Abstract
Purpose
MedAustron mission is to cure cancer by providing advanced patient care, clinical trials, applied and basic research, and know-how transfer. The facility is constantly striving to improve the therapy method of ion beam therapy, to increase its effectiveness and to make the treatment accessible to more people.
Methods
The MedAustron particle therapy accelerator facility is located in Austria and delivers protons in the energy range 60–250 MeV and carbon ions 120–400 MeV/n for tumor treatment to four irradiation rooms. Clinical treatment includes two rooms with fixed beam lines horizontal and horizontal/vertical and a third room with a rotating beam line, the proton gantry. A fourth irradiation room is dedicated to research delivering carbon and helium beams and where proton beams up to 800 MeV are also provided.
Results
The facility has been built, the commissioning has been completed and MedAustron is now successfully operating at its full functionality. Since the first patient treatment in December 2016, more than 2,000 patients have been treated at MedAustron.
Conclusions
In this paper, we provide an overview of the facility including the world-wide first so-called rotator system used, synchronously with the gantry, to improve the quality of the beam delivered at the patient. Furthermore, we discuss about the ongoing projects for improvement of the facility, the areas of research and potential topics for collaboration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The MedAustron accelerator delivers proton and carbon ion beams for cancer treatment to three irradiation rooms with the goal of treating 800 patients per year. The center also offers a dedicated beam line and infrastructures to national and international research institutes.
Following the commissioning of the gantry beamline, all rooms at MedAustron are now operational. Since the first patient in 2016, more than 2,000 patients have been treated with protons and carbon ions using over 50,000 single fractions with an average weekly machine uptime during clinical operation > 96%. Accelerator parameters are defined by requirements for clinical treatment and research and an example for the fixed and gantry beam lines is shown in Table 1.
2 Facility overview
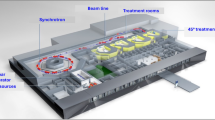
MedAustron is located in Wiener Neustadt, Lower Austria region, approximately 50 km south of the Austrian capital city Vienna, as shown in Fig. 1. It is one of the only six proton and carbon ion therapy centers in the world.
At today, the facility totals ca. 300 employees from 20 different countries with expertise in designing, building, commissioning, certifying and operating a particle therapy facility.
3 Facility History
In the early years of feasibility study, the facility was conceived as a neutron spallation source. It was only in the mid-90’ that it was reoriented to a clinical therapy facility for cancer treatment and research, then renamed MedAustron to emphasize the medical nature and the country of origin. In the same decade, an important milestone was the research study effort culminated with the Proton-Ion Medical Machine Study PIMMS document [1,2,3] that was the basis for the CNAO and MedAustron facilities. After a period of feasibility and design studies the company was founded in 2007 when the manufacturing of accelerator components started, followed by the construction of the buildings that terminated in 2012. In the following year, the installation and commissioning of the accelerator was begun and then completed in 2016 leading to the first successful patient treatment in December of the same year. At the same time, the research program also started in the dedicated irradiation room (IRs). The timeline of the facility history is shown in Fig. 2 layout.
As the clinical treatments were progressively extended for longer times during the week days, the beam commissioning of the remaining particle types and irradiation rooms including the gantry had to be performed during the available night and weekend hours.
Thus, the accelerator components and beam commissioning of all beam lines and particle types was completed in 2023, when the facility successfully reached its full functionality. Milestones of the facility over the years are shown in the Fig. 3 schematic.
4 The technology behind
A bird’s eye view of a MedAustron facility model is shown in Fig. 4.
Two sources are used to generate proton and carbon ion beams. A third source generates helium for research purposes and is also used as spare for the other particle types used for clinical operation.
