Abstract
In today’s world, there is a wide array of materials engineered at the nano- and microscale, with numerous applications attributed to these innovations. This review aims to provide a concise overview of how nano- and micromaterials are utilized for enzyme immobilization. Enzymes act as eco-friendly biocatalysts extensively used in various industries and medicine. However, their widespread adoption faces challenges due to factors such as enzyme instability under different conditions, resulting in reduced effectiveness, high costs, and limited reusability. To address these issues, researchers have explored immobilization techniques using nano- and microscale materials as a potential solution. Such techniques offer the promise of enhancing enzyme stability against varying temperatures, solvents, pH levels, pollutants, and impurities. Consequently, enzyme immobilization remains a subject of great interest within both the scientific community and the industrial sector. As of now, the primary goal of enzyme immobilization is not solely limited to enabling reusability and stability. It has been demonstrated as a powerful tool to enhance various enzyme properties and improve biocatalyst performance and characteristics. The integration of nano- and microscale materials into biomedical devices is seamless, given the similarity in size to most biological systems. Common materials employed in developing these nanotechnology products include synthetic polymers, carbon-based nanomaterials, magnetic micro- and nanoparticles, metal and metal oxide nanoparticles, metal–organic frameworks, nano-sized mesoporous hydrogen-bonded organic frameworks, protein-based nano-delivery systems, lipid-based nano- and micromaterials, and polysaccharide-based nanoparticles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
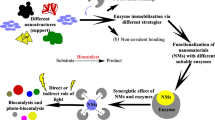
The ongoing industrial revolution highlights a significant meaning of biocatalysis construction. In recent times, various micro- and nanoscale materials have been tested for immobilizing enzymes to enhance their valuable properties (Bilal et al. 2019). The field of nanobiotechnology has emerged as nanotechnology products find increasing utility in biomedicine (Saji et al. 2010).
Enzymes play a crucial role as green biocatalysts, finding extensive applications in industries and medicine. However, their widespread use is impeded by several factors, including the instability of enzyme preparations under different conditions, leading to reduced efficiency, higher costs, and the inability to reuse enzymes. Immobilization techniques have been explored as a solution to address these challenges (Yan et al. 2012; Senko et al. 2019; Razumovsky et al. 2008).
To achieve successful enzyme immobilization, certain conditions must be met:
-
i.
The enzyme must demonstrate stability under the specific reaction conditions.
-
ii.
When using cross-linking agents for immobilization, they should interact with the enzyme’s functional groups outside its active site. Alternatively, if this is not feasible, the cross-linking reagent should be as large as possible to prevent entry into the active site.
-
iii.
During immobilization, the enzyme’s active site should be protected by binding it with its substrate, inhibitors, or cofactors.
-
iv.
The chosen method of immobilization should be compatible with the nature of the catalyzed reaction and the characteristics of the substrate.
-
v.
The procedure to remove unbound enzyme should not adversely affect the immobilized enzyme.
-
vi.
The mechanical strength and physical properties of the carrier material should be carefully considered (Reis et al. 2019; Jesionowski et al. 2014; Cantone et al. 2013).
Developing an immobilization method that fulfills all these requirements is undoubtedly challenging and often requires making compromises. Exploring the structural and functional characteristics of enzymes can contribute to advancing our theoretical understanding of the mechanisms behind enzyme-carrier interactions. This knowledge aids in controlling enzyme-binding processes and selecting effective matrices for immobilization, thereby preserving enzyme activity and stability. Moreover, it helps identify promising microenvironments for optimal enzyme functionality (Holyavka et al. 2016; Ashmarina et al. 1984).
Nanotechnology has experienced significant growth, leading to the availability of a wide range of nanoscale materials. These materials have found diverse applications, and immobilizing enzymes on nano- and micromaterials has emerged as a promising approach for biocatalysts in both medicine and industry (Ansari and Husain 2012). Immobilization offers the potential to enhance enzyme stability against variations in temperature, solvents, pH levels, pollutants, and impurities (Cherednikova et al. 1980; Holyavka et al. 2019).
However, in the present day, enabling enzyme reusability and stability is not the sole objective of immobilization; furthermore, it has been acknowledged as potent tool for enhancing various enzyme abilities. Although immobilization can improve biocatalyst performance and characteristics, it may also induce structural changes that can influence catalytic activity (Cipolatti et al. 2016; Holyavka et al. 2017). Consequently, ongoing research is necessary to explore methods for controlling the functional, kinetic, physical, structural, and chemical characteristics of biocatalysts, as well as techniques for their stabilization (Ó’Fágáin 2003; Shalova et al. 2007; Artyukhov et al. 2010). In some cases, immobilization can be associated with enzyme purification, which helps offset the costs related to the process (Barbosa et al. 2015).
In recent years, a technique called “nanoimmobilization” has gained popularity. Immobilizing enzymes on nanoparticles (NPs) offers several advantages compared to native enzymes, including broader pH and temperature usability, enhanced thermal stability, and potential changes in selectivity and specificity (Ansari and Husain 2012; Verma et al. 2016). As a result, nano- and microparticles have been extensively studied for enzyme immobilization (Tang et al. 2007; Sahoo et al. 2011; Ghosh et al. 2012). The increasing number of publications in this field demonstrates the growing interest in utilizing nano- and microscale particles for enzyme immobilization, primarily due to their inherent characteristics.
The progress in nanotechnology has resulted in the creation of diverse methods for immobilizing enzymes onto nanoscale materials, including nanoparticles, nanotubes, mesoporous materials, and nanofibrous membranes (Takimoto et al. 2008; Zhu and Sun 2012; Zhao et al. 2013). Consequently, much discussion revolves around the use of nanomaterials and their advantages in interacting with biocatalysts. The benefits of nano- and microimmobilization include reduced mass transfer resistance, efficient enzyme loading, high surface area, and minimized diffusional problems. However, it is important to consider the disadvantages, such as the cost of the fabrication process, high expenses associated with large-scale applications, and challenges related to the separation of the reaction medium (Cipolatti et al. 2014a). Careful consideration must be given to the selection of nanosupports, as some supports may be difficult to handle but prevent diffusion problems (Tan et al. 2023). Furthermore, in cases where the support lacks porosity, the enzyme becomes exposed to the surrounding medium (Zhao et al. 2006). Several studies have examined nanomaterials as platforms for immobilizing enzymes and biomolecules, where their pros and cons have been extensively discussed (Ahmad and Sardar 2015; Leitgeb et al. 2016; Stine 2017; Fathi et al. 2019; An et al. 2020).
In the field of medicine, particularly nanomedicine, micro- and nanocatalysts have found wide-ranging applications. Nanomedicine employs nanotechnology to treat, diagnose, monitor, and control biological systems. The core focus of nanotechnology is the creation of nano-objects, like nanoparticles (Williams and Adams 2007). Micro- and nanoparticles possess unique characteristics that make them highly promising for drug delivery purposes (Faraji and Wipf 2009). They have the ability to encapsulate or adsorb medical agents, providing protection against immediate enzymatic degradation within the biological environment. The properties of nanoparticles can be influenced by factors such as the preparation process, size, and pH of the medium (Mora-Huertas et al. 2010; Vauthier and Bouchemal 2009).
Significant advancements have been achieved in nanomedicine by targeting nanoparticles to specific organs, marking a major breakthrough in mentioned area of study (Nguyen et al. 2015; Uddin et al. 2016). Organs often exhibit considerable spatial heterogeneity in diseases, with healthy and pathological regions coexisting within the same organ (González-García et al. 2002; Ju et al. 2014). Recently, there has been a growing interest in the development of effective food-grade oral nano-delivery systems. These systems aim to encapsulate, protect, and deliver nutraceuticals, enhancing their bioavailability, preventing diseases, and promoting human health and well-being (Hu and Huang 2013).
Nanoparticles have emerged as viable alternatives to conventional antibacterial drugs. Synthetic polymer-based nano- and microparticles (MPs) are extensively used to develop innovative drug formulations, allowing for targeted delivery and controlled release of biologically active compounds over time (Sur et al. 2019). Moreover, the properties of nanoparticles are determined collectively by the ensemble of particles distributed in the dispersion medium, underscoring the critical role of the microenvironment in drug delivery properties (Egebro Birk et al. 2021). Nano- and microparticles exhibit high drug loadings due to their large interfacial area, enabling rational drug use while minimizing toxic effects. However, the elimination of drugs from the body becomes challenging for such pharmaceutical forms due to steric hindrance, which hampers the passage of polymer particles through kidney tubules (Zielinska et al. 2020). One potential solution to this problem involves designing micro- and nanoparticles using biodegradable carriers that can be metabolized and eliminated from the human body without resistance. Chitosan stands out as the most promising polymer in this regard.
Nano- and microscale materials seamlessly integrate into biomedical devices due to the inherent similarity in size with many biological systems. The materials commonly employed in the development of these nanotechnology products encompass inorganic and metal nanoparticles, carbon nanotubes, liposomes, metallic surfaces (Liu et al. 2016), as well as synthetic polymers, polymer nanofibers, graphene films, ZnO-based materials, protein-based nano-delivery systems, polysaccharide-based nanoparticles, and hybrid organic–inorganic nanoflowers. By employing physical or chemical methods and leveraging specific biological reactions like the ligand-receptor or antigen–antibody interactions, and DNA-DNA hybridization, it becomes feasible to interact biomolecules with NPs. The physics (McNamara et al. 2010; Yim et al. 2010), thermodynamics (Menzies and Jones 2010), surface chemistry (Castner and Ratner 2002; Moyano and Rotello 2011), and their toxicological effects collectively affect the potential applications of nanomaterials.
This paper is devoted to discussion of novel biocatalysts based on enzymes in complexes with nano- and micromaterials (Fig. 1).
Synthetic polymers
Polymeric supports, including poly(styrene), poly(acrylates), poly(methyl methacrylate) (PMMA), poly(acrylamide), and poly(urea-urethane), are widely used for enzyme immobilization. Additionally, inorganic supports like aluminum oxide, silica gel, apatite, and glass are commonly employed due to their high thermal and mechanical stabilities, low toxicity, and resistance to microbial and/or solvent attacks (Hartmann and Kostrov 2013; Magner 2013; Zhou and Hartmann 2013; Garcia-Galan et al. 2014).
In the literature, several techniques for immobilizing enzymes on polymeric supports have been documented (Besteti et al. 2014; Nicoletti et al. 2015). For instance, PMMA has been recognized as an effective support for immobilizing lipase B from Candida antarctica. The immobilized enzyme on PMMA exhibited a relative activity of 40% after 20 recycling cycles, whereas the free lipase displayed only 5% relative enzyme activity (Valerio et al. 2015). Another approach involved the synthesis of poly(urea-urethane) nanoparticles modified with poly(ethylene glycol) which was a new support for lipase B from Candida antarctica. The authors reported achieving a high esterification activity of 21 U mg−1. Furthermore, an improvement in the thermal stability of the immobilized enzyme was observed (Cipolatti et al. 2014b).
Polymer nanofibers hold significant prospects as enzyme carrier, in situ formation of composites reinforced with nanofiber, biocatalysis/separation applications, and biosensors. Nanofibers possess less sizes (indicating a larger specific surface area), higher porosity (diffusion resistance reduction), enhanced conductivity, and simple fabrication processes (Ghosh et al. 2012; Asgher et al. 2014) compared to conventional membranes.
An illustration of covalent immobilization involves lipase from Candida rugosa on nanofiber membranes of poly(vinyl alcohol-co-ethylene) activated with glutaraldehyde (PVA-co-PE). The study exhibited improved thermal and storage stability, and also pH tolerance, of the lipase immobilized on PVA-co-PE nanofibers (Zhu and Sun 2012). Likewise, the immobilization of L-asparaginase on polyaniline nanofibers led to enhanced enzyme activity and stability (Ghosh et al. 2012).
Carbon-based nanomaterials
Carbon-based nanomaterials, including graphene, carbon dots, and carbon nanotubes hold immense promise for enzyme immobilization due to their promising characteristics. The above-mentioned nanomaterials possess high conductivity and biocompatibility, a large surface area, rendering them highly suitable for sensitive and selective biomolecule detection. Furthermore, the surface properties of carbon-based nanomaterials can be easily tailored to create specific sorption sites for enzyme. Overall, carbon-based nanomaterials have emerged as a compelling platform for developing biosensors with enhanced accuracy, portability, and sensitivity, poised to revolutionize various fields such as food safety, medical diagnostics, and environmental monitoring (Wei et al. 2022; Anwar et al. 2023).
Fullerenes
Due to their low sizes, quantum confinement, and distinctive shape, fullerenes exhibit exceptional properties not present in macro-sized materials. Scientists have investigated their chemical and physical traits, morphology, and potential for functionalization. Fullerenes’ solubility in different media further enhances their application potential by facilitating the production of uniform sheets essential for coatings and electrodes (Paukov et al. 2023).
Graphene stands out as the most well-studied carbon nanoform, consisting of carbon atoms in sp2-hybridized state placed in 2D sheet arranged in a honeycomb lattice (Wick et al. 2014). It boasts a substantial area of the surface, robust mechanical properties, and remarkable physical parameters, such as optical, electrical, and thermal characteristics. Because of its exceptionally large surface area, graphene proves to be a highly effective substrate for enzyme immobilization. Additionally, its high conductivity facilitates a direct reactions of the enzyme with the electrodes (Palanisamy et al. 2017). Considering its exceptional attributes, graphene is regarded as an ideal carrier for enzyme immobilization, as the increased surface area enables a more robust wrapping of the enzyme, while its greater mechanical strength enhances enzyme recyclability.
The exceptional above-mentioned qualities of graphene have made it an outstanding choice for enzyme immobilization (Thakur et al. 2011). Nevertheless, the use of graphene as a support has brought about various concerns related to potential toxic effects, low solubility, clumping issues, and challenges in fabrication (Gokhale et al. 2023).
Graphene oxide (GO) and reduced graphene oxide are particularly noteworthy in enzyme immobilization (Hermanova et al. 2015; Shola et al. 2022). GO, with the same physical properties as for graphene, is considered an ideal carrier for immobilizing enzymes. Its high oxygen content and various functional groups, such as carboxyl, hydroxyl, and epoxy, make it a perfect material for enzyme immobilization. Notable enzymes immobilized on graphene oxide include lipase (Povedano et al. 2017; Fotiadou et al. 2019), acetylcholine esterase (Zhang et al. 2019), trametes versicolor laccase (Rouhani et al. 2020), glucose oxidase (Liu et al. 2019), lactate oxidase (Pakchin et al. 2018), and peroxidase (Rezaei et al. 2018).
Graphene oxide possesses many oxygen-containing substituents, like COOH, C–O–C, and OH-groups, which are promising for further modification. Its outstanding physical and chemical endurance makes it a perspective carrier (Obayomi et al. 2022). GO magnetic nanocomposites are slightly modified to maintain topological integrity and stability during enzyme interactions (Georgakilas et al. 2016; Thakur et al. 2011). However, reduced graphene oxide (rGO) offers limited control over its shape and dimension, has a less extensive surface, is more prone to having inclusions/residual groups, and may experience deterioration in concrete strength (Long 2023).
