tested by RT-nested-PCR using specific primers direct to VP1 (AN88 TACTGGACCACCTGGNGGNAYRWACAT and AN89 CCAGCACTGACAGCAGYNGARAYNGG) (Nix et al. 2006). Seroneutralization and immunocytochemistry tests performed according to standard WHO methods (WHO 2004) were also used to confirm and extend results of viral typing obtained. Details of the virus typing by seroneutralization and immunocytochemistry methods were described in our previous paper (Battistone et al. 2014a).
Data analysis was performed using the statistical software SPSS, version 14.01 for Windows (SPSS Inc., Chicago, IL, USA). All data were presented as the mean ± SD. The level of significance was set at p ≤ 0.05.
Results
Sewage samples spiked with 2 × 105 and 2 × 103 CCID50 of Sabin type 1 polio suspension, respectively, produced CPE on the first RD cells passage, while using 20 CCID50 of virus CPE was seen only on the second passage. The sample spiked with 2 CCID50 of Sabin type 1 polio was CPE negative. The resulting detection limit of 20 CCID50/sample is in line with the WHO requirements for a satisfactory environmental surveillance of polio and other enteroviruses (WHO 2003).
Seven hundred and thirty-one samples collected from 3 WWTPs were subjected to virological investigation (Table 1). One hundred and sixty-one (22.0%) of the samples were CPE positive on RD cells (140 inlet samples, 19.2%, and 21 outlet samples, 2.9%), whereas no samples showed CPE on L20B cells. RT–nested-PCR with primers directed to the VP1 confirmed the presence of enteroviruses in all positive samples. The percentage of CPE-positive inlet samples differed between collectors: “Cuma” showed 85 positive samples (52.8%), “San Giovanni Teduccio” 35 samples (21.7%), and “Napoli Est” 20 samples (12.4%). Instead, CPE-positive samples after treatment were nine samples (5.6%) from “Cuma” and “San Giovanni Teduccio”, while only three samples (1.9%) from “Napoli Est” (Table 1).
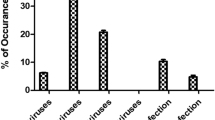
The seasonal distribution of 12 viral serotypes isolated from the three collectors, before and after sewage treatment, is reported in Fig. 1. The most of the enteroviruses isolated from inlet and outlet the wastewater treatment plant were isolated in summer, following by autumn, winter, and spring. The results show significant correlations between positive samples and the months (p value = 0.0064), such as the season (p value = 0.0025); while no significant correlation was found between the serotype and the months or season (p value = 0.1421 and 0.2986 respectively). In particular, among the 140 non-polio enterovirus isolated from inlet sewage, 97 were Coxsackieviruses type B (69.3%) and 43 were Echoviruses (30.7%). Among these, Coxsackievirus B3 (CVB3) and Coxsackievirus B5 (CVB5) were most prevalent (30 samples; 21.4%), followed by Coxsackievirus B4 (CVB4) (29 samples; 20.7%), Echovirus 6 (Echo6) (16 samples; 11.4%), and Echovirus 11 (Echo11) (16 samples; 11.4%). Other serotypes were isolated less frequently (Fig. 1). The 21 samples tested after treatment contained 6 CVB4, 5 CVB3, 3 Echo11, and 2 Echo6; while other serotypes were isolated less frequently.
Four Coxsackievirus, 2 CVB3 collected in July 2012 and July 2014 and 2 CVB4 collected in August and October 2010, isolated from inlet wastewater from 2 sewers of Naples (“Cuma” and “San Giovanni Teduccio”) were also identified in four outlet sample collected in the same month and location. In addition the same situation was found for one Echo11 that was detected before and after the physical–chemical treatment (“San Giovanni Teduccio”) in August 2012.
Discussion
The environmental monitoring of Enterovirus is necessary to better understand the epidemiology and to identify the possible viral contamination source (Prevost et al. 2015; Qiu et al. 2015; Osuolale and Okoh 2017); however, the research on environmental matrices is difficult, time consuming, and more expensive. Faster and more economic molecular techniques, for example, conventional and real-time RT–PCR, allow for a sensitive detection of different viral serotypes in wastewaters, without assessing viral infectivity (Donia et al. 2010; Adeniji and Faleye 2014). In this study, we used conventional viral isolation in order to evaluate infectious enteroviruses in pre- and posttreated sewage in three WWTPs of Naples city, in Southern Italy. This work was conducted within the national environmental surveillance of poliovirus and other enteroviruses launched in 2005, coordinated by the Italian Ministry of Health and the Istituto Superiore di Sanità, which complements the AFP (Acute Flaccid Paralyses) surveillance, in accordance with the WHO polio-eradication initiative (Battistone et al. 2014a).