Three Supernanogan Electron Cyclotron Resonance Ion Sources (ECRIS) are designed to provide \({H}_{3}^{+}\), 12C4+ and \({He}^{2+}\) beams to a low energy transfer line (LEBT) and subsequently to a Linac, shown in Fig. 5, consisting of a 400 keV/n RFQ and an interdigital H-mode (IH) DTL that accelerates the beam to 7 MeV/n. A multi-turn injection from the Medium Energy Transfer Line (MEBT) accumulates particles into the synchrotron, Fig. 6. Following RF capture and acceleration, the beam is extracted from the synchrotron by a third-order resonant slow extraction mechanism, driven by a so-called resonant sextupole and a betatron core magnet.
The high energy transfer beam line (HEBT) shown in Fig. 7, transports the beam from the synchrotron and deflects it either to the research room or to one of three treatment rooms with the assistance of achromatic beam-deflection modules. Deflection branches are parallel to each other. Each deflection module contains switching dipole magnets. With all switching dipole magnets off, the beam is sent straight to the beam dump at the end of the extraction beam line.
Towards the end of the HEBT, there is an integral part of the gantry beamline: the rotator, a 9.9 m long straight section containing seven identical quadrupoles and independently powered. This configuration has been chosen by MedAustron to match the slowly extracted asymmetric beams to the rotating gantry. The rotator is connected to the gantry via an achromatic deflection module. Thus effectively, the gantry beam line is located 20 m downstream of the rotator. Such a beam-transport line has been commissioned for the first time world-wide.
Most importantly, by using the rotator system, all beam parameters at the gantry isocenter become independent from the gantry rotation angle. The beam commissioning covered several performance related tests of the rotator, the gantry, and finally of the whole beamline. The first proof-of-concept of a rotator system has been accomplished. The gantry beamline commissioning has been successfully completed and the line is now in clinical operation since the year 2022.
A picture of the rotator and the gantry structures are shown respectively in Fig. 8 and Fig. 9.
The world-wide first rotator system (white structure) in the HEBT. Seven quadrupoles (orange) are mounted on this mechanical supporting structure that is rotated by half of the gantry angle. The gantry beam line is located downstream of the rotator. With a rotator, the beam parameters at the room isocenter are independent from the gantry rotation angle
One of the irradiation rooms of a fixed beam line and the patient positioning system are shown in Fig. 10. The automatic-controlled couch fulfils the requirements to placing the patient in the correct position and to monitoring the position continuously. An “imaging ring” on the couch provides X-ray and computerized tomography CT images to ensure sub-millimiter patient positioning.
The particle accelerator and all systems connected are subject to strict quality assurance on a daily basis and are constantly being further developed.
The facility includes four irradiation rooms (IR) as summarized also in Table 2: room 1 dedicated to research; room 2 with two beamlines, horizontal and vertical; room 3 with a horizontal beamline and room 4 hosting a proton gantry based on a PSI design [4, 5]. The fixed beamlines of IR1, IR2 and IR3 provide protons, carbon ions [6, 7], while the helium ion beam is currently under commissioning in IR1.
5 Indications treated at MedAustron
Particle beam therapy makes use of the physical effect where a beam of charged particles deposits most of its kinetic energy at a defined distance within the body. The penetration depth depends on the beam energy in a well-defined and reproducible correlation. This physical effect allows for a lower exposure of healthy tissues to radiation with a reduction of side effects and long-term damages with respect to conventional photon radiotherapy. In addition, irradiating the tumor from different directions also contributes to reduce the exposure of healthy tissue to radiation.
Thus, tumors in close proximity to radiosensitive organs are the main field of application of particle therapy. Paediatric tumors and local recurrences are also important patient groups in the treatment with charged particles. More information is also available at [8].
An overview of the indications by category and related fractional percent of the tumors treated at the MedAustron is shown in Fig. 11.
The biological effect of proton therapy is very similar to that of conventional photon therapy; thus, the chances of cure at the same dose are similar after proton therapy as after conventional irradiation. Though, due to a lower exposure of healthy tissues to radiation, in special situations the total dose can be increased during proton therapy and consequently an improvement in local control can be achieved [9]. For these reasons, proton therapy renders the use of high doses of radiation a viable option.