Graphene films, which represent the first material found in nature with a one-atom-thick structure, are obtained from graphite and consist of a single layer of C-atoms arranged in a honeycomb lattice, akin to unrolled single-wall carbon nanotubes or a colossal flat molecule of fullerenes (Novoselov et al. 2004, 2005; Mas-Balleste et al. 2011). Biomolecules can primarily interact with graphene-based nanomaterials through different types of weak physical interactions, such as van der Waals forces, electrostatic forces, and π-π stacking (Pavlidis et al. 2014). Several strategies for enzyme immobilization on modified graphene nanoparticles have been proposed in the literature, including the synthesis of graphene-by-graphene oxide, which yields nanomaterial enriched with various oxygen-containing groups (C = O, COOH, C–O–C, O–O, OH. etc.) (Vashist and Luong 2015).
In a research performed by Jiang et al. (2014), they investigated the trypsin immobilization on dendrimer-grafted graphene oxide nanosheets using glutaraldehyde as an activator for covalent binding. It was suggested that the resulting enzymatic formulation holds promise for effective proteome identification. Similarly, cellulase and other enzymes have also been successfully immobilized on graphene supports. Gokhale et al. (2013) developed a nano magneto-responsive carrier using graphene, achieved through a self-assembly of oppositely charged polyelectrolytes and maghemite-magnetite nanoparticles on 2D graphene. They observed that enzymes immobilized on nanographene structures exhibit variations in catalytic efficiency, operational stability, and potential applications when compared to traditional derivatives (Pavlidis et al. 2014).
Carbon nanotubes (CNTs) are tubular carbon allotrope forms with a graphite composition. These nanomaterials have garnered significant interest due to their structure, biocompatibility, and impressive physical properties, such as electrical, thermal, and mechanical, making them promising for enzyme immobilization (Gubaidullin et al. 2022; Zueva et al. 2023). CNTs offer an interesting support material for enzymes, as they disperse well in solution and allow broad functionalization (Tan et al. 2023). They produced as single-walled carbon nanotubes (SWCNTs), where one graphitic layer is wrapped, or multi-walled carbon nanotubes (MWCNTs), which make of numerous coaxial graphitic nanotubes (Iijima 1991). SWCNTs and MWCNTs are extensively researched for enzyme immobilization because of their distinct physical properties and high biocompatibility. (Ji et al. 2010; Tan et al. 2012). MWCNTs are widely used in biosensor production. These CNTs can be easily modified by the formation of COOH- or NH2- groups, or coupled with other structures such as polymers or metal NPs to tune desirable properties. (Zeng et al. 2016). The excellence of using CNTs is a relatively large area of surface, high stability, biocompatibility, and reusability (Liu et al. 2020). However, handling them can be challenging, and their economic feasibility may be a concern (Naha et al. 2023). Both MWCNTs and SWCNTs have served as matrices for immobilizing various enzymes, such as tyrosinase, choline oxidase, bilirubin oxidase, amylase, lysozyme, and glucose dehydrogenase (Yaari et al. 2020; Blazek and Gorski 2020; Gentil et al. 2017; Neupane et al. 2019; Boussema et al. 2018).
Carbon nanofibers
Both carbon nanofibers (CNFs) and polymeric nanofibers are frequently utilized as nanocarriers for enzyme immobilization. These nanofibers exhibit distinctive properties, including uniform dispersion formation and high enzyme recovery, giving them an advantage over other nanocarriers for nanoenzyme assembly. Moreover, their high interconnectivity and porosity result in minimal mass transfer limitations, further enhancing their suitability (Misson et al. 2015).
CNFs are distinct cylindrical nanostructures that come in various forms of graphene layers. Based on the form of these layers, CNFs can be divided into herringbone (cup-stacked), tubular, or platelet. They possess several advantages over carbon nanotubes, including cost-effectiveness, ease of mass production, a high ratio of surface-active groups, and a substantial functional surface area (Shanta et al. 2017). On the other hand, polymer nanofibers exhibit promising properties, such as a high ratio of surface area to bulk volume, perfect mechanical characteristics, and versatile surface modification ability (Li and Wan 2017).
Quantum dots are nanoscale semiconductor crystals with distinctive optical properties, composed not only of carbon (Alshammari et al. 2023; Pourmadadi et al. 2023) or graphene but also inorganic materials (Cui et al. 2018). Typically, quantum dots have a small size, are chemically inert, are amenable to various surface modifications, are cost-effective, and are biocompatible. Their key advantage lies in their photoluminescent properties (Wu et al. 2017). For instance, an electrochemical enzyme biosensor based on carbon quantum dots was developed for hydrogen peroxide measurement. This biosensor utilized immobilized on carbon dots horseradish peroxidase in a glassy carbon electrode (Su et al. 2018). Additionally, a sensor incorporating carbon dots modified magnetite particles was capable of detecting NADH in the 0.2–5 mM concentration range (Canevari et al. 2017). Despite high physical and chemical stabilities and easy detectability, carbon quantum dots have some limitations, including non-compatibility with certain catalysts, low recyclability, potential safety concerns, and reduced enzymatic stability (Khan et al. 2023). Nevertheless, enzymes immobilized on quantum dots have been employed for biosensing applications, such as using quantum dots CdS-chitosan-tyrosinase for detecting phenolic compounds (Han et al. 2015) and graphene quantum dots-laccase for detecting catecholamine neurotransmitters (Baluta et al. 2018).
Metal and metal oxide nanoparticles
Metal nanoparticles are frequently studied as carriers for enzyme immobilization due to their capacity for functionalization and their large area of surface (Somturk et al. 2015; Li et al. 2019a). In this context, Dedania et al. (2020) conducted a study using titanium dioxide nanoparticles to immobilize D-psicose 3-epimerase from Agrobacterium tumefaciens, with glutaraldehyde as the cross-linking agent. The immobilized D-psicose 3-epimerase on TiO2 exhibited a t1/2 of 180 min at 60 °C, surpassing the activity of the free enzyme (which had a t1/2 of 3.99 min at 50 °C). Under optimal conditions (pH 6.0, 60 °C, and presence of Mn2+), the immobilized enzyme demonstrated a significant fructose conversion of 36:64 at equilibrium. However, its activity diminished rapidly after reuse, retaining only 50% and 20% of its initial activity after 5 and 8 cycles, respectively.
Among all metallic nanoparticles, gold and silver NPs exhibit the most fascinating physical properties for application in biosensor creation (Mayer and Hafner 2011). These NPs offer numerous excellences such as easy production, high stability, and reproducibility (Iftikhar et al. 2019). Moreover, their size allows them to penetrate cell membranes, so they are suitable for applications in live organisms. As a result, Au and Ag NPs become the main components in biosensors for numerous biological or medical utilizing. The bioprocesses for creating AgNPs are cost-effective, eco-friendly, and innovative ways reducing synthetic complexity (Husain et al. 2023; Kilic et al. 2023).
Gold nanoparticles have attracted considerable attention in catalysis; however, their practicality as supports is often questioned due to economic concerns. Some instances of enzymes immobilized on gold nanoparticles include α-amylase (Homaei and Saberi 2015), peroxidase (Qiu et al. 2009), cellulase (Cheng and Chang 2013), and superoxide dismutase (Thandavan et al. 2013). The decision to use gold nanoparticles as carriers are based on the specific using, considering the high cost of gold as a metallic catalyst, which may make it impractical for producing compounds with low aggregate value. Examples of applications utilizing gold nanospheres, nanocages, and nanoshells can be found in the literature (Liao et al. 2006).
Gold nanoparticles possess distinctive characteristics when compared to other nanoparticles, as highlighted by Cipolatti et al. (2016):
-
i.
They offer easy utilization and recovery while providing a high area of surface.
-
ii.
Their porous structure provides good adsorption and allows for high enzyme binding.
-
iii.
The NP size can be adjusted in a wide range (from nm to μm), accommodating various enzyme molecule sizes and functions.
-
iv.
They exhibit perfect biocompatibility.
-
v.
Processed “green chemistry” conditions, gold nanoparticles have highly clean surfaces, eliminating potential interference effects on enzymes from side impurities.
Gold nanoparticles have garnered interest for various medical applications, such as radiotherapy (Hainfeld et al. 2004) and drug delivery (Joshi et al. 2006), due to their high area of surface and physical properties. In drug delivery, these nanoparticles can carry bio(macro)molecules, including nucleic acids proteins. For effective therapy, the high-throughput release of biological activity substances governed by internal factors like pH values or glutathione concentrations, or external factors like temperature, is crucial. Apart from their enhanced absorption and scattering capabilities (Hillyer and Albrecht 2001), easily synthesized gold nanoparticles exhibit relative inertia, high biocompatibility, and the ability to conjugate with various bio(macro)molecules (Stern et al. 2012). Their ample area of surface allows them immobilize biomolecules without compromising the bioactivity of the linked species (Joshi et al. 2006).
Moving on to ZnO-based materials, metal oxide NPs are the most spread inorganic nanomaterials, including ZrO2, TiO2, Fe2O3, Al2O3, CeO2, and MoO3 (Chavali and Nikolova 2019). Zinc oxide (ZnO) with diverse nanostructures fabricated using various techniques has found extensive use in enzyme immobilization. Among the methods employed, the wet chemical route is particularly popular for producing different ZnO nanostructures, including nanoparticles, nanorods, and nanosheets. These ZnO nanostructures have been proposed as platforms for immobilizing cholesterol oxidase through physical adsorption. Nano-ZnO possesses several advantageous traits, such as being non-toxic, biologically compatible, displaying good adsorption, high catalytic efficiency and electron transfer rate, and ease of production, making it a favorable material for immobilizing biomolecules (Zhu et al. 2007a, b; Hu et al. 2011; Guzik et al. 2014).
Magnetic micro- and nanoparticles
Magnetic micro- and NPs have gained significant attention, primarily due to their mechanical strength and the ease with which they can be recovered from the reaction medium (Netto et al. 2013). An insightful review by Netto et al. (2013) discusses the application of superparamagnetic NPs as promising carriers for enzyme immobilization. However, “naked” magnetic NPs do not effectively conjugate with proteins or enzymes, necessitating surface modification. The literature offers various methods for surface modification of magnetic nanoparticles. These include polymer coating, reaction with the glutaraldehyde or 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDAC) activators (Xie and Ma 2010), coupling with carbohydrates like agarose (Chen et al. 2014) or chitosan (Zang et al. 2014).
Several recent researches have explored the enzymes’ magnetic modification using various materials and methods. For example, Pospiskova and Safarik (2015) achieved low-temperature magnetic modification by utilizing magnetic nano- and micro-sized iron oxide particles obtained through a microwave-assisted synthesis. They successfully binded trypsin and lipase with magnetization. Chen et al. (2014) investigated the β-glucosidase immobilization on magnetic Fe3O4 NPs in the presence of agarose, resulting in increased enzyme thermostability. Soozanipour et al. (2015) presented modified magnetic nanoparticles produced by covalent xylanase immobilization through using silica-coated modified magnetite NPs activated by cyanuric chloride. Raita et al. (2015) focused on immobilizing Thermomyces lanuginosus lipase on magnetic nanoparticles. In addition to magnetic nanoparticles, nanoporous nanocrystalline surfaces of metal oxide has been proposed as novel carrier for enzyme immobilization (Ansari and Husain 2012).
Metallic nanoparticles possess higher magnetization in comparison to their oxidic counterparts. Nevertheless, their high reactivity and toxicity can restrict their utilization in fields such as biotechnology or biomedicine. To overcome these concerns, NPs are coated with silica or polymers with different structure (Soozanipour et al. 2015). However, magnetic polymer-coated NPs may become notably non-stable at elevated temperatures, as the polymer’s instability is further affected by the NPs’ catalytic characteristics (Lu et al. 2013).
Numerous polymers and polyelectrolytes have been investigated to stabilize magnetite NPs, both through in situ addition during NPs’ synthesis and as post-processing modification (Bolivar et al. 2009; Valls-Chivas et al. 2017). Biocompatible polymers commonly used for this purpose include chitosan (Kalkan et al. 2012), dextran (Peng et al. 2015), alginate (Castelló et al. 2015), PEG (Butterworth et al. 2001), polyvinyl alcohol (PVA) (Chastellain et al. 2004), and PEI (Virgen-Ortíz et al. 2017). Charged polymers have gained popularity for stabilizing magnetite particles because their resulting coating significantly enhances dispersion stability and allows the adsorption of oppositely charged species. Polyelectrolytes are a type of polymer that contains ionizable groups dissociating into charged polymeric backbones (macroions) and counterions in polar solvents (Barrat and Joanny 1995). These counterions condense on the polymer chain in solution (Manning 1969). The charge density of the polyelectrolyte depends on various factors, including intrinsic factors like the structure or type of ionizable group and extrinsic factors like ionic strength, the nature and concentration of the counterion, pH, or temperature (Drifford and Delsanti 2001; Visakh et al. 2014). Ionic strength is crucial in polyelectrolyte conformation. In the absence of or low ionic strength, the polyelectrolyte adopts a “stretched” conformation due to repulsion between the charged groups. In contrast, at high ionic strength, the polyelectrolyte adopts a “folded” conformation due to charge shielding, as explained by Kokufuta and Takahashi (1986).
Magnetic nanoparticles find application in facilitating the handling of certain biocatalysts that have physical properties unsuitable for industrial processes, such as crosslinking enzyme aggregates (CLEAs) (Garcia-Galan et al. 2014). Some researchers have suggested incorporating magnetic nanoparticles into CLEAs to enhance their handling. In certain cases, this approach has also led to improved enzyme properties through interactions between the nanoparticles and enzyme molecules (Talekar et al. 2012; Kopp et al. 2014; Khorshidi et al. 2016). This technology enables the production of a robust catalyst in a straightforward manner, with matrix-free immobilization, and even the possibility of utilizing semi-purified enzymes (Liu et al. 2015; Shaarani et al. 2016).
Despite widespread application of CLEAs in various enzymatic processes, they present challenges when it comes to biocatalyst recovery. In practical use, CLEAs tend to form stable suspensions that are difficultly separating from the reaction mixture through common ways such as filtration or centrifugation, leading to increased aggregate size (Kopp et al. 2014). Moreover, this can hinder internal mass transfer. Combining magnetic nanoparticles with CLEAs addresses these issues by facilitating separation and enabling the use of reduced-sized aggregates. Moreover, the high area of surface of NPs enhances enzyme recovery, thereby improving utilization parameters in continuous industrial processes (Zhang et al. 2015a).
In an effort to minimize the toxicity caused by various meds, especially cytostatic drugs, researchers have explored magnetic nanomaterials as their carriers (Xie et al. 2009; Wang et al. 2010). Magnetic NPs have been extensively studied as promising drug delivery vehicles for several years. However, their prolonged clearance time and tendency to accumulate in specific tissues, such as the liver and kidney, have led to some side effects, such as inflammatory (Wahajuddin 2012). Despite these challenges, scientists have been actively working on innovative approaches to functionalize nanoparticle structures, enhancing potential targeting and transport efficiency, biodegradability and biocompatibility, and magnetic properties. These enhancements could lead to a reduction in the quantity of required administration (Emerich et al. 2002). The method involves directly injecting drug-loaded magnetic nanoparticles to the affected site, where they can be kept by an external magnetic field and controllably release the drug (Amirfazli 2007; Stocke et al. 2015). This approach opens up exciting possibilities for the theragnostic utilization of nanocarriers in chronic lung diseases (da Silva et al. 2017).