From the three WWTP studied, no poliovirus, either wild type or vaccine-derived (Sabin-Like, SL), was isolated, confirming the absence of wild polio or vaccine-associated paralytic poliomyelitis (VAPP) cases, i.e., confirming the high immunization coverage in Italy. Since 2002 in Italy, the use of Salk-inactivated poliovirus vaccine (IPV)vaccination, which replaced oral attenuated Sabin vaccine (OPV) use, has gradually reduced the Sabin poliovirus circulation in the population and consequently in the environment (Patti et al. 2008; WHO 2011; Battistone et al. 2014b; Foiadelli et al. 2016). Nevertheless, our data show clearly that the circulation of many different enterovirus serotypes are common in the populations linked to the WWTPs investigated.
The most detected serotypes were CVB3 and CVB5 (21.4% each), CVB4 (20.7%,), followed by Echo6 and Echo11 (11.4% each). Usually, these viruses have been detected during viral meningitis outbreaks or other diseases, as reported in many environmental surveillance studies (Richter et al. 2011; Tao et al. 2014) and in particular, CVB5 is considered an emerging serotype causing meningitis outbreaks recurring through the years (Rezig et al. 2004; Cordey et al. 2017). During the study period, no similar outbreaks were identified in area studied, and the identification of 12 different enteroviruses indicates that these are some of main circulating serotypes, not excluding, however, other different HEV serotypes circulating in the environment. In fact, not all HEVs can be grown on RD cells, and every year the number of enteroviruses are steadily increasing and can be detected only by molecular methods. However, direct use of RT–PCR methods may be hampered by the presence of enzymes inhibitors in sewage samples, which may not be fully removed during RNA extraction.
The seasonal distribution of virus-positive samples between months and years overall suggest that HEVs circulate during all year in the city of Naples. However, the majority of positive samples was detected in summer, as reported by different studies in other countries with temperate climate (Costán-Longares et al. 2008; Sdiri-Loulizi et al. 2010; Peci et al. 2014; Tao et al. 2014).
The presence of enterovirus in samples collected after treatment suggest that the conventional chemical–physical WWTPs monitored are not sufficiently adequate to reduce the presence of infectious HEVs, causing a possible risk for public health for the long-term persistence in the environmental. In our previous study, the percentage of CPE-positive inlet samples showed five positive samples in “Napoli Est” (15.6%) and two samples in “San Giovanni Teduccio” (6.2%) (Battistone et al. 2014a). In the present study, instead, the percentage of positive samples show a significant increase in “San Giovanni Teduccio” (21.7%), while a slight decrease in “Napoli Est” (12.4%). The percentage of CPE-positive outlet samples show a significant increase in “San Giovanni Teduccio” (0% vs 5.6%), while in “Napoli Est,” a similar percentage of outlet positive samples was detected (1.6% vs 1.9%). However, data shown in this study are insufficient to make a proper and comprehensive assessment to evaluate the efficacy of processes used by the WWTPs to reduce the Enterovirus load. Certainly, technological improvements in wastewater treatment should also be in the future directed toward the reduction in viral contamination. Nonetheless, our data showed a decrease from 19.2 to 2.9% of HEV-positive samples, between upstream- and downstream-collected waters suggesting a significant, though incomplete, reduction in infectious viruses by treatments performed by the WWTPs. The number of positive enteroviruses encountered, indicate a high concentration of these viruses in sewage waters, reflecting a large circulation in the population.
Conclusion
No vaccines or antiviral agents are currently available for non-polio enteroviruses. Therefore, monitoring of¤ environmental enteroviruses contamination and WWTP performance are the only currently available aid to determine the circulation of pathogenic strains within the population and to assess risks of possible outbreaks due to waterborne and and foodborne diseases. The improvement of environmental surveillance before and after the water treatment to other cities of Italy, especially in areas more densely populated and where the risk of polio importation is higher is desirable. This activity could help also to investigate possible correlations between the non-polio enteroviruses circulating in the environment and the clinical cases.