Additionally, the low dose bath which is present in the irradiation with photons can be minimized to a large extent with protons and carbon ions due to the absence of the exit dose (dose beyond the tumor in beam direction) and a smaller dose in the entrance of the beam. Consequently, large volumes of normal tissue get a significant amount of lower dose.
On the other hand, the therapy with carbon ions provides several unique physical and radiobiological advantages, high conformity along with high relative biologic effectiveness (RBE) and linear energy transfer (LET) with different damaging effects on the tumor DNA with respect to protons and photons.
Particle therapy depending on the tumor, can be combined with systemic therapy, as it is with conventional radiotherapy as well. Particle therapy can also be performed after surgery, or in situations where surgery is associated with high risks particle therapy can also be performed as an option.
6 Research at MedAustron
In addition to patient treatment in clinical studies, the MedAustron accelerator facility offers a wide range of research opportunities.
Different aspects of research are addressed at MedAustron: on one hand, translational research addresses topics close to medical applications, on the other hand, particle beams can also generally be used in radiation physics and applied particle physics. Medical University of Vienna and Vienna University of Technology are the priority partner institutes for research projects, whose implementation is decided by an independent advisory board. There is also close cooperation in scientific work with the Institute for High Energy Physics of the Austrian Academy of Sciences, University of Applied Sciences Wiener Neustadt and Medical University of Graz. An executive committee supports practical research work on site and accompanies research progress.
Recently, two professorships have been appointed at the Department of General and Translational Oncology and Haematology at the Karl Landsteiner University for Health Sciences, Austria. Prof. Markus Stock from MedAustron in the Division Medical Physics and Prof. Piero Fossati from MedAustron in the Division Radiation Oncology. Together, they form a Research Cluster on Cancer with Prof. Klaus Podar and the Division of Molecular Oncology and Hematology.
Three main field of research are undergoing at MedAustron.
Clinical research
Dedicated to registry study and clinical studies, creating more evidence in particle therapy. MedAustron is also involved in a collaborative study program on clinical research and studies PROSPER [10]. The PROSPER study is a tricontinental, nonrandomized, prospective, three-arm, pragmatic trial evaluating the same indication with treatments performed using different particle species, namely proton and carbon ions. The comparative studies are conducted in collaboration with the Mayo Clinic campuses in Arizona, Florida, and Minnesota, U.S.A.
Interdisciplinary oncology research
The teaching and research site of the Karl Landsteiner University support the interdisciplinary research in oncology that includes two main branches:
-
Medical Physics
-
Radiation Oncology
Medical Physics research focuses on the development and optimization of medical physics methods including dosimetry and micro-dosimetry, Monte-Carlo simulations, Big Data, radiation plan optimization, software development and process management.
Radiation Oncology research concentrates on the optimization of patient treatments with radiotherapy by applying the highest standards of care and by performing clinical research while fostering the translation of preclinical research in a clinical setting.
Translational and scientific research
It is based on four research pillars: Applied Particle and Medical Physics, Technological Innovations and Clinical implementations, Biophysics and Molecular Radiobiology and Accelerator Physics, as shown in Fig. 12. Each of them includes a subset of research topics that are worked on in cooperation with Medical Universities Vienna & Graz, Technical University Vienna, HEPHY and FH Wiener Neustadt.
7 Development projects
Several development projects are ongoing or planned to improve the performances and enhance the treatment capabilities.
Currently, helium ions are being commissioned and will be available for research purposes in IR1 by the end of 2024.
This year will also be possible to treat a new indication with the dedicated project implementation for eye treatment and improve the treatment quality by the activation of respiratory gating. Furthermore, a new beam delivery system is now under commissioning to support the planned projects to reach 800 treated patients per-year goal.
On a longer term, improvements for enhanced treatment quality consider the implementation of RF Knock-out driven extraction, multi-energy per single-spill treatments and dynamic intensity control.