From a thermodynamic point of view, the enzyme adsorption process onto charged solid or rigid surfaces is expected to lower the free energy of the system, driven by entropy as the quantity of opposite charge interaction increases (Netto et al. 2013). Consequently, this process may alter the enzyme conformational equilibrium towards more disordered, e.g., partially folded states, resulting in a greater number of attractive electrostatic interactions. The particle surface morphology also significantly influences enzyme conformational changes; a solid increased surface area has a more impact on the enzyme’s secondary structure, preserving its tertiary structure (Lundqvist et al. 2004; Vertegel et al. 2004). Conversely, the curvature of the nanoparticle’s surface plays a crucial role in the enzyme’s adaptability without compromising its conformational stability. A more curved surface, such as a smaller spherical geometry, allows the enzyme to better adjust to the surface. As the particle size rises, its curve reduces becoming flatter, favoring the enzyme adsorption in more extended conformation leading to a decrease in its stability (Valls-Chivas et al. 2017).
To mitigate the above-mentioned effects, the polymer use is a common approach to stabilize rigid NPs, ensuring greater flexibility of the particle-solution interfaces. The interaction between enzymes and the surface is influenced by these polymers, which are known to enhance enzyme stability. Both non-ionic polymers and ionic polymers, such as polyelectrolytes, have demonstrated this effect (Andersson and Hatti-Kaul 1999; Padilla-Martínez et al. 2015). Notably, PEI has been recognized as an effective membrane destabilizer for the different type of cells, facilitating permeabilization of cells (Andersson and Hatti-Kaul 1999; Godbey et al. 1999). Additionally, it suppresses the multimeric enzyme dissociation, thereby enhancing protein stability (Virgen-Ortíz et al. 2017). Many studies have utilized PEI coating to provide the enzyme adsorption. For example, glucose oxidase, catalyzing the glucose oxidation to gluconolactone, was binding with PEI to enhance the thermal stability of the enzyme, increasing it from 50 °C for the free enzyme to 70 °C (Padilla-Martínez et al. 2015). In contrast, non-ionic polymers, like PEG, have shown to improve storage stability in the temperature range of 4–30 °C (Costa et al. 2002).
Singh et al. (2018) explored the xylanase immobilization on both “naked” and silica-coated magnetite NPs. Their study underlined the surface coating importance with inert silica, as the coated NPs possessed higher resistance to destruction. Consequently, the immobilized xylanase exhibited superior hydrolytic activity across a wide range of pH and temperature values and compared to the one immobilized on the “naked” magnetic NPs. Similarly, Nematian et al. (2020) conducted a study of immobilization of lipase using GO-coated and “naked” nanoparticles. The enzyme recovery of the coated NPs was nearly three times higher, with 24 wt.% versus 70 wt.% of the uncoated ones. Moreover, based on the determined kinetic parameters (Kcat/Km), the authors summarized that the hydrolytic activity of lipase enhanced when it was coupled with the GO-coated surface.
Metal–organic frameworks
Metal–organic frameworks (MOFs) are a class of porous or crystalline hybrid materials that feature a regular arrangement of positively charged metal ions surrounded by organic “linker” molecules. These metal ions serve as nodes, connecting the arms of the linkers to form a repeating cage-like structure (Sun et al. 2014). MOFs are characterized by their extremely high porosity and large area of interior surfaces (Lian et al. 2011; Wu et al. 2015). The combination of organic materials and mesoporous are promising in capture and encapsulation of enzymes (Liu et al. 2017a; Du et al. 2022; Hu et al. 2022; Wang et al. 2022), enabling a diverse range of downstream applications through co-immobilization at the surface interface and enhancing enzyme performance through structural arrangements (Ren et al. 2020; Feng et al. 2021, 2022; Zhong et al. 2021).
Magnetic metal–organic frameworks are unique porous materials because of their huge pore volumes, greater surface areas, crystalline structure, and the ability to modify pore diameters through well-defined active sites, as well as their capacity to hold organic ligands (Jiang and Xu 2011; Doherty et al. 2014; Bilal et al. 2023). The characteristics and topology of MOFs can be adjusted to incorporate different metal atoms and/or ligands, allowing for the formation of various bonds between MOF carriers and enzymes, including hydrogen and covalent bonding, van der Waals forces, and coordinative interactions (Majewski et al. 2017). With their large pore volume and modifiability, MOFs have recently appeared as exceptional carriers for immobilizing enzymes (Li et al. 2023), because of their exposed surfaces (Morris et al. 2014; Mehta et al. 2016; Doonan et al. 2017; Lian et al. 2017).
The encapsulation of proteins into MOFs was pioneered by the Lyu group (Lyu et al. 2014; Liu et al. 2021), and Wang et al. identified ZIF-8 as the ideal choice for immobilization and encapsulation (Wang et al. 2019). MOFs exhibit significant potential for immobilizing diverse enzymes for specialized applications (Huang et al. 2020; Liang and Liang 2020; Issaka et al. 2023). Enriched with metal sites, MOFs function as enzyme activators, thereby enhancing the biocatalytic performance of enzymes (Issaka et al. 2022). The integration of multienzymes into MOFs for cascade catalysis further boosts catalytic activity, minimizing the impact of mass transfer resistance (Xu et al. 2021). These distinctive advantages empower MOFs to immobilize enzymes and nanoenzymes, enhancing the catalytic efficiency of enzymatic reactions (Hu et al. 2020; Zhang et al. 2022).
Gao et al. (2021) utilized solvothermal synthesis to grow Fe-MOF around Fe3O4 nanoparticles, resulting in Fe3O4/Fe-MOF with a core–shell structure and an enlarged pore diameter. This unique combination of magnetic properties from Fe3O4 and the ranked porous structure was ideal for enzyme immobilization, such as chloroperoxidase (CPO) or horseradish peroxidase (HRP). The immobilized enzymes demonstrated remarkable enhancement of temperature stability and reusability. For example, HRP-Fe3O4/Fe-MOF retained an impressive 94.1% of its catalytical capacity even after 10 repeated uses. Moreover, CPO/HRP-Fe3O4/Fe-MOFs demonstrate highly effectiveness in rapidly (about 15 min) decomposition organic wastewater pollutants like isoproturon or 2,4-dichlorophenol.
Xu et al. (2022) obtained Fe3O4-COOH@UiO-66-NH2 composite with core–shell structure for porcine pancreatic lipase (PPL) immobilization. The composite possessed several advantages, including a high specific surface area, a high concentration of active sites, and superparamagnetic characteristics (Ke et al. 2016). Under optimal conditions, the PPL recovery reached 247.8 mg g−1, and the probable catalytical capacity was 101.5%. The immobilized enzyme also demonstrated increased stability. Remarkably, a practical approach was developed utilizing Fe3O4-COOH@UiO-66-NH2@PPL and UPLC-Q-TOF–MS/MS to identify bioactive compounds found in natural raw materials.
Motamedi et al. (2021) enhanced the catalytical ability of the urate oxidase (Uox) enzyme from Aspergillus flavus by its immobilizing on the magnetic MOF (Nim-MOF) nanomaterial surface. The obtained Uox@Nim-MOF formulation retained around 60% of its initial function keeping it up to 70 °C; the soluble enzyme lost nearly 100% of catalytical capacity at 40–45 °C. A stabilization process led to a determination of the activation energy (Ea) of urate oxidase equaled 58.81 kJ mol−1, which is approximately half of the one for free enzyme.
Wei et al. (2021) devised a method to create magnetic reusable MOFs for capturing His-tagged enzymes from cell lysates without further purification. The immobilized β-glucuronidase, compared to its native form, exhibited enhanced stability at varying temperatures and pH levels. The immobilized β-glucuronidase retained 80% of its initial relative activity even after 8 cycles. The MOFs’ capability for immobilization increased to over 90% after only three use cycles. This magnetic targeted MOF system proved to be an effective and sustainable approach with promising applications in catalysis and industry.
In their research, Sargazi et al. (2018) successfully synthesized Ta-MOF@Fe3O4 core–shell nanostructures under optimized conditions using a rapid and effective method that employed ultrasonic assistance for the reverse micelle reaction. They immobilized Km12 lipase from Bacillus licheniformis on the Ta-MOF@ Fe3O4 substrate, which provided an excellent solid platform due to its large area of surface, unsaturated metal centers, and free COOH-groups. The immobilized lipase demonstrated significantly improved stability and prolonged activity in reaction with high substrate concentrations. Ta-MOF@Fe3O4-lipase holds great potential for biotechnological applications, such as biotransformation and biodiesel production.
In a recent breakthrough, Ji et al. (2021) achieved the efficient encapsulation of lipase (CRL) and Fe3O4 NPs within ZIF-8 using a mild and rapid one-pot approach, leading to CRL/MNP@ZIF-8 with an impressive enzyme recovery of 88.4%. Notably, this was the first instance of both MNPs and lipase being encapsulated in ZIF-8 using a co-precipitation method. On a similar note, Lin et al. (2019) successfully developed a ranked magnetic porous MOF with the core–shell structure and utilized it for amidase immobilization. The material obtained parameters such as strong magnetic response, and the type of structure and high porosity contributed to the immobilized enzyme’s outstanding catalytic capacity and enhanced thermal stability and resistance to organic solvents. Remarkably, even after 15 reuse cycles, the immobilized lipase retained a remarkable 98.2% of its catalytical capacity during (S)-4-fluorophenylglycine preparation.
Zhong et al. (2020) accomplished the successful synthesis of a magnetic zeolite imidazolate framework-8 (iron oxide@ZIF-8@Trypsin) using a one-pot synthesis method. Through reaction system pH adjustment, they facilitated the growth of ZIF-8 around citric acid-modified iron oxide, effectively immobilizing trypsin. The resulting iron oxide@ZIF-8@Trypsin exhibited a remarkable 2.6 times higher activity than free enzyme, thanks to its high enzyme recovery and expansive area of surface. Moreover, its low magnetic response (43 emu g−1) made it convenient for easy use and recycling. The promising results for application in proteomic analysis was demonstrated using Fe oxide@ZIF-8@Trypsin as a micro-reactor for efficient protein digestion in complex samples of human blood serum.
Li et al. (2022) successfully manufactured MOF-based mimic enzymes through a straightforward and efficient process. They utilized NH2-MIL-88B(Fe)@MRGO to biomimetically detect glucose.
Amari et al. (2021) accomplished the immobilization of laccase on Fe3O4-NH2@MIL-101(Cr). Remarkably, the Fe3O4-NH2@MIL-101(Cr) laccase exhibited exceptional stability, retaining its immobilization capacity and regenerating a remarkable quantity of lost activity even under extreme temperatures and varying pH levels. The immobilized system displayed remarkable durability, with stored for 28 days laccase remaining 88% of its initial activity and maintaining a residual activity of 49% at 85 °C. Additionally, the immobilized laccase demonstrated its efficiency in biodegrading RB and AR dyes in water.
In a similar vein, Wan et al. (2021) achieved the in situ α-glucosidase immobilization through association with Fe3O4@CS@ZIF-8. The immobilized enzyme showcased improved stability at various pH levels and temperatures, as well as reusability, with 74% of the overall enzyme catalytical capacity remaining after ten cycles. Notably, the IC50 for acarbose was measured at 0.57 M, and the Michaelis–Menten constant for the immobilized α-glucosidase was 0.47 mM.
Nano-sized mesoporous hydrogen-bonded organic frameworks
Porous organic framework (POF) materials, including metal–organic frameworks (MOFs), covalent organic frameworks (COFs), and hydrogen-bonded organic frameworks (HOFs), have demonstrated some advantages as carriers for enzyme immobilization (Huang et al. 2017, 2020; Liao et al. 2017; Sheldon et al. 2021; Wu et al. 2022). These POF materials’ robust framework provides excellent protection to enzymes under environmental conditions, ensuring their structural integrity (Chen et al. 2020; Tang et al. 2021a, 2021b; Huang et al. 2022). Additionally, the ultrahigh porosity and tunable apertures of POF materials provide efficient substrate and product transport (Li et al. 2013).
Hydrogen-bonded organic frameworks (HOFs) represent a typical POF material, assembled through hydrogen-bonding interactions between molecular building blocks (Hisaki et al. 2019; Wang et al. 2020; Yang et al. 2020; Das et al. 2022). HOFs offer additional merits, such as mild synthesis or processing conditions and good enzyme compatibility, making them a promising emerging support for enzyme immobilization (Morshedi et al. 2017; White 2021; Chen et al. 2022). Notably, certain HOFs constructed with amidinium/carboxylate dimers and carboxy dimers, such as BioHOF-1, TA-HOF, and PFC-1, have been reported for enzyme immobilization, displaying excellent stabilities and superior recyclability (Yin et al. 2018; Liang et al. 2019; Tang et al. 2021a, 2021b; Wied et al. 2022). However, some of these HOFs have pore sizes below 2 nm and particle sizes over 1 µm, leading to enzyme@HOFs retaining only 20–70% of catalytic activity of free enzyme.
Drawing upon the intrinsic ultrahigh porosity of HOF materials, it is postulated that optimizing the transfer distance and enlarging the transfer channels within HOFs could effectively mitigate diffusion limitations, thereby enhancing the apparent enzymatic activity following the diffusion flux equation (Yu et al. 2023). Considering the volume of enzymes, often larger than 6 nm, and especially when dealing with nicotinamide cofactors (NADH/NAD+), which have dynamic molecular diameters of around 1.7 nm as substrates, the ideal immobilized enzyme carrier is hypothesized to possess pore channels ranging from 2 to 6 nm and particle sizes less than 100 nm (Li et al. 2016; Wang and Liao 2021).
In a recent study, a nano-sized mesoporous HOF (nmHOF) for the in situ immobilization of enzymes was successfully synthesized. The building blocks used were tetrakis(4-aminophenyl) methane (TAM) and 1,3,6,8-tetrakis(p-benzoic acid) pyrene (H4TBAPy). The incorporation of H4TBAPy, with its large π-conjugated system, extended the length of the building block and prevented structure interpenetration. During the nucleation process, the –COOH/–NH2 residues in enzymes played a crucial role, triggering the rapid formation of TaTb nmHOF. The resulting enzyme@TaTb possessed a pore size of approximately 2.4 nm and a particle size ranging from 19.9 ± 6.4 nm to 56.4 ± 20.1 nm. For instance, lactate dehydrogenase (LDH), a typical NADH-dependent enzyme, was in situ immobilized in TaTb to create LDH@TaTb, which exhibited enzymatic activity nearly approaching that of the free LDH. Moreover, TaTb nmHOF proved effective in immobilizing α-amylase (α-Amy) and horseradish peroxidase (HRP), both of which displayed significantly enhanced activities compared to counterparts in ZIF-8 and BioHOF-1 (Li et al. 2023).