References
Adeniji, J. A., & Faleye, T. O. (2014). Isolation and identification of enteroviruses from sewage and sewage-contaminated water in Lagos, Nigeria. Food and Environmental Virology, 6(2), 75–86. https://doi.org/10.1007/s12560-014-9137-5.
Battistone, A., Buttinelli, G., Bonomo, P., Fiore, S., Amato, C., Mercurio, P., et al. (2014a). Detection of enteroviruses in influent and effluent flow samples from wastewater treatment plants in Italy. Food and Environmental Virology, 6(1), 13–22. https://doi.org/10.1007/s12560-013-9132-2.
Battistone, A., Buttinelli, G., Fiore, S., Amato, C., Bonomo, P., Patti, A. M., et al. (2014b). Sporadic isolation of sabin-like polioviruses and high-level detection of non-polio enteroviruses during sewage surveillance in seven Italian cities, after several years of inactivated poliovirus vaccination. Applied and Environmental Microbiology, 80(15), 4491–4501.
Cordey, S., Schibler, M., L’Huillier, A. G., Wagner, N., Gonçalves, A. R., Ambrosioni, J., et al. (2017). Comparative analysis of viral shedding in pediatric and adult subjects with central nervous system-associated enterovirus infections from 2013 to 2015 in Switzerland. Journal of Clinical Virology, 89, 22–29. https://doi.org/10.1016/j.jcv.2017.01.008.
Costán-Longares, A., Mocé-Llivina, L., Avellón, A., Jofre, J., & Lucena, F. (2008). Occurrence and distribution of culturable enteroviruses in wastewater and surface waters of north-eastern Spain. Journal of Applied Microbiology, 105(6), 1945–1955. https://doi.org/10.1111/j.1365-2672.2008.03954.x.
Di Lonardo, A., Buttinelli, G., Amato, C., Novello, F., Ridolfi, B., & Fiore, L. (2002). Rapid methods for identification of Poliovirus isolates and determination of polio neutralizing antibody titers in human sera. Journal of Virological Methods, 101, 189–196.
Donia, D., Bonanni, E., Diaco, L., & Divizia, M. (2010). Statistical correlation between enterovirus genome copy numbers and infectious viral particles in wastewater samples. Letters in Applied Microbiology, 50, 237–240.
Farthing, M. J. G. (1989). Viruses and the gut. Walwyn Garden City: Smith Kline & French.
Foiadelli, T., Savasta, S., Battistone, A., Kota, M., Passera, C., Fiore, S., et al. (2016). Nucleotide variation in Sabin type 3 poliovirus from an Albanian infant with agammaglobulinemia and vaccine associated poliomyelitis. BMC Infectious Diseases, 16, 277. https://doi.org/10.1186/s12879-016-1587-y.
Fong, T. T., & Lipp, E. K. (2005). Enteric viruses of humans and animals in aquatic environments: Health risks, detection, and potential water quality assessment tools. Microbiology and Molecular Biology Reviews, 69(2), 357–371.
Griffin, D. W., Donaldson, K. A., Paul, J. H., & Rose, J. B. (2003). Pathogenic human viruses in coastal waters. Clinical Microbiology Reviews, 16(1), 129–143. https://doi.org/10.1128/CMR.16.1.129-143.2003.
Hewitt, J., Leonard, M., Greening, G. E., & Lewis, G. D. (2011). Influence of wastewater treatment process and the population size on human virus profiles in wastewater. Water Research, 45(18), 6267–6276. https://doi.org/10.1016/j.watres.2011.09.029.
Iaconelli, M., Muscillo, M., Della Libera, S., Fratini, M., Meucci, L., De Ceglia, M., et al. (2017). One-year surveillance of human enteric viruses in raw and treated wastewaters, downstream river waters, and drinking waters. Food and Environmental Virology, 9(1), 79–88. https://doi.org/10.1007/s12560-016-9263-3.
Kaas, L., Gourinat, A. C., Urbès, F., & Langlet, J. (2016). A 1-year study on the detection of human enteric viruses in New Caledonia. Food and Environmental Virology, 8(1), 46–56. https://doi.org/10.1007/s12560-015-9224-2.