MedAustron also actively contributes to international collaborations such as EuroSIG and HITRI + for the design study of a superconducting ion accelerator and gantry.
8 Knowledge transfer
Over the last fifteen years, MedAustron has acquired a solid and extensive experience in the field of particle therapy accelerator and certification of medical devices.
MedAustron technical expertise and its innovative technology are available for scientific and commercial purposes throughout a variety of technology transfer options: R&D collaborations, MedAustron technology in start-ups, service, consultancy and licensing in all aspects related to:
-
Concept and planning
-
Hardware and Software development
-
Maintenance and Services
-
Commissioning and Operation
-
Certification
-
Training of accelerator personnel and operators
-
Radiation Protection
MedAustron grants licences to commercial and academic partners for the exploitation of its technologies. MedAustron expertise and infrastructures represent an opportunity for companies and academics in need of a specific high-tech service. Experts in many areas of particle accelerator aspects are available to provide advice or specific studies to particle accelerator projects for medical applications.
9 Certification aspects of heavy ion therapy machines
Once a particle therapy accelerator has been developed, there are certain requirements to be fulfilled to be able to bring it to the market as a medical device. The safety of the patient, the operating and service personnel, the environment affected by electromagnetic field and radiation need to be testified for conformity with the standard directives.
The Conformité Européenne (CE) Mark is defined as the European Union’s (EU) mandatory conformity marking for regulating the goods traded within the European Economic Area (EEA) since 1985. The CE Marking serves as a declaration from the manufacturer that their product complies with the essential requirements of the relevant European health, safety and environmental legislation laid out in the different product directives. Once a product has been awarded the CE Marking, then it is able to move freely within the European Free Trade Association (EFTA) and the European Union (EU).
Manufacturers play a crucial role in ensuring that products placed on the extended single market of the European Economic Area (EEA) are safe. They are responsible for checking that their products meet EU safety, health, and environmental protection requirements. It is the manufacturer’s responsibility to carry out the conformity assessment, set up the technical files, issue the EU declaration of conformity, and affix the CE marking to a product. Only then can this product be traded on the EEA market.
MedAustron, having the expertise on the certification aspects of heavy ion therapy machines, can support with obtaining the CE marking according to the Medical Device Regulations (MDR) and obtaining the declaration of conformity. This process requires preparing the technical documentation, testifying the safety and environmental protection, dealing with regulatory affairs and the aspects related to the risk management to ultimately certify the technology as a medical product and be able to treat patients.
10 Summary and outlook
Since more than seven years, patient treatment is ongoing at the MedAustron facility in Austria, using proton and carbon ion beams, with a continued ramp up in the number of patients throughput towards the final goal of 800 patients per year.
Protons and carbon ions with high intensities and transmissions are available for patient treatment and research in all three rooms with fixed beam lines, while protons are available in the rotating gantry beam line. The machine is remarkably stable in terms of its beam parameters robustness and reproducibility.
The proton gantry beamline including the world-wide first rotator is clinically operational since already two years. The rotator, designed, built, installed, and tested at MedAustron, is the first and the only hardware implementation of its kind all over the world. The rotator concept has been successfully proven to work resulting in a beam spot at the gantry isocenter to be round and independent from the gantry rotation angle.
Recently, carbon commissioning has also been completed in a second irradiation room, which completed the commissioning of the facility and MedAustron is now operating at its full functionality.
In addition to clinical operations, one irradiation room is fully dedicated to research, available to national and international research groups. The research room is delivering carbon, recently helium ion beams and proton beams up to 800 MeV are also provided.
A number of performance improvement projects are ongoing including eye treatment that will be available on a short term and the activation of respiratory gating. A new beam delivery system is under commissioning to facilitate reaching the 800 patients per-year goal. On a longer term, multi-energy extraction in a single beam is part of the improvement projects for enhanced treatment quality.
Data availability
The data used to support the findings of this work are available from the corresponding author upon request.