Protein-based nano-delivery systems
Nanoparticulate delivery systems composed of proteins (such as gelatin, albumin, and gliadin) offer low antigenicity, biodegradability, and biocompatibility. Moreover, the protein primary structure of these nanoparticles and their numerous available functional groups allow for the covalent attachment of targeting ligands, shielding substances, or drugs to the surface of the nanoparticles (Diab et al. 2012).
Food proteins exhibit remarkable functional properties, such as emulsification, gelation, foaming, and water-binding capacity (Chen et al. 2006; Elzoghby et al. 2011, 2012), making them highly attractive for the development of protein nanocarriers for drug or nutraceutical delivery (Shikama et al. 2010). Their advantages include biodegradability, non-antigenicity, high nutritional value, abundance from renewable sources, and excellent binding capabilities with various drugs or nutraceuticals (Fändrich et al. 2001). Moreover, protein nanoparticles are easily prepared and scalable for manufacturing (Elzoghby et al. 2011, 2012). Being metabolizable naturally occurring components, protein nanoparticles undergo hydrolysis by digestive enzymes, producing bioactive peptides with potential physiological effects in vivo (Chen et al. 2006).
Lipid-based nano- and micromaterials
Liposomes are versatile vesicular carriers, varying in size from some micrometers to tens of nanometers. Comprising one or more phospholipid bilayers, liposomes provide a suitable environment for the encapsulation of both lipophilic and hydrophilic drugs. Extensive testing in animal studies and human volunteers has confirmed their tolerability and safety, with no adverse effects reported. Liposomes are known for their outstanding biocompatibility, low toxicity, and high safety profile (Jesorka and Orwar 2008).
Apart from their role as drug delivery vehicles, liposomes can be utilized as biomimetic models to investigate the interaction between membranes and hydrophobic drugs and proteins. Specifically, proteoliposomes are artificial systems that imitate lipid membranes (liposomes) by incorporating or inserting proteins. Over the past decade, these systems have gained prominence in biophysical studies on lipid-protein interactions and biotechnological applications. Proteoliposomes offer an advantage over natural membrane systems as they can be designed with a smaller number of lipidic and protein components, simplifying experiment design and interpretation (Ciancaglini et al. 2012; Ramos et al. 2017).
Solid lipid nanoparticles have emerged as promising nanocarriers, offering several advantages over polymeric nanoparticles and liposomes. These benefits include enhanced drug stability and drug loading capacity and ease of scaling up and sterilization. Typically ranging from 50 to 1000 nm in size, solid lipid nanoparticles consist of lipids that remain in a solid state at both body and room temperatures, and they are dispersed in aqueous media with or without surfactant (Müller et al. 2000). These nanoparticles use physiologically tolerated lipid components, such as triglycerides, partial glycerides, fatty acids, steroids, and waxes, which contribute to reduced potential for acute and chronic toxicity (Souto and Müller 2007).
Polysaccharide-based nanoparticles
Over the past five decades, there has been a growing trend in utilizing colloidal carriers based on naturally origin polysaccharides like alginate, carrageenans, chitosan, cellulose, pectin, lignin and starch (Chen and Schopfer 1999; Makshakova et al. 2022; Makshakova and Zuev 2022; Makarova et al. 2022, 2023; Makshakova et al. 2023; Faizullin et al. 2023). This growing popularity can be attributed to their cost-effectiveness, biocompatibility, and low toxicity (Laurienzo 2010). These polymers also exhibit mucoadhesive properties, allowing them to persist at the drug absorption site, making them appealing matrices for oral nanoparticles. Among these biopolymers, chitosan has garnered significant attention due to its absorption-enhancing effect and biodegradability, making it a promising matrix for tuning of the drug or biomacromolecule intestinal transport. The mucoadhesion provides an extended contact period of the drugs and absorption sites, while also temporarily opening tight contact in the mucosal epithelium to promote paracellular transport. These behaviors are attributed to the electrostatic attraction of positively charged chitosan and negatively charged mucosal epithelium (Illum et al. 1994).
Chitosan is an appealing choice for enzyme immobilization due to its surface containing functional groups, such as NH2 and OH groups. It exhibits favorable parameters such as biodegradability, biocompatibility, and cost-effectiveness, as it is the second most abundant biopolymer after cellulose (Juang et al. 2001; Klein et al. 2012; Chen et al. 2014). Enzyme immobilization chitosan particles can be obtained in wide size range from macro- to nanoscales using different technique such as ionic gelation, precipitation, and emulsion cross-linking (Tang et al. 2007; Biro et al. 2008).
Chitosan possesses distinct characteristics such as high viscosity, polyelectrolyte nature, film-forming ability, metal chelation, and unique optical and structural characteristics. Its versatility makes it a popular choice as a support for enzyme immobilization, offering a wide range of geometric configurations including nanofibers, nanoparticles, hydrogels, powders, flakes, and membranes (Zhu and Sun 2012; Shukla et al. 2013).
Besides its excellent absorption parameters, the convenience of preparing nanoparticles in aqueous media has made chitosan the preferred carbohydrate for delivering hydrophilic macromolecules, resulting in a growing number of research studies on chitosan-based oral nanoparticles. Most chitosan nanoparticle preparation methods involve dissolving chitosan in acidic aqueous solution with pH < 6.5. One advantage of this technique is avoiding the use of organic solvents that might affect the encapsulated drug. Additionally, the water solubility of chitosan facilitates the strong incorporation of polar hydrophilic drugs, dissolving in the aqueous phase and interacting with chitosan through electrostatic attraction or hydrogen bonding. This is particularly beneficial, as such meds often poorly interact with frequently used hydrophobic polymers like poly(caprolactone) and polylactic acid due to their low affinity. Furthermore, chitosan has a wide range of molecular weights and deacetylation degrees, allowing to design NPs with adjustable size and surface charge, which can influence their pathway through the gastrointestinal barrier and the encapsulated drug release (Diab et al. 2012).
Contribution of the Department of Biophysics and Biotechnology of Voronezh State University to the development of novel biocatalysts based on cysteine proteases in complexes with nano- and micromaterials
Cysteine proteases constitute a family of hydrolytic enzymes responsible for catalyzing the hydrolysis reactions of proteins and peptides. They are widely found in both animal and plant organisms. Proteolytic enzymes, including cysteine proteases, find extensive applications in various industries, such as food, pharmaceuticals, and cosmetics. Ficin, papain, and bromelain are among the plant proteases that have demonstrated significant potential (Bekhit et al. 2014).
Proteolytic enzymes are known for their biological antiseptic properties, possessing important characteristics like antitoxic and anti-inflammatory effects. They can hydrolyze toxic microbial protein metabolites present in infected wounds, thereby promoting accelerated epithelialization and faster formation of granulation tissue. The high specificity of enzyme catalysis contributes to impressive product yields and nearly waste-free production processes (Gonzalez-Rabade et al. 2011).
In our study, we focused on ficin (EC 3.4.22.3), papain (EC 3.4.22.2), and bromelain (EC 3.4.22.32) obtained from Sigma. Azocasein from Sigma served as the substrate for hydrolysis. We utilized micro (Mps)- and nanoparticles (Nps) of medium (MMCh, 200 kDa)- and high (HMCh, 350 kDa)-molecular-weight chitosans as carriers for immobilization. These micro- and nanoparticles of chitosans were produced with and without ascorbic acid (AsA).
Production of chitosan particles involved dissolving 300 mg of chitosan in 100 ml of a 0.3% acetic acid solution under mechanical stirring. Afterwards, a 3% NaOH solution was added at a rate of 5 ml/min while stirring continuously until a white precipitate formed and the pH exceeded 11. The solution was then filtered through a 0.45-μm filter, and the filtrate was washed with distilled water until reaching a neutral pH. For microparticles, ultrasound treatment was applied using a Qsonica Sonicators disintegrator (Japan) for 5 min at 20 kHz, while for nanoparticles, it was done for 10 min at 40 kHz. When chitosan micro- and nanoparticles were synthesized in the presence of ascorbic acid, 50 mg of ascorbic acid was added after chitosan dissolution.
The size of the micro- and nanoparticles was measured using a Nano Zetasizer ZS instrument (Malvern-Panalytical, UK). The size and zeta potential of the micro- and nanoparticles of medium- and high-molecular-weight chitosans prepared both without and with ascorbic acid are presented in Table 1.
To produce cysteine proteases complexes with chitosan micro- and nanoparticles, an enzyme solution with a concentration of 2 mg mL−1 was mixed in equal volumes with the dispersion of chitosan micro- and nanoparticles and kept at room temperature for 2 h. The residual activity of the enzyme complexes with the micro- and nanoparticles was evaluated after incubating them in a 0.05 M tris–HCl buffer, pH 7.5, at 37 °C for 7 days. The catalytic activity was measured at specific time intervals from 1 to 168 h by quantifying the amount of a colored reaction product resulting from the cleavage of the azocasein substrate.
The proteolytic activity of the enzymes was evaluated as described by Malykhina et al. (2022). The unit of catalytic activity was defined as the amount of enzyme that hydrolyzed 1 μM of azocasein per minute under the experimental conditions. The efficiency of enzyme immobilization was expressed as the percentage of preserved proteolytic activity of the enzyme after immobilization, relative to the catalytic activity of the enzyme in the solution (considered as 100%).
All experimental studies were performed with 8 replicates. The statistical significance of the differences between the control and experimental indicators was determined using the Student’s t-test, considering a significance level of p < 0.05, as all indicators exhibited a normal distribution.
Catalytical activity assay
When ficin formed complexes with MMChMp and HMChMp, the biocatalyst activity was approximately 75–80% compared to the free enzyme. However, when ficin complexes were formed with MMChAsMp and HMChAsMp, the activity increased by about 5%. For ficin complexes with MMChNp and HMChNp, the activity of the biocatalysts was over 80% compared to the free enzyme. Additionally, when ficin complexes were formed with MMChAsNp and HMChAsNp, the catalytic ability increased by 15–18% (Fig. 2a).
Catalytic (proteolytic) activity (left) and residual catalytic activity (center and right) of ficin (a, d, g), papain (b, e, h), and bromelain (c, f, i): free (1); in a complex with MMChMp (2); MMChAsMp (3); HMChMp (4); HMChAsMp (5); MMChNp (6); MMChAsNp (7); HMChNp (8); HMChAsNp (9). The activity of free enzyme under optimum hydrolysis conditions (left) and activity of the samples without their pre-incubation and under optimal hydrolysis conditions (center and right) were taken as 100% (Olshannikova et al. 2022; Redko et al. 2022; Goncharova et al. 2023)
In the case of papain complexes, when MMChMp and HMChMp were used as carrier, the biocatalyst activity ranged between 92 and 99% compared to the free enzyme. After interacting with MMChAsMp, the activity of papain increased by 18%, and by 10% after forming complexes HMChAsMp. When papain complexes were formed with MMChNp and HMChNp, the activity of the biocatalysts was approximately 95% compared to the free enzyme. The activity of papain remained almost unchanged after interacting with MMChAsNp and increased by 16% after forming complexes with HMChAsNp (Fig. 2b).
It was observed that when forming bromelain complexes with MMChMp and HMChMp, the biocatalyst activity was 94% and 76%, respectively, compared to the free enzyme. After interacting with MMChAsMp, the activity of bromelain increased by 11%. However, the activity remained at the initial level after forming complexes with HMChAsMp or MMChNp. When bromelain complexes were created with HMChNp, the activity of the biocatalysts was 83% compared to the free enzyme. Additionally, the activity of bromelain increased by 20% after interacting with MMChAsNp and by 8% with HMChAsNp (Fig. 2c).
Stability of cysteine proteases
In this study, the stability of cysteine proteases in complexes with chitosan micro- and nanoparticles was investigated. The experiments were conducted in a 0.05 M tris–HCl buffer at pH 7.5 and a temperature of 37 °C. The results indicated that all samples, including free enzymes and complexes with MMChMp, HMChMp, MMChAsMp, HMChAsMp, MMChNp, HMChNp, MMChAsNp, and HMChAsNp, showed a decline in activity over a seven-day period.
After 168 h of incubation, ficin in the solution retained only approximately 8% of its catalytic activity. Ficin complexes with MMChMp, HMChMp, MMChNp, and HMChNp exhibited 21, 9, 27, and 16% of their catalytic ability, respectively (Fig. 2d). Notably, complexes with MMChAsNp and HMChAsNp retained 39% and 18% of their proteolytic activity, respectively. Starting from 4 h of incubation, complexes of ficin with all carriers showed higher stability compared to the free enzyme (Fig. 2g).
Soluble papain retained 15% of its catalytic activity after 168 h of incubation. Papain complexes with MMChMp and HMChMp exhibited approximately 30% of their catalytic ability. The formation of papain complexes with MMChMp and HMChMp, both with and without ascorbic acid, increased the stability of the enzyme starting from 120 and 96 h, respectively (Fig. 2e). After 168 h of incubation, bromelain in the solution retained only 13% of its catalytic activity. Bromelain complexes with MMChMp and HMChMp exhibited approximately 35–40% of their catalytic ability. The complexes of bromelain with MMChMp, prepared both with and without ascorbic acid, were more stable than the free enzyme starting from 24 h of incubation. Similarly, the complexes with HMChMp showed increased stability starting from 8 h of incubation (Fig. 2f).
The stability of cysteine proteases was also evaluated when forming complexes with MMChNp and HMChNp. After 168 h of incubation, papain in complexes with MMChNp and HMChNp exhibited 29% and 34% of their catalytic ability, respectively; bromelain complexes with MMChNp and HMChNp retained approximately 30–35% of their catalytic ability. The formation of bromelain complexes with MMChNp and HMChNp, prepared both with and without ascorbic acid, increased the stability of the samples after 4 and 8 h of incubation, respectively (Fig. 2h and i).
In summary, the formation of complexes between cysteine proteases (ficin, papain, bromelain) and chitosan micro- and nanoparticles resulted in increased enzyme activity in certain cases. For example, ficin complexes with HMChaAsNp showed an 18% increase in activity. A similar result was observed for the papain complex with MMChaAsMp. Additionally, the binding of bromelain to MMChaAsNp showed a 20% increase in enzyme activity. Moreover, the stability of all three cysteine proteases was enhanced after complexation with micro- and nanoparticles.
Thus, immobilization on micro- and nanoparticles of chitosan increases the efficiency of biocatalysts based on cysteine proteases and their prospects for industry, increasing catalytic activity up to 20% and stability, starting from 8 h of incubation in a buffer solution at 37 °C.
Hybrid organic–inorganic nanoflowers
In recent times, there has been a surge in interest surrounding a new class of organic–inorganic hybrid nanoflowers, thanks to their distinctive properties, which position them as a promising and innovative technique for enzyme immobilization (Wu et al. 2016; Escobar et al. 2017). These nanoflowers consist of nanostructures that combine both organic and inorganic materials, with the inorganic component often being metal ions like Cu2+, Mn2+, or Ca2+, and the organic component comprising DNA and proteins. Although flower-shaped inorganic compounds have long been utilized in catalysis and analysis, the organic–inorganic nanoflowers signify a comparatively recent innovation (Zeng and Xia 2012).
These hybrid nanoflowers offer several advantages over conventional immobilization methods. Their synthesis is straightforward, and they have a larger surface area compared to spherical nanoparticles. Furthermore, they exhibit higher stability and catalytic activity when compared to both free enzymes and immobilized enzymes. This is attributed to their low mass transfer resistance and the cooperative interactions between the enzyme and the nanoflowers (Ge et al. 2012).
Drawing inspiration from this innovative technique, many researchers have tried to synthesize protein-inorganic hybrid nanoflowers applying various metal ions such as copper(II), as well as other metal ions like Zn2+, Ca2+, Ni2+, Co2+, and Mn2+, among others (Patel et al. 2018). Additionally, these nanoflowers have been combined with a wide variety of different enzymes, opening up new possibilities for enzyme immobilization and biocatalytic applications (Batule et al. 2015).
The enzymes commonly used in engineering organic–inorganic hybrid nanoflowers include trypsin, laccase, NADH oxidase, glucoamylase (Nadar et al. 2016), urease, lactoperoxidase, isomerase, and glucose oxidase (Bilal et al. 2019). In recent years, there have been reports on the fabrication and characterization of multi-enzyme-integrated flower-shaped hybrid nanomaterials (Rai et al. 2018). These hybrid nanocomposites, with their diverse functionalities stemming from both the enzyme/protein and inorganic components, hold tremendous potential for applications in various fields such as industrial biocatalysis, environmental management, biofuel production, biofuel cells, biosensors, and bio-related analytical devices (Altinkaynak et al. 2016, 2018; Celik et al. 2018; Jiang et al. 2018; Zhu et al. 2018, Fu et al. 2019).
Numerous reviews have delved into the captivating realm of organic–inorganic hybrid nanoflowers, examining various aspects of their development and applications. For instance, Cui and Jia (2017) and Cui et al. (2017) delved into functional biomolecule-inorganic hybrid nanoflowers, exploring their synthesis factors and utilizing in drug delivery systems, biosensors or biocatalysis, and chemical analytical science. Lei et al. (2018) focused on immobilization of biomacromolecules using self-assembled organic–inorganic hybrid nanoflowers and metal–organic frameworks as novel immobilization supports. The numerous using of nanoflowers in nanotechnology for biomedical research and patents were discussed by Negahdary and Heli (2018) and Shende et al. (2018).
Copper-based hybrid nanoflowers
Initially proposed by Ge et al. (2010), copper-based hybrid nanoflowers were accidentally formed when CuSO4 was added to PBS with pH 7.4 containing BSA, resulting in flower-like microstructures in the precipitate after 3 days. Later, a modified approach replaced bovine serum albumin with Cu(II) ions and other proteins or enzymes, such as carbonic anhydrase, laccase, lactalbumin, and lipases. The formation of these nanoflowers is driven by the coordination between the proteins and Cu2+ ions. The interaction between N-atoms present in the protein’s amide bonds and specific amino acid residues, like histidine, can form complexes with Cu(II) (Rulı́šek and Vondrášek 1998). Remarkably, all the tested protein/enzyme-Cu3(PO4)2 hybrid nanoflowers exhibited significantly higher catalytic potential than their free enzymes, except for lipase, which is non-metal-contained and demonstrated similar activity to the free enzyme. Additionally, incorporating enzymes into the hybrid nanoflowers improved their storage stability and recycling ability. For example, laccase nanoflowers displayed much slower degradation in PBS with pH 7.4 and room temperature compared to free enzyme. The enhanced catalytic properties of the nanoflowers-incorporated enzymes can be attributed to the nanoflower large area of surface, which reduced mass transfer limitations. Moreover, the multiplicative effects of the enzyme molecules associated with the nanocomposite and the laccase-Cu2+ interfaces in the above-mentioned microenvironment likely contribute to the enhanced performance of laccase. Building on this pioneering research, many enzymes have been explored as organic components to create copper-based hybrid nanoflowers with enhanced catalytic properties and/or stabilities compared to native enzymes (Cui and Jia 2017).
In a successful synthesis conducted by Altinkaynak et al. (2017), they produced innovative flower-shaped hybrid nanoflowers by combining Turkish black radish peroxidase with Cu(II). The resulting peroxidase-Cu2+ biocatalyst exhibited improved catalytical capacity and stability across a broad pH diapason. Impressively, it achieved an efficiency of over 90% in degrading Victoria blue dye within a 60-min timeframe.
In a different investigation, Cui et al. (2016) produced an innovative immobilization technique to develop surfactant-activated lipase-inorganic flower-shaped hybrid nanoconstructs, aiming to enhance lipase’s catalytic capacity. The newly designed lipase-based biocatalytic system demonstrated an impressive 460% increase in catalytic capacity compared to free lipase. Furthermore, it demonstrated remarkable durability and repeatability in several cycles, owing to its uniformity and robust mechanical parameters. The enhanced catalytic capacity was attributed to the enzyme’s stabilized active conformation within the hybrid nanoflower, enabled by appropriate interfacial activation. The larger surface area of the nanoflower also reduced mass transfer resistances, ensuring enhanced substrate accessibility to the enzyme’s active sites. The surfactants’ use effectively prevented the formation of bulky lipase aggregates, resulting in uniform and well-dispersed molecular architectures.
In spite of the promising properties of hybrid nanoflowers, such as maintaining high enzyme activity and stability, their reusability is often limited due to the fragility of their flower-shaped structures. The various orderly shaped petals can break in centrifugation steps during reuse, limiting their practical applications on a larger scale (Lee et al. 2015, 2017). To overcome these limitations, Lee et al. (2017) proposed a simple and effective way to creating reusable enzyme-inorganic nanoflowers by applying reaction with glutaraldehyde (GLU) to improve the binding of the enzyme molecules within the synthesized nanoflowers. While GLU treatments have been used to immobilize various enzymes, this study was the first attempt to develop protein-inorganic nanoflowers using GLU treatment. The GLU-treated lipase nanoflowers, synthesized using lipase from Candida rugosa and Cu3(PO4)2, exhibited enhanced robustness and recycling capability. In comparison to non-covalent lipase-incorporated nanoflowers, which lost approximately 90% of their activity after four use cycles, the GLU-lipase-nanoflowers retained over 70% of their original activity while maintaining their flower-like structure. Similarly, a GLU-laccase-metal hybrid nanoflower system demonstrated an enzyme recovery of 78.1%, activity recovery up to 204%, and a 2.2-time increase in catalytic efficiency compared to free enzyme.
Calcium-based hybrid nanoflowers
In recent years, calcium-based hybrid nanoflowers have gained significant attention for their diverse applications. Yin et al. (2015) presented a simple and efficient method to synthesize α-chymotrypsin-inorganic hybrid nanostructures using calcium phosphate (Ca3(PO4)2) as the inorganic component. These hybrid nanoflowers comprised microscale particles with nanosized flower-like petals, exhibiting exceptional stability and regenerability. The immobilized biocatalytic system demonstrated remarkable enzyme activity during repeated protein digestion cycles, surpassing its free enzyme counterpart. Through a one-pot green approach, Concanavalin A/invertase/CaHPO4 hybrid nanoflowers were successfully synthesized, where Con-A and invertase were mixed with calcium ions in phosphate-buffered saline (Ye et al. 2016). This hybrid nanobiocatalytic system displayed a significant increase in enzyme activity and efficient loading of invertase. Wang et al. (2013) reported the rational design of CaHPO4–α-amylase hybrid nanobiocatalytic systems with three different morphologies, including nanoflowers, nanoplates, and parallel hexahedrons. Liu et al. (2017a, 2017b) developed a self-repairing metal–organic hybrid nanocatalyst coated with sodium alginate to reinforce immobilized chloroperoxidase, utilizing Ca3(PO4)2 as an inorganic component. Furthermore, Wang et al. (2014) explored the application of chitosan/calcium pyrophosphate microflowers as dye adsorption carriers and for catalase immobilization. The microflowers were synthesized using a coupled biomimetic mineralization and ionotropic gelation. In a different context, Bhavsar and Amiji (2007) introduced a novel hybrid oral carrier called “nanoparticles-in-microsphere oral system NiMOS,” based on a gelatin and poly(epsilon-caprolactone) matrix, for oral gene delivery.
Manganese-based hybrid nanoflowers
These materials have emerged as a promising catalyst support material for fuel cells, with manganese (II) phosphate and bovine serum albumin being utilized in their synthesis (Zhang et al. 2015b, 2015c). These manganese phosphates have garnered considerable interest due to their unique electrochemical properties, as highlighted in previous studies (Fadnis and Kulshrestha 1982; Ferdov et al. 2007; Brahim and Boughzala 2013; Jin et al. 2014). In their work, Rai et al. (2018) introduced a purified L-arabinose isomerase ordered flower-shaped spherical assembly in a manganese-based hybrid nanocomposite, specifically for the D-tagatose obtaining. The produced nanocomposite demonstrated high recyclability and regenerability during multiple uses, along with remarkable stability over a month of storage. Additionally, it exhibited highly D-galactose high conversion to desirable product, positioning them as excellent candidates for efficient biosynthesis of D-tagatose. Furthermore, Munyemana et al. (2018) employed an environmentally friendly biomineralization technique to create protein-manganese phosphate hybrid nanocomposite materials. Collagen was used as a biotemplate, shaping the structure of the nanomaterial into the flower-like ones with branched petals. Apart from acting as a scaffold for nucleating manganese phosphates, collagen also acted as an holding the petals together adhesive. The newly obtained manganese-based nanocomposite demonstrated promising catalytic potential for the treatment of water.
Zinc-based hybrid nanoflowers
These nanomaterials present a promising and eco-friendly alternative to copper-ion-based synthesis, offering a faster reaction rate without compromising proteins, making them more efficient and environmentally friendly (Zhang et al. 2016a, b, c). The reduced synthesis time is particularly valuable for nanotechnology applications. In their research, Zhang et al. (2016a, b, c) successfully developed a lipase/Zn3(PO4)2 hybrid nanoflower as an immobilized enzyme nanobiocatalyst, showcasing a significant increase in hydrolytic activity up to147%, and greater stability compared to free lipase. Additionally, they synthesized flower-like papain/Zn3(PO4)2 hybrid nanocomposite, which displayed enhanced catalytic properties, thermal stability, repeatability, and extended storage profile compared to free papain. In another study, Zhang et al. (2018) introduced a simple way to obtain a novel flower-shaped bovine serum albumin (BSA)/Zn3(PO4)2/Fe3O4 magnetic hybrid composite. This particle exhibited excellent retention of over 90% of its initial adsorption capability even after ten consecutive repetitions, attributed to its magnetic property. Additionally, zinc oxide nanoflower petals were investigated for their potential in mediating amyloid degradation in vitro using a zeta potential-based technique (Girigoswami et al. 2019). Nanoparticles and nanoflowers, such as zinc-based hybrid nanoparticles, are of particular interest due to their potential to cross the blood–brain barrier, making them a promising avenue for treating neurodegenerative diseases. Zinc’s effect on the amyloid β-aggregate appearing makes it an essential component in developing anti-amyloid agents, as demonstrated using a model huminsulin amyloid (Girigoswami et al. 2019).
Cobalt-based hybrid nanoflowers
These hybrid nanocomposites have not garnered as much attention as other biomolecule-inorganic nanoflower hybrid structures; nevertheless, they hold great potential for catalysis. Cobalt (II) ions play a critical role in enhancing the performance of enzymes like D-psicose 3-epimerase (DPEase) due to their allosteric effect (Kim et al. 2006). López-Gallego and Yate (2015) presented a selective way to obtain bioinorganic hybrid nanoflowers based on Co3(PO4)2 and alcohol dehydrogenases which is His-tagged enzyme. However, until recently, there was no study devoted to similar technique of DPEase immobilization until Zheng et al. (2018) described a smart bio-mineralization way to create DPEase-inorganic hybrid nanoflowers using Co3(PO4)2 as the inorganic matrix. The obtained nanobiocatalyst exhibited a remarkable 7.2-time higher activity (pH 8.5 and 60 °C) compared to soluble enzyme. Additionally, the new DPEase formulation demonstrated enhanced pH and thermal stabilities, preserving about 90% of initial catalytic capacity after six reuses, indicating excellent reusability. Kim et al. (2016) investigated the mechanism of growth of BSA/cobalt hybrid nanoflowers using a straightforward way. The study showed that BSA serves as a Co and P atoms’ coordination template, leading to the protein-metal hybrid nanocomposite creation. Morphological examination over varying reaction durations showed the sequential transformation of BSA cobalt phosphate nanoparticles into 1D and 2D nanopetals, ultimately forming nanoflowers. In another recent study, Lu et al. (2019) reported a simple strategy for fabricating a 3-D nitrogen-doped holey graphene hydrogel decorated with NiCo2O4 nanoflowers (NHGH/NiCo2O4) using a one-pot hydrothermal method followed by calcination. NHGH/NiCo2O4 nanoflowers exhibited high sensitivity, electrical conductivity, and electrocatalytic activity, along with a large area of surface and assessable active sites, making them efficient in detecting glucose and H2O2.
Iron-based hybrid nanoflowers
Ocsoy et al. (2015) were pioneers in developing iron-based hybrid nanoflowers by combining iron(II) ions acting as the inorganic fragment with horseradish peroxidase as organic component to create flower-like hybrid nanocomposites with greatly enhanced catalytic capacity and operation and storage stability. The research showed that introducing of iron(II) ions into the hybrid nanoflower revealed approximately 512% and 710% increased catalytic capacity in relation to guaiacol when stored at 4 °C and 20 °C, respectively, and the activity decrease was only 2.9% and 10% of the initial one following storage at 4 °C and 20 °C, respectively, for up to 1 month. These significant improvements in catalytic activity were attributed to the enhancing enzyme concentration in the nanocomposite, more favorable enzyme conformation, less mass transfer hindrances, and the effect of Fe2+ on HRP enzyme activity. The flower-like hybrid nanocomposites’ elevated activity and stability have prompted further studies for potential biosensor applications. The choice of Fe2+ ions as the inorganic component in this study was influenced by its role as an activator of the catalytic capacity of H2O2-dependent HRP transformations (Chen and Schopfer 1999; Meng et al. 2004; Chalkias et al. 2008).
Selenium and other non-metal-based hybrid nanoflowers
Selenium and other non-metal-based hybrid nanoflowers have not received extensive attention compared to the numerous studies on ordered arrangement nanocomposites/nanoflowers involving various metal ions (Yang et al. 2012; Yin et al. 2004; Luesakul et al. 2016). Nevertheless, selenium-based nanoparticles have recently gained rising interest as components for drug delivery systems because of their bioactivity, low toxicity, and perfect bioavailability (Wu et al. 2012; Kumar et al. 2014; Yu et al. 2016). Recently, significant attention has been directed to creating controllable selenium NPs with various architectures and morphologies, such as nanospheres, nanotubes, nanowires, nanoribbons, and nanoplates. Integrating functionalized biopolymers into the nanoparticle formation process enhances stability, controls shape, and size and imparts exceptional properties to these nanoconstructs. A notable recent study by Nonsuwan et al. (2018) introduced a simple and bio-inspired way to synthesize organic–inorganic hybrid pullulan/SeNPs microflowers for the first time. In this synthesis process, SeNPs served as the inorganic fragment, while pullulan acted as the organic component, providing a replacement of frequently used proteins due to its easy modification to achieve desired properties. The formation of SeNPs stabilized through folic acid–decorated cationic pullulan (FA-CP) resulted in highly ordered flower-like assemblies, with the critical factors for forming such structures being the FA-CP concentration and the Na2SeO3 to cysteine molar ratios. The resulting nanocomposite demonstrated three times higher doxorubicin adsorption, compared to sphere-shaped SeNPs. This study opens up new possibilities for the development of selenium-based hybrid nanoflowers with potential applications in drug delivery and other biomedical fields.
Multi-enzyme co-immobilized nano-assemblies
Co-immobilization of multiple enzymes has emerged as a promising concept in bio-catalysis engineering (Bilal et al. 2021). This innovative approach integrates different catalytic conversions to produce numerous industrially important chemicals, mimicking in vivo multi-enzymatic reactions (Brady and Jordaan 2009; Ansari and Husain 2012; Britton et al. 2018; Huffman et al. 2019). Researchers are actively working on designing in vitro multi-enzyme-based functional biocatalytic systems using various strategies, including co-immobilization, enzyme fusion, and enzyme-scaffold conjugates (Mayer et al. 2001; Hwang and Lee 2019; Yang et al. 2019).
In the field of multi-enzyme technology, two or more enzymes are co-localized onto suitable carriers or integrated into single enzyme molecules with the presence of a linking agent (Li et al. 2014; Ren et al. 2019). This co-immobilization process reduces mass transfer resistance, leading to improved enzyme activity through efficient substrate channeling, enhanced reusability, and increased stability (Brady and Jordaan 2009; Hwang and Lee 2019; Arana-Pena et al. 2020). A wide range of functionalized nanostructured materials has been utilized to develop multi-enzyme immobilized nanobiocatalytic systems, such as carbon nanotubes, nanoparticles, graphene, metal–organic frameworks, silica, nanocomposites, and nanofiber scaffolds. These materials protect enzymes from harsh conditions like high temperatures and heavy metals, ensuring their stability and performance (Zhu et al. 2007a, b; Sheldon 2010; Wang and Jiang 2011; Ma et al. 2012; Tran et al. 2012; Sheldon and Woodley 2018; Ren et al. 2019).
The co-immobilization process involving an activator agent, often glutaraldehyde, allows two or more enzymes to be co-localized on suitable carriers without the need for different support systems (Ren et al. 2019). This method optimizes enzyme productivity through effective substrate channeling, improved renewability, and enhanced consistency (Hwang and Lee 2019; Arana-Pena et al. 2020). With the enhancement of sustainable organic synthesis and production methods, biocatalysis provides a captivating route in that same direction (Bie et al. 2022). The synergistic effect of multiple enzymes immobilized on various nanosupports opens up possibilities for producing synthetic drugs, analgesics, nutritional supplements, biofuels, and other valuable products (Wahab et al. 2020; Gkantzou et al. 2021).
Multi-enzymatic pathways facilitate the accomplishment of multi-step one-pot reactions, providing various advantages compared to single enzyme immobilization or free enzyme methods. These benefits encompass fewer process steps, decreased production of by-products, enhanced summarized reaction efficiency, shorter reaction periods, and economic advantages. In situ recovery of expensive co-factors, if any, becomes possible, and the equilibrium can be substantially altered (Gruber et al. 2017; Li et al. 2019b; Jiang et al. 2020). The process of co-immobilization further boosts both the thermal and mechanical stability of enzymes, safeguarding against enzyme denaturation.
Various examples of multi-enzyme system immobilization named co-immobilization on different nanosupports include pectinase, cellulase, xylanase/magnetic iron oxide NPs (Muley et al. 2018), Candida antarctica lipase B and Thermomyces lanuginosus lipase/epoxy-functionalized silica gel (Shahedi et al. 2021), glucose oxidase, catalase/porous magnetic microspheres (Liu et al. 2022), decarboxylase, oxygenase/mesoporous zeolite (Ni et al. 2022), trypsin, and chymotrypsin/amino-functionalized zeolite SiO2 (Dogan et al. 2021).
The co-immobilization of enzymes poses specific challenges that require careful consideration. One crucial factor to address is the size of the enzymes, which can be influenced by the largest enzyme present. This may affect peptide loading, substrate availability, and other factors unless a carrier-free strategy is adopted (Kazenwadel et al. 2015; Quin et al. 2017). When non-porous nanostructures are utilized as carriers, the enzymes may simply distribute on the carrier’s surface (membrane) (Arana-Pena et al. 2020). In cases where enzymes work synergistically, such as in the hydrolysis of biopolymers, complete reconfiguration of fats and oils, multistage reactions involving sequential modification of a substrate to yield a different product, or reactions requiring a cofactor and a co-factor regenerating enzyme, co-immobilization is highly recommended (Li et al. 2019b; Velasco-Lozano 2020).
An illustration of the advantages brought about by co-immobilization is evident when considering the pairing of the desired enzyme with catalase. This method has demonstrated a near-complete mitigation of the deleterious consequences arising from hydrogen peroxide, which is produced as a secondary product in scenarios where an intermediary compound proves to be unstable. A case in point is the synthesis of α-oxoacids using amino acid oxidases through the oxidative deamination of amino acids, as explored by Bie et al. in 2022.
Nanomaterial toxicity during enzyme immobilization
The extensive utilization of synthetic nanoparticles with uncertain toxicological implications poses potential risks to both the environment and human health (Ayub et al. 2023). There is a potential for human and environmental exposure to nanoparticles throughout their entire life cycle, from production (Mohajerani et al. 2019) to disposal (Khan and Shanker 2015). Recent attention has been drawn to concerns about the potential adverse effects of nanoparticles on both human health and the environment (Stefani and Dobson 2003).
Silver nanoparticles, as an example, enter the aquatic ecosystem through consumer products and pose a threat to aquatic species. In comparison to silver nanoparticles, copper nanoparticles exhibit a higher level of toxicity towards bacteria and aquatic organisms (Gondwal and Joshi nee Pant 2018). Similarly, carbon nanoparticles, particularly in their raw powdered state, can also pose risks. It is imperative to conduct specific assessments for nanoparticles as a precautionary measure before employing them as nanosupports. The utilization of metal complexes is restricted due to their significantly higher cost, and they often necessitate the use of hazardous solvents as reaction solutions, which can lead to additional adverse consequences. Assessing the impact of nanoparticles on the environment and establishing exposure levels during production are of utmost importance. In an assessment of the harmful effects of naproxen and untreated diclofenac solutions, Artemia salina was employed as a test organism (Zdarta et al. 2019). The results indicated that only 20% and 25% of naproxen and diclofenac concentrations were effective, suggesting the toxicity of these solutions. A substantial improvement was observed when using NanoBio catalysts for immobilization, with encapsulated laccase demonstrating the least toxic and most compatible method, achieving effective values ranging from 60 to 85%. To enable the widespread use of nanoparticles while safeguarding health and the environment, it is essential for academic and industrial research sectors, in collaboration with regulatory agencies, to assess both the advantages and risks associated with nanoparticles (Mohajerani et al. 2019).
There is a need to focus on biological approaches for nanoparticle synthesis because chemical methods come with certain drawbacks (Jiang et al. 2009). Despite significant progress in enzyme immobilization techniques, the development of new methods that are environmentally friendly and non-toxic poses a substantial challenge. This challenge may necessitate exploring alternative solutions, such as green nanoparticle synthesis, plasma technology, and the integration of novel technologies with immobilization processes (Ahmed et al. 2016).
The eco-friendly approach to nanoparticle synthesis is a superior, efficient, one-step procedure known for its environmental sustainability. This method commonly yields stable materials, making it highly favored in the chemical industries (Syed et al. 2016). While plasma technologies and similar approaches may be costly and less convenient, they hold the potential to enable the establishment of multi-point adhesion of industrially immobilized enzymes on the support substrate surface (Ayub et al. 2023).
Advantages and disadvantages of engineered complexes enzymes-nanomaterials
Nanomaterials exhibit numerous advantageous characteristics, such as the presence of diverse functional groups, insolubility during reactions, reusability, straightforward recovery, a high surface area-to-volume ratio, heightened enzyme affinities, and more. These features make nanoparticles an attractive option for enzyme immobilization. Nonetheless, despite these advantages, there exist technical limitations when employing nanomaterials for enzyme immobilization, alongside potential approaches to address these issues. The straightforward selection of the most suitable combination of nanomaterials and enzymes, guided by the physical properties of various biomolecules and carrier supports, stands in contrast to dealing with larger matrices. The reduced mass transfer resistance in nanomaterial-coupled biocatalysts enhances enzyme activity. The use of nanomaterial-based matrix supports offers increased flexibility in reaction mixtures due to their easy handling and operational efficiency. In a recent investigation, Huang et al. introduced an innovative magnetic chitin nanofiber composite as a scaffold for enzyme immobilization, employing chymotrypsin as a representative enzyme (Huang et al. 2018).
The straightforward separation and retrieval of magnetic nanoparticles using an external magnetic field eliminate the necessity for high-speed centrifugation methods. This leads to a reduction in enzyme processing expenses and a substantial improvement in enzymatic stability, enabling the continuous reuse of immobilized enzymes (Hwang and Gu 2013). However, prior to implementing immobilized enzymes in biotechnology applications, it is imperative to carefully evaluate the potential advantages and disadvantages associated with nanomaterial-based matrix supports. The initial stages, encompassing synthesis, pre-processing, surface group modification, and ensuring the exceptional stability and extended shelf life of nanomaterial-based support matrices, present distinct technical challenges. For example, certain procedures demand specific reaction conditions, including precise pH and temperature control, to prevent precipitation and the formation of aggregates during reactions. The issue of aggregate formation can be resolved by applying a polymer shell coating to nanomaterials (Assa et al. 2017). Moreover, the direct manipulation of nanomaterials with substantial toxicity can give rise to health and environmental concerns. For instance, carbon nanotubes in their pure powdered form may pose potential hazards (Gupta et al. 2019).
Creating a toxicity analysis protocol for particular nanomaterials as a precautionary measure before employing them as nano supports is of utmost importance. Furthermore, vigilantly monitoring the environmental repercussions of nanomaterials and identifying critical thresholds is crucial. Prior to the development of sustainable products of interest, it is essential to possess a comprehensive comprehension of the interaction between nanomaterials and proteins (Majumdar and Keller 2021). These considerations emphasize the necessity for collaboration among academic, industrial, regulatory, and legislative stakeholders to elucidate both the potential benefits and limitations of nanomaterials. This collaborative effort aims to maximize their large-scale utilization while simultaneously mitigating risks to public health and environmental well-being.
Conclusions and trends
Contemporary industry and scientific endeavors are embracing eco-conscious trends, marked by a deliberate move away from the reliance on hazardous organic compounds, solvents, and heavy metal salts. The latter components are integral to the composition of many catalysts utilized in industrial processes. Within this context, the adoption of enzyme biocatalysts holds the potential to significantly expedite the complete elimination of toxic compound usage. Furthermore, enzymes wield the capacity to tackle a multitude of pressing medical challenges, among which the escalating resistance of pathogenic microorganisms to commonly prescribed antibiotics is of paramount concern.
However, akin to all biological entities, enzymes display inherent limitations including low stability and constrained reusability. To surmount these hurdles, the immobilization of enzymes onto nanosized substrates emerges as a promising approach. This strategy not only bolsters enzyme resilience and longevity but also presents a transformative avenue for the widespread integration of biocatalysts across diverse spheres of human activity, from industry to medicine. As such, this innovative approach possesses the potential to revolutionize the landscape of sustainable and efficient processes, offering substantial benefits to both industry and healthcare.
Biocatalysts have a significant impact on various industries, including biotechnology, medicine, pharmaceuticals, and tanning, due to their high substrate specificity and ease of production. However, the industrial utilization of enzymes is often hampered by their low reusability and decreased stability during biochemical reactions. To tackle these challenges, enzyme immobilization has emerged as a promising solution. The methods for enzyme immobilization are extensively studied, and ongoing research aims to gain a more comprehensive understanding of the underlying mechanisms.
Exploring the distinctive characteristics of enzyme immobilization on various carriers offers opportunities to create novel continuous industrial processes for targeted product production through heterogeneous catalysis. Currently, a diverse range of carriers is available for enzyme immobilization. However, careful selection of the most suitable carrier, conditions, and immobilization methods for each specific enzyme employed in industrial processes is essential. This customized approach ensures optimal performance and efficiency in industrial applications.
The efficacy of enzymes under immobilization is influenced not only by the immobilization process but also by comprehension of the dynamic interplay at the enzyme-solid support interfaces. This understanding significantly aids in advancing and regulating reaction rates with immobilized enzymes. Nanotechnology has ushered in a novel era in the realm of enzyme immobilization using polymeric supports. A multitude of scholarly works illustrate that enzyme immobilization within a polymeric matrix can yield superb enzyme stability and the capacity for multiple cycles of reutilization. When selecting a support material, cost should not be the sole consideration; the potential to choose optimal operating conditions or explore different process options should also be taken into account.
Biopolymers derived from food sources are fascinating and important materials, representing some of the most complex examples of soft condensed matter that we encounter daily (Donald 2004). These natural bio-polymeric soft materials are not only biodegradable and biocompatible but also possess biofunctional properties (Mezzenga et al. 2005; Ubbink et al. 2008). Various nanostructured vehicles, such as association colloids, lipid-based nanoencapsulators, nanoemulsions, biopolymeric nanoparticles, nanotubes, and nanofibers, formed with food-grade ingredients, including food biopolymers (proteins, carbohydrates), fats, low-molecular-weight surfactants, and co-polymers (protein-carbohydrate conjugates), have been employed for delivering a wide range of functional ingredients in pharmaceuticals and foods (Huang et al. 2010; McClements 2011).
With the increasing interest in cascade enzymatic reactions and in vitro synthetic biology, the co-immobilization of multienzymes on micro- and nanoparticles has become a viable possibility. As techniques for media development progress, numerous materials and combinations have emerged as promising options for enzyme immobilization. Further research will be required to advance co-immobilized multi-enzyme support systems that demonstrate high catalytic efficiency, renewability, and practical implementation.
One promising application is enzyme pro-drug therapy, where immobilized enzymes are utilized to convert prodrugs into active drugs at the target site, enhancing therapeutic potency and reducing drug toxicity, particularly in cancer treatment (Sharifi et al. 2020). In the future, immobilized enzymes are likely to be utilized as biocatalysts against malignant cells, offering an engineered enzyme-based targeted anticancer drug system with the potential to effectively destroy cancer cells and serve as therapeutic protease nano-reactors.