La Rosa, G., Fratini, M., della Libera, S., Iaconelli, M., & Muscillo, M. (2012). Emerging and potentially emerging viruses in water environments. Annali dell’Istituto Superiore di Sanità, 48(4), 397–406. https://doi.org/10.4415/ann_12_04_07.
Laxmivandana, R., Yergolkar, P., Gopalkrishna, V., & Chitambar, S. D. (2013). Characterization of the non-polio enterovirus infections associated with acute flaccid paralysis in South-Western India. PLoS ONE, 8(4), e61650. https://doi.org/10.1371/journal.pone.0061650.
Lin, J., & Ganesh, A. (2013). Water quality indicators: bacteria, coliphages, enteric viruses. International Journal of Environmental Health Research, 23, 484–506. https://doi.org/10.1080/09603123.2013.769201.
Nix, W. A., Oberste, M. S., & Pallansch, M. A. (2006). Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. Journal of Clinical Microbiology, 44(8), 2698–2704.
Osuolale, O., & Okoh, A. (2017). Human enteric bacteria and viruses in five wastewater treatment plants in the Eastern Cape, South Africa. Journal of Infection and Public Health, S1876–0341(17), 30024-2. https://doi.org/10.1016/j.jiph.2016.11.012.
Patti, A. M., Martini, V., Calvani, A., Vulcano, A., Zotti, C., Sudano, L., et al. (2008). La sorveglianza della poliomelite in Italia: stato immunitario della popolazione di età 0-14 anni. Annali di Igiene, 20(5), 15–22.
Peci, A., Winter, A. L., Eshaghi, A., Marchand-Austin, A., Olsha, R., Lombardi, N., et al. (2014). Coxsackieviruses in Ontario, January 2005 to December 2011. International Journal of Infectious Diseases, 25, 136–141. https://doi.org/10.1016/j.ijid.2014.04.013.
Pellegrinelli, L., Binda, S., Chiaramonte, I., Primache, V., Fiore, L., Battistone, A., et al. (2013). Detection and distribution of culturable Human Enteroviruses through environmental surveillance in Milan, Italy. Journal of Applied Microbiology, 115(5), 1231–1239. https://doi.org/10.1111/jam.12321.
Petrinca, A. R., Donia, D., Pierangeli, A., Gabrieli, R., Degener, A. M., Bonanni, E., et al. (2009). Presence and environmental circulation of enteric viruses in three different wastewater treatment plants. Journal of Applied Microbiology, 106, 1608–1617. https://doi.org/10.1111/j.1365-2672.2008.04128.x.
Prado, T., Silva, D. M., Guilayn, W. C., Rose, T. L., Gaspar, A. M., & Miagostovich, M. P. (2011). Quantification and molecular characterization of enteric viruses detected in effluents from two hospital wastewater treatment plants. Water Research, 45(3), 1287–1297. https://doi.org/10.1016/j.watres.2010.10.012.
Prevost, B., Lucas, F. S., Goncalves, A., Richard, F., Moulin, L., & Wurtzer, S. (2015). Large scale survey of enteric viruses in river and waste water underlines the health status of the local population. Environment International, 79, 42–50. https://doi.org/10.1016/j.envint.2015.03.004.
Qiu, Y., Lee, B. E., Neumann, N., Ashbolt, N., Craik, S., Maal-Bared, R., et al. (2015). Assessment of human virus removal during municipal wastewater treatment in Edmonton, Canada. Journal of Applied Microbiology, 119(6), 1729–1739. https://doi.org/10.1111/jam.12971.
Rezig, D., Yahia, A. B., Abdallah, H. B., Bahri, O., & Triki, H. (2004). Molecular characterization of coxsackievirus B5 isolates. Journal of Medical Virology, 72, 268–274.
Rhoades, R. E., Tabor-Godwin, J. M., Tsueng, G., & Feuer, R. (2001). Enterovirus infections of the central nervous system review. Virology, 411(2), 288–305. https://doi.org/10.1016/j.virol.2010.12.014.
Richter, J., Tryfonos, C., & Christodoulou, C. (2011). Circulation of enteroviruses in Cyprus assessed by molecular analysis of clinical specimens and sewage isolates. Journal of Applied Microbiology, 111, 491–498.
Sano, D., Amarasiri, M., Hata, A., Watanabe, T., & Katayama, H. (2016). Risk management of viral infectious diseases in wastewater reclamation and reuse: Review. Environment International, 91, 220–229. https://doi.org/10.1016/j.envint.2016.03.001.