Code availability
Not applicable
References
Badano L, Benedikt M, Bryant P, Crescenti M, Holý P, Maier A, Pullia M, Reimoser S, Rossi S. Proton-ion medical machine studies (PIMMS) parts I and II respectively CERN Technical Report Nos. CERN-PS-99-010-DI (1999) and CERN-PS-2000-007-DR (2000). https://cds.cern.ch/record/2858081?ln=en, https://cds.cern.ch/record/2858080?ln=en.
Amaldi U, Magrin G. The path to the italian national centre for ion therapy. Ed. Mercurio Cardo S.c.r.l; 2005. ISBN 978-88-95522-44-9.
Benedikt M, et al. Overview of the MedAustron design and technology choices. In: Proc. Int. Particle Accelerator Conf. Kyoto, Japan: (IPAC’10); 2010. p. 109–11.
Pedroni E, et al. The PSI Gantry 2: a second generation proton scanning gantry. Z Med Phys. 2004;14(1):25–34.
Benedikt M. Optics design of the extraction lines for the MedAustron hadron therapy centre. In: Nucl Instrum Methods Phys Res A, vol. 539. 2005. p. 25–36.
Schmitzer C, et al. Carbon Commissioning of the MedAustron Therapy Accelerator. In: Proc. 9th Int. Particle Accelerator Conf. Vancouver, BC, Canada: (IPAC’18); 2018. p. 457–9. https://doi.org/10.18429/JACoW-IPAC2018-MOPML027.
Kurfürst C, et al. Status of the MedAustron beam commissioning with protons and carbon ions. In: Proc. 9th Int. Particle Accelerator Conf. Vancouver, BC, Canada: (IPAC’18); 2018. p. 666–8. https://doi.org/10.18429/JACoW-IPAC2018-TUPAF004.
Indications treated at MedAustron, website: https://www.medaustron.at/en/indications/.
Kiseleva V, Gordon K, Vishnyakova P, Gantsova E, Elchaninov A, Fatkhudinov T. Particle therapy: clinical applications and biological effects. Life (Basel). 2022;12(12):2071. https://doi.org/10.3390/life12122071.
Hoppe BS, Petersen IA, Wilke BK, DeWees TA, Imai R, Hug EB, Fiore MR, Debus JN, Fossati P, Yamada S, Orlandi E, Zhang Q, Bao C, Seidensaal K, May BC, Harrell AC, Houdek MT, Vallow LA, Rose PS, Haddock MG, Ashman JB, Goulding KA, Attia S, Krishnan S, Mahajan A, Foote RL, Laack NN, Keole SR, Beltran CJ, Welch EM, Karim M, Ahmed SK. Pragmatic, prospective comparative effectiveness trial of carbon ion therapy, surgery, and proton therapy for the management of pelvic sarcomas (soft tissue/bone) involving the bone: the prosper study rationale and design. Cancers. 2023;15(6):1660. https://doi.org/10.3390/cancers15061660.
Acknowledgements
This article has been written on behalf of the MedAustron Technical Accelerator and the Medical Physics Divisions that we would like to Thank. We are thankful to Michael Benedikt and Ulrich Dorda for the optics design including the rotator and the original gantry optics and to Marco Pullia and Márius Pavlovič for the support with the beam commissioning.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Mauro Pivi. The first draft of the manuscript was written by Mauro Pivi. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study does not involve humans or animals.
Consent to participate
N.A.
Consent to publish
N.A.
Informed consent
An informed consent to publish has been obtained from all individual mentioned for whom identifying information is included in this article. In particular, informed consent was obtained from the individual Professors of whom names are cited in this article, to publish their identifying information. Informed consent was obtained from the MedAustron Institution to publish the data in the tables and figures of this article. In particular, informed consent has been obtained to publish the data in Fig. 11 of this article.
Conflict of interest
The author has no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pivi, M.T.F. The MedAustron particle therapy accelerator facility. Health Technol. 14, 919–928 (2024). https://doi.org/10.1007/s12553-024-00840-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12553-024-00840-z