The potential for enzyme immobilization is vast and cannot be fully enumerated, but it is evident that there is a growing interest, particularly in micro- and nanomaterials. The primary objective of immobilization is to enhance biocatalyst features, leading to increased productivity and reduced process costs. The selection of micro- or nanomaterial as a support will depend on the specific reaction in which it will be employed, considering enzyme properties, stability, and process characteristics such as pH and temperature.
Considering the elements detailed in this overview, the incorporation of immobilized enzymes onto micro- and nanomaterials should be actively contemplated by scientists in their reactions. Auspicious benefits and prospects for a wide array of applications are presented by these methodologies.
Data availability
Not applicable.
Code availability (software application or custom code)
Not applicable.
References
Ahmad R, Sardar M (2015) Enzyme immobilization: an overview on nanoparticles as immobilization matrix. Anal Biochem 4:178–186. https://doi.org/10.4172/2161-1009.1000178
Ahmed S, Saifullah AM, Swami BL, Ikram S (2016) Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J Radiation Res Appl Sci 9(1):1–7. https://doi.org/10.1016/j.jrras.2015.06.006
Alshammari GM, Al-Ayed MS, Abdelhalim MA, Al-Harbi LN, Qasem AA, Yahya MA (2023) Development of luminescence carbon quantum dots for metal ions detection and photocatalytic degradation of organic dyes from aqueous media. Environ Res 226:115661. https://doi.org/10.1016/j.envres.2023.115661
Altinkaynak C, Kocazorbaz E, Özdemir N, Zihnioglu F (2018) Egg white hybrid nanoflower (EW-hNF) with biomimetic polyphenol oxidase reactivity: synthesis, characterization and potential use in decolorization of synthetic dyes. Int J Biol Macromol 109:205–211. https://doi.org/10.1016/j.ijbiomac.2017.12.072
Altinkaynak C, Tavlasoglu S, Kalin R, Sadeghian N, Ozdemir H, Ocsoy I, Özdemir N (2017) A hierarchical assembly of flower-like hybrid Turkish black radish peroxidase-Cu2+ nanobiocatalyst and its effective use in dye decolorization. Chemosphere 182:122–128. https://doi.org/10.1016/j.chemosphere.2017.05.01
Altinkaynak C, Tavlasoglu S, Ozdemir N, Ocsoy I (2016) A new generation approach in enzyme immobilization: organic-inorganic hybrid nanoflowers with enhanced catalytic activity and stability. Enzyme Microb Technol 93–94:105–112. https://doi.org/10.1016/j.enzmictec.2016.06.011
Amari A, Alzahrani FM, Alsaiari NS, Katubi KM, Rebah FB, Tahoon MA (2021) Magnetic metal organic framework immobilized laccase for wastewater decolorization. Processes 9:774–788. https://doi.org/10.3390/pr9050774
Amirfazli A (2007) Nanomedicine: magnetic nanoparticles hit the target. Nat Nanotechnol 2:467–468. https://doi.org/10.1038/nnano.2007.234
An J, Li G, Zhang Y, Zhang T, Liu X, Gao F, Peng M, He Y, Fan H (2020) Recent advances in enzyme-nanostructure biocatalysts with enhanced activity. Catalysts 10(3):338. https://doi.org/10.3390/catal10030338
Andersson MM, Hatti-Kaul R (1999) Protein stabilising effect of polyethyleneimine. J Biotechnol 72:21–31. https://doi.org/10.1016/S0168-1656(99)00050-4
Ansari SA, Husain Q (2012) Potential applications of enzymes immobilized on/in nano materials: a review. Biotechnol Adv 30:512–523. https://doi.org/10.1016/j.biotechadv.2011.09.005
Anwar A, Imran M, Iqbal HMN (2023) Smart chemistry and applied perceptions of enzyme-coupled nano-engineered assemblies to meet future biocatalytic challenges. Coord Chem Rev 493:215329. https://doi.org/10.1016/j.ccr.2023.215329
Arana-Pena S, Carballares D, Morellon-Sterlling R, Berenguer-Murcia A, Alcantara AR, Rodrigues RC, Fernandez-Lafuente R (2020) Enzyme co-immobilization: always the biocatalyst designers choice…or not? Biotechnol Adv 107584–107621. https://doi.org/10.1016/j.biotechadv.2020.107584
Artyukhov VG, Kovaleva TA, Kholyavka MG, Bityutskaya LA, Grechkina MV (2010) Thermal inactivation of free and immobilized inulinase. Appl Biochem Microbiol 46:385–389. https://doi.org/10.1134/S0003683810040034
Asgher M, Shahid M, Kamal S, Iqbal HMN (2014) Recent trends and valorization of immobilization strategies and ligninolytic enzymes by industrial biotechnology. J Mol Catal B: Enzym 101:56–66. https://doi.org/10.1016/j.molcatb.2013.12.016
Ashmarina LI, Muronetz VI, Nagradova NK (1984) Immobilized glyceraldehyde-3-phosphate dehydrogenase forms a complex with phosphoglycerate kinase. Biochem Int 9:511–521
Assa F, Jafarizadeh-Malmiri H, Ajamein H, Vaghari H, Anarjan N, Ahmadi O, Berenjian A (2017) Crit Rev Biotechnol 37(4):492–509. https://doi.org/10.1080/07388551.2016.1185389
Ayub J, Saeed MU, Hussain N, Zulfiqar I, Mehmood T, Iqbal HMN, Bilal M (2023) Designing robust nano-biocatalysts using nanomaterials as multifunctional carriers - expanding the application scope of bio-enzymes. Top Catal 66:625–648. https://doi.org/10.1007/s11244-022-01657-8
Baluta S, Lesiak A, Cabaj J (2018) Graphene quantum dots-based electrochemical biosensor for catecholamine neurotransmitters detection. Electroanalysis 30(8):1781–1790. https://doi.org/10.1002/elan.201700825
Barbosa O, Ortiz C, Berenguer-Murcia A, Torres R, Rodrigues RC, Fernandez-Lafuente R (2015) Strategies for the one-step immobilization–purification of enzymes as industrial biocatalysts Biotechnol. Adv 33:435–456. https://doi.org/10.1016/j.biotechadv.2015.03.006
Barrat J-L, Joanny F (1995) Theory of Polyelectrolyte Solutions. Wiley-Blackwell, Hoboken, NJ. https://arxiv.org/pdf/cond-mat/9601022.pdf
Batule BS, Park KS, Kim MI, Park HG (2015) Ultrafast sonochemical synthesis of protein-inorganic nanoflowers. Int J Nanomedicine 10:137–142. https://doi.org/10.2147/IJN.S90274
Bekhit AA, Hopkins DL, Geesink G, Bekhit AA, Franks P (2014) Exogenous proteases for meat tenderization. Crit Rev Food Sci Nutr 54:1012–1031. https://doi.org/10.1080/10408398.2011.623247
Besteti MD, Cunha AG, Freire DMG, Pinto JC (2014) Core/Shell polymer particles by semibatch combined suspension/emulsion polymerizations for enzyme immobilization. Macromol Mater Eng 299:135–143. https://doi.org/10.1002/mame.201300023
Bhavsar MD, Amiji MM (2007) Gastrointestinal distribution and in vivo gene transfection studies with nanoparticles-in-microsphere oral system (NiMOS). J Control Release 119:339–348. https://doi.org/10.1016/j.jconrel.2007.03.006
Bie J, Sepodes B, Fernandes PCB, Ribeiro MHL (2022) Enzyme immobilization and co-immobilization: main framework, advances and some applications. Processes 10(3):494–525. https://doi.org/10.3390/pr10030494
Bilal M, Asgher M, Shah SZH, Iqbal HMN (2019) Engineering enzyme-coupled hybrid nanoflowers: the quest for optimum performance to meet biocatalytic challenges and opportunities. Int J Biol Macromol 135:677–690. https://doi.org/10.1016/j.ijbiomac.2019.05.206
Bilal M, Hussain N, Juliana A-P, Almulaiky YQ, Iqbal HMN (2021) Multi-enzyme co-immobilized nano-assemblies: bringing enzymes together for expanding bio-catalysis scope to meet biotechnological challenges. Int J Biol Macromol 186:735–749. https://doi.org/10.1016/j.ijbiomac.2021.07.064
Bilal M, Rashid EU, Munawar J, Iqbal HMN, Cui J, Zdarta J, Ashraf SS, Jesionowski T (2023) Magnetic metal-organic frameworks immobilized enzyme-based nano-biocatalytic systems for sustainable biotechnology. Int J Biol Macromol 237:123968–123986. https://doi.org/10.1016/j.ijbiomac.2023.123968
Biro E, Nemeth AS, Sisak C, Feczko T, Gyenis J (2008) J Biochem Biophys Methods 70:1240–1246. https://doi.org/10.1016/j.jprot.2007.11.005
Blazek T, Gorski W (2020) Oxidases, carbon nanotubes, and direct electron transfer: a cautionary tale. Biosens Bioelectron 163:112260–112266. https://doi.org/10.1016/j.bios.2020.112260
Bolivar JM, Rocha-Martin J, Mateo C, Cava F, Berenguer J, Fernandez-Lafuente R, Guisan JM (2009) Coating of soluble and immobilized enzymes with ionic polymers: full stabilization of the quaternary structure of multimeric enzymes. Biomacromol 10(4):742–747. https://doi.org/10.1021/bm801162e
Boussema F, Gross AJ, Hmida F, Ayed B, Majdoub H, Cosnier S, Maaref A, Holzinger M (2018) Dawson-type polyoxometalate nanoclusters confined in a carbon nanotube matrix as efficient redox mediators for enzymatic glucose biofuel cell anodes and glucose biosensors. Biosens Bioelectron 109:20–26. https://doi.org/10.1016/j.bios.2018.02.060
Brady D, Jordaan J (2009) Advances in enzyme immobilization. Biotechnol Lett 31:1639–1650. https://doi.org/10.1007/s10529-009-0076-4
Brahim FB, Boughzala H (2013) Crystal structure, vibrational spectra and thermal analysis of a new centrosymmetric transition metal phosphate compound, Mn(H2PO4)2·4H2O. J Mol Struct 1034:336–345. https://doi.org/10.1016/j.molstruc.2012.10.050
Britton J, Majumdar S, Weiss GA (2018) Continuous flow biocatalysis. Chem Soc Rev 47:5891–5918. https://doi.org/10.1039/c7cs00906b
Butterworth M, Illum L, Davis S (2001) Preparation of ultrafine silica- and peg-coated magnetite particles. Colloids Surf A Physicochem Eng Asp 179(1):93–102. https://doi.org/10.1016/S0927-7757(00)00633-6
Canevari TC, Cincotto FH, Gomes D, Landers R, Toma HE (2017) Magnetite nanoparticles bonded carbon quantum dots magnetically confined onto screen printed carbon electrodes and their performance as electrochemical sensor for NADH. Electroanalysis 29(8):1968–1975. https://doi.org/10.1002/elan.201700167
Cantone S, Ferrario V, Corici L, Ebert C, Fattor D, Spizzo P, Gardossi L (2013) Efficient immobilization of industrial biocatalysts: criteria and constraints for the selection of organic polymeric carriers and immobilization methods. Chem Soc Rev 42:6262–6276. https://doi.org/10.1039/c3cs35464d
Castelló J, Gallardo M, Busquets MA, Estelrich J (2015) Chitosan (or alginate)-coated iron oxide nanoparticles: a comparative study. Colloids Surf A Physicochem Eng Asp 468:151–158. https://doi.org/10.1016/j.colsurfa.2014.12.031
Castner DG, Ratner BD (2002) Biomedical surface science: foundations to frontiers. Surface Sci 500(1–3):28–60. https://doi.org/10.1016/S0039-6028(01)01587-4
Celik C, Tasdemir D, Demirbas A, Kati A, Gul OT, Cimen B, Ocsoy I (2018) Formation of functional nanobiocatalysts with a novel and encouraging immobilization approach and their versatile bioanalytical applications. RSC Adv 8:25298–25303. https://doi.org/10.1039/c8ra03250e
Chalkias NG, Kahawong P, Giannelis EP (2008) Activity increase of horseradish peroxidase in the presence of magnetic particles. J Am Chem Soc 130:2910–2911. https://doi.org/10.1021/ja7102263
Chastellain M, Petri A, Hofmann H (2004) Particle size investigations of a multistep synthesis of PVA coated superparamagnetic nanoparticles. J Colloid Interface Sci 278:353–360. https://doi.org/10.1016/j.jcis.2004.06.025
Chavali MS, Nikolova MP (2019) Metal oxide nanoparticles and their applications in nanotechnology. SN Appl Sci 1:607–637. https://doi.org/10.1007/s42452-019-0592-3
Chen G, Huang S, Kou X, Zhu F, Ouyang G (2020) Embedding functional biomacromolecules within peptide-directed metal-organic framework (MOF) nanoarchitectures enables activity enhancement. Angew Chem Int Ed 59:13947–13954. https://doi.org/10.1002/anie.202005529
Chen G, Tong L, Huang S, Huang S, Zhu F, Ouyang G (2022) Hydrogen-bonded organic framework biomimetic entrapment allowing non-native biocatalytic activity in enzyme. Nat Commun 13:4816. https://doi.org/10.1038/s41467-022-32454-2
Chen H, Zhang J, Dang Y, Shu G (2014) Optimization for immobilization of β-galactosidase using plackett-burman design and steepest ascent method. J Chem Pharm Res 6(4):612–616. https://doi.org/10.1016/j.enzmictec.2011.06.010
Chen L, Remondetto GE, Subirade M (2006) Food protein-based materials as nutraceutical delivery systems. Trends Food Sci Technol 17:272–283. https://doi.org/10.1016/j.tifs.2005.12.011
Chen SX, Schopfer P (1999) Hydroxyl-radical production in physiological reactions: a novel function of peroxidase. Eur J Biochem 260:726–735. https://doi.org/10.1046/j.1432-1327.1999.00199.x
Cheng C, Chang KC (2013) Development of immobilized cellulase through functionalized gold nano-particles for glucose production by continuous hydrolysis of waste bamboo chopsticks. Enzyme Microb Technol 53:444–451. https://doi.org/10.1016/j.enzmictec.2013.09.010
Cherednikova TV, Muronetz VI, Nagradova NK (1980) Study of subunit interactions in immobilized d-glyceraldehyde-3-phosphate dehydrogenase. Biochim Biophys Acta (BBA) - Enzymol 613:292–308. https://doi.org/10.1016/0005-2744(80)90084-4
Ciancaglini P, Simão AMS, Bolean M, Millán JL, Rigos CF, Yoneda JS, Colhone MC, Stabeli RG (2012) Proteoliposomes in nanobiotechnology. Biophys Rev 4:67–81. https://doi.org/10.1007/s12551-011-0065-4
Cipolatti EP, Silva MJA, Klein M, Feddern V, Feltes MMC, Oliveira JV, Ninow JL, de Oliveira D (2014a) Current status and trends in enzymatic nanoimmobilization. J Mol Catal b: Enzym 99:56–67. https://doi.org/10.1016/j.molcatb.2013.10.019
Cipolatti EP, Valerio A, Henriques RO, Moritz DE, Ninow JL, Freire DMG, Manoel EA, Fernandez-Lafuente R, de Oliveira D (2016) Nanomaterials for biocatalyst immobilization - state of the art and future trends. RSC Adv 6:104675–104692. https://doi.org/10.1039/c6ra22047a
Cipolatti EP, Valerio A, Nicoletti G, Theilacker E, Araujo PHH, Sayer C, Ninow JL, de Oliveira D (2014b) Immobilization of Candida antarctica lipase B on PEGylated poly(urea-urethane) nanoparticles by step miniemulsion polymerization. J Mol Catal B: Enzym 109:116–121. https://doi.org/10.1016/j.molcatb.2014.08.017
Costa SA, Tzanov T, Carneiro AF, Paar A, Gübitz GM, Cavaco-Paulo A (2002) Studies of stabilization of native catalase using additives. Enzyme Microb Technol 30:387–391. https://doi.org/10.1016/S0141-0229(01)00505-1
Cui J, Feng Y, Lin T, Tan Z, Zhong C, Jia S (2017) Mesoporous metal-organic framework with well-defined cruciate flower-like morphology for enzyme immobilization. ACS Appl Mater Interfaces 9(12):10587–10594. https://doi.org/10.1021/acsami.7b00512
Cui J, Jia S (2017) Organic-inorganic hybrid nanoflowers: a novel host platform for immobilizing biomolecules. Coord Chem Rev 352:249–263. https://doi.org/10.1016/j.ccr.2017.09.008
Cui J, Zhao Y, Liu R, Zhong C, Jia S (2016) Surfactant-activated lipase hybrid nanoflowers with enhanced enzymatic performance. Sci Rep 6:27928–27941. https://doi.org/10.1038/srep27928
Cui L, Li C-C, Tang B, Zhang C-Y (2018) Advances in the integration of quantum dots with various nanomaterials for biomedical and environmental applications. Analyst 143(11):2469–2478. https://doi.org/10.1039/c8an00222c
da Silva AL, Cruz FF, Rocco PRM, Morales MM (2017) New perspectives in nanotherapeutics for chronic respiratory diseases. Biophys Rev 9:793–803. https://doi.org/10.1007/s12551-017-0319-x
Das MC, Pal SC, Chen B (2022) Emerging microporous HOF materials to address global energy challenges. Joule 6:22–27. https://doi.org/10.1016/j.joule.2021.12.005
Dedania SR, Patel VK, Soni SS, Patel DH (2020) Immobilization of Agrobacterium tumefaciens D-psicose 3-epimerase onto 462 titanium dioxide for bioconversion of rare sugar. Enzyme Microb Technol 140:109605. https://doi.org/10.1016/j.enzmictec.2020.109605
Diab R, Jaafar-Maalej C, Fessi H, Maincent Ph (2012) Engineered nanoparticulate drug delivery systems: the next frontier for oral administration? AAPS J 14:688–702. https://doi.org/10.1208/s12248-012-9377-y
Dogan D, Sezer S, Ulu A, Köytepe S, Ateş B (2021) Preparation and characterization of amino-functionalized Zeolite/SiO2 materials for trypsin-chymotrypsin co-immobilization. Catal Lett 151(8):2463–2477. https://doi.org/10.1007/s10562-021-03636-2
Doherty CM, Buso D, Hill AJ, Furukawa S, Kitagawa S, Falcaro P (2014) Using functional nano- and microparticles for the preparation of metal-organic framework composites with novel properties. Acc Chem Res 47:396–405. https://doi.org/10.1021/ar400130a
Donald A (2004) Food for thought. Nat Mater 3:579–581. https://doi.org/10.1038/nmat1207
Doonan C, Ricco R, Liang K, Bradshaw D, Falcaro P (2017) Metal-organic frameworks at the biointerface: synthetic strategies and applications. Acc Chem Res 50:1423–1432. https://doi.org/10.1021/acs.accounts.7b00090
Drifford M, Delsanti M (2001) Polyelectrolyte solutions with multivalent added salts: stability, structure, and dynamics. physical chemistry of polyelectrolytes. Marcel Dekker, New York, pp 135–162. https://www.taylorfrancis.com/chapters/edit/10.1201/9781482270686-6/polyelectrolyte-solutions-multivalent-addedsalts-stability-structure-dynamics-maurice-drifford-michel-delsanti
Du Y, Jia X, Zhong L, Jiao Y, Zhang Z, Wang Z, Feng Y, Bilal M, Cui J, Jia S (2022) Metal-organic frameworks with different dimensionalities: An ideal host platform for enzyme@MOF composites. Coord Chem Rev 454:214327. https://doi.org/10.1016/j.ccr.2021.214327
Egebro Birk S, Boisen A, Hagner NL (2021) Polymeric nano- and microparticulate drug delivery systems for treatment of biofilms. Adv Drug Deliv Rev 174:30–52. https://doi.org/10.1016/j.addr.2021.04.005
Elzoghby AO, Abo El-Fotoh WS, Elgindy NA (2011) Casein-based formulations as promising controlled release drug delivery systems. J Control Release 153:206–216. https://doi.org/10.1016/j.jconrel.2011.02.010
Elzoghby AO, Samy WM, Elgindy NA (2012) Albumin-based nanoparticles as potential controlled release drug delivery systems. J Control Release 167:168–182. https://doi.org/10.1016/j.jconrel.2011.07.031
Emerich DF, Snodgrass P, Lafreniere D, Dean RL, Salzberg H, Marsh J, Perdomo B, Arastu M, Winn SR, Bartus RT (2002) Sustained release chemotherapeutic microspheres provide superior efficacy over systemic therapy and local bolus infusions. Pharm Res 19:1052–1060. https://doi.org/10.1023/a:1016434926649
Escobar S, Velasco-Lozano S, Lu CH, Lin YF, Mesa M, Bernal C, Lopez-Gallego F (2017) Understanding the functional properties of bio-inorganic nanoflowers as biocatalysts by deciphering the metal-binding sites of enzymes. J Mater Chem B 5:4478–4486. https://doi.org/10.1039/x0xx00000x
Fadnis AG, Kulshrestha SK (1982) Kinetics of oxidation of adonitol by manganese (III) pyrophosphate. React Kinet Catal Lett 19:267–269. https://doi.org/10.1007/BF02074043
Faizullin D, Valiullina Y, Salnikov V, Zelenikhin P, Zuev Y, Ilinskaya O (2023) Fibrin-rhamnogalacturonan I composite Gel for therapeutic enzyme delivery to intestinal tumors. Int J Mol Sci 24:926. https://doi.org/10.3390/ijms24020926
Fändrich M, Fletcher MA, Dobson CM (2001) Amyloid fibrils from muscle myoglobin. Nature 410:165. https://doi.org/10.1038/35065514
Faraji AH, Wipf P (2009) Nanoparticles in cellular drug delivery. Bioorg Med Chem 17:2950–2962. https://doi.org/10.1016/j.bmc.2009.02.043
Fathi M, Karim M, Khoigani SR, Mosayebi V (2019) Bioactive molecules in food: use of nanotechnology for immobilization and entrapment of food applicable enzymes. Springer, Cham, pp 2037–2061. https://doi.org/10.1007/978-3-319-78030-652
Feng Y, Du Y, Kuang G, Zhong L, Hu H, Jia S, Cui J (2022) Hierarchical micro- and mesoporous ZIF-8 with core-shell superstructures using colloidal metal sulfates as soft templates for enzyme immobilization. J Colloid Interface Sci 610:709–718. https://doi.org/10.1016/j.jcis.2021.11.123
Feng Y, Hu H, Wang Z, Du Y, Zhong L, Zhang C, Jiang Y, Jia S, Cui J (2021) Three-dimensional ordered magnetic macroporous metal-organic frameworks for enzyme immobilization. J Colloid Interface Sci 590:436–445. https://doi.org/10.1016/j.jcis.2021.01.078
Ferdov S, Lopes AM, Lin Z, Ferreira RAS (2007) New template-free layered manganese (III) phosphate: hydrothermal synthesis, ab initio structural determination, and magnetic properties. Chem Mater 19:6025–6029. https://doi.org/10.1021/Cm701951w
Fotiadou R, Patila M, Hammami MA, Enotiadis A, Moschovas D, Tsirka K, Spyrou K, Giannelis EP, Avgeropoulos A, Paipetis A, Gournis D, Stamatis H (2019) Development of effective lipase-hybrid nanoflowers enriched with carbon and magnetic nanomaterials for biocatalytic transformations. Nanomaterials 9(6):808–825. https://doi.org/10.3390/nano9060808
Fu M, Xing JZ, Ge Z (2019) Preparation of laccase-loaded magnetic nanoflowers and their recycling for efficient degradation of bisphenol A. Sci Total Environ 65:2857–2865. https://doi.org/10.1016/j.scitotenv.2018.10.145
Gao X, Zhai Q, Hu M, Li S, Jiang Y (2021) Hierarchically porous magnetic Fe3O4/Fe-MOF used as an effective platform for enzyme immobilization: a kinetic and thermodynamic study of structure-activity. Catal Sci Technol 11:2446–2455. https://doi.org/10.1039/D0CY02146F
Garcia-Galan C, Barbosa O, Hernandez K, dos Santos JCS, Rodrigues RC, Fernandez-Lafuente R (2014) Evaluation of styrene-divinylbenzene beads as a support to immobilize lipases. Molecules 19:7629–7645. https://doi.org/10.3390/molecules19067629
Ge J, Lei J, Zare RN (2012) Protein–inorganic hybrid nanoflowers. Nat Nanotechnol 7:428–432. https://doi.org/10.1038/nnano.2012.80
Ge MY, Han LY, Wiedwald U, Xu XB, Wang C, Kuepper K, Ziemann P, Jiang JZ (2010) Monodispersed NiO nanoflowers with anomalous magnetic behavior. Nanotechnology 21:425702–425707. https://doi.org/10.1088/0957-4484/21/42/425702
Gentil S, Lalaoui N, Dutta A, Nedellec Y, Cosnier S, Shaw WJ, Artero V, Le Goff A (2017) Carbon-nanotube-supported bio-inspired nickel catalyst and its integration in hybrid hydrogen/air fuel cells. Angew Chem Int Ed 56(7):1845–1849. https://doi.org/10.1002/anie.201611532
Georgakilas V, Tiwari JN, Kemp KC, Perman JA, Bourlinos AB, Kim KS, Zboril R (2016) Noncovalent Functionalization of Graphene and Graphene Oxide for Energy Materials, Biosensing, Catalytic, and Biomedical Applications. Chem Rev 116(9):5464–5519. https://doi.org/10.1021/acs.chemrev.5b00620
Ghosh S, Chaganti SR, Prakasham RS (2012) Polyaniline nanofiber as a novel immobilization matrix for the anti-leukemia enzyme l-asparaginase. J Mol Catal B: Enzym 74:132–137. https://doi.org/10.1016/j.molcatb.2011.09.009
Girigoswami A, Ramalakshmi M, Akhtar N, Metkar SK, Girigoswami K (2019) ZnO Nanoflower petals mediated amyloid degradation-an in vitro electrokinetic potential approach. Mater Sci Eng C 101:169–178. https://doi.org/10.1016/j.msec.2019.03.086
Gkantzou E, Chatzikonstantinou AV, Fotiadou R, Giannakopoulou A, Patila M, Stamatis H (2021) Trends in the development of innovative nanobiocatalysts and their application in biocatalytic transformations. Biotechnol Adv 51:107738–107764. https://doi.org/10.1016/j.biotechadv.2021.107738
Godbey WT, Wu KK, Mikos AG (1999) Poly(ethylenimine) and its role in gene delivery. J Control Release 60:149–160. https://doi.org/10.1016/s0168-3659(99)00090-5
Gokhale AA, Lu J, Lee I (2013) Immobilization of cellulase on magnetoresponsive graphene nano-supports. J Mol Catal b: Enzym 90:76–86. https://doi.org/10.1016/j.molcatb.2013.01.025
Gokhale KM, Walekar C, Manje S (2023) Novel curcumin analogues: synthesis and therapeutic applications. Curr Overview Pharm Sci 5:86–116. https://doi.org/10.9734/bpi/cops/v5/17813D
Goncharova SS, Redko YuA, Lavlinskaya MS, Sorokin AV, Holyavka MG, Kondratyev MS, Artyukhov VG (2023) Biocatalysts based on papain associates with chitosan nanoparticles. Condens Matter Interphases 25:173–181. https://doi.org/10.17308/kcmf.2023.25/11098
Gondwal M, Joshi nee Pant G (2018) Synthesis and catalytic and biological activities of silver and copper nanoparticles using Cassia occidentalis. International Journal of Biomaterials, 2018https://doi.org/10.1155/2018/6735426
González-García I, Solé RV, Costa J (2002) Metapopulation dynamics and spatial heterogeneity in cancer. Proc Natl Acad Sci U S A 99:13085-13089https://doi.org/10.1073/pnas.202139299
Gonzalez-Rabade N, Badillo-Corona JA, Aranda-Barradas JS, Oliver-Salvador MC (2011) Production of plant proteases in vivo and in vitro – a review. Biotechnol 29:983–996. https://doi.org/10.1016/j.biotechadv.2011.08.017
Gruber P, Marques MPC, O’Sullivan B, Baganz F, Wohlgemuth R, Szita N (2017) Conscious coupling: the challenges and opportunities of cascading enzymatic microreactors. Biotechnol J 12(7):1700030–1700043. https://doi.org/10.1002/biot.201700030
Gubaidullin AT, Makarova AO, Derkach SR, Voron’ko NG, Kadyirov AI, Ziganshina SA, Salnikov VV, Zueva OS, Zuev YuF (2022) Modulation of molecular structure and mechanical properties of k-carrageenan-gelatin hydrogel with multi-walled carbon nanotubes. Polymers 14:2346. https://doi.org/10.3390/polym14122346
Gupta N, Gupta SM, Sharma SK (2019) Carbon nanotubes: synthesis, properties and engineering applications. Carbon Lett 29(5):419–447. https://doi.org/10.1007/s42823-019-00068-2
Guzik U, Hupert-Kocurek K, Wojcieszynska D (2014) Immobilization as a strategy for improving enzyme properties-application to oxidoreductases. Molecules 19:8995–9018. https://doi.org/10.3390/molecules19078995
Hainfeld JF, Slatkin DN, Smilowitz HM (2004) The use of gold nanoparticles to enhance radiotherapy in mice. Phys Med Biol 49:309–315. https://doi.org/10.1088/0031-9155/49/18/n03
Han E, Yang H, He Z, Cai J, Zhang X, Dong X (2015) Development of tyrosinase biosensor based on quantum dots/chitosan nanocomposite for detection of phenolic compounds. Anal Biochem 486:102–106. https://doi.org/10.1016/j.ab.2015.07.001
Hartmann M, Kostrov X (2013) Immobilization of enzymes on porous silicas-Benefits and challenges. Chem Chem Soc