Sdiri-Loulizi, K., Hassine, M., Aouni, Z., Gharbi-Khelifi, H., Chouchane, S., Sakly, N., et al. (2010). Detection and molecular characterization of enteric viruses in environmental samples in Monastir, Tunisia between January 2003 and April 2007. Journal of Applied Microbiology, 109, 1093–1104. https://doi.org/10.1111/j.1365-2672.2010.04772.x.
Seppälä, E., Sillanpää, S., Nurminen, N., Huhtala, H., Toppari, J., Ilonen, J., et al. (2016). Human enterovirus and rhinovirus infections are associated with otitis media in a prospective birth cohort study. Journal of Clinical Microbiology, 85, 1–6. https://doi.org/10.1016/j.jcv.2016.10.010.
Simmons, F. J., & Xagoraraki, I. (2011). Release of infectious human enteric viruses by full-scale wastewater utilities. Water Research, 45(12), 3590–3598. https://doi.org/10.1016/j.watres.2011.04.001.
Staggemeier, R., Heck, T. M., Demoliner, M., Ritzel, R. G., Röhnelt, N. M., Girardi, V., et al. (2017). Enteric viruses and adenovirus diversity in waters from 2016 Olympic venues. Science of the Total Environment, 586, 304–312. https://doi.org/10.1016/j.scitotenv.2017.01.223.
Steyer, A., Gutiérrez-Aguirre, I., Rački, N., Glaser, S. B., Humar, B. B., Stražar, M., et al. (2015). The detection rate of enteric viruses and clostridium difficile in a waste water treatment plant effluent. Food and Environmental Virology, 7(2), 164–172. https://doi.org/10.1007/s12560-015-9183-7.
Tao, Z., Wang, H., Li, Y., Liu, G., Xu, A., Lin, X., et al. (2014). Molecular epidemiology of human enterovirus associated with aseptic meningitis in Shandong Province, China, 2006-2012. PLoS ONE, 9(2), e89766. https://doi.org/10.1371/journal.pone.0089766.
Tian, H., Zhang, Y., Shi, Y., Li, X., Sun, Q., Liu, L., et al. (2017). Epidemiological and aetiological characteristics of hand, foot, and mouth disease in Shijiazhuang City, Hebei province, China, 2009-2012. PLoS ONE, 12(5), e0176604. https://doi.org/10.1371/journal.pone.0176604.
van der Linden, L., Wolthers, K. C., & van Kuppeveld, F. J. M. (2015). Replication and inhibitors of enteroviruses and parechoviruses. Viruses, 7, 4529–4562. https://doi.org/10.3390/v7082832.
WHO. (2003). Guidelines for environmental surveillance of Poliovirus circulation. V&B/03.03. Geneva: World Health Organization.
WHO. (2004). Polio laboratory manual./IVB/04.10. Geneva: World Health Organization.
Wieczorek, M., Ciąćka, A., Witek, A., Kuryk, Ł., & Żuk-Wasek, A. (2015). Environmental surveillance of non-polio enteroviruses in Poland, 2011. Food and Environmental Virology, 7(3), 224–231. https://doi.org/10.1007/s12560-015-9195-3.
Wikswo, M. E., Khetsuriani, N., Fowlkes, A. L., Zheng, X., Peñaranda, S., Verma, N., et al. (2009). Increased activity of Coxsackievirus B1 strains associated with severe disease among young infants in the United States, 2007-2008. Clinical Infectious Diseases, 49(5), e44–e51. https://doi.org/10.1086/605090.
Wyn-Jones, A. P., & Sellwood, J. (2001). Enteric viruses in the aquatic environment. Journal of Applied Microbiology, 91, 945–962.
Acknowledgements
This work was supported by grants from the Italian Ministry of Health, CCM, “AFP surveillance in Italy. Research of Poliovirus and other enteroviruses in immunodeficient patients and in the environment” (2008–2010).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pennino, F., Nardone, A., Montuori, P. et al. Large-Scale Survey of Human Enteroviruses in Wastewater Treatment Plants of a Metropolitan Area of Southern Italy. Food Environ Virol 10, 187–192 (2018). https://doi.org/10.1007/s12560-017-9331-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12560-017-9331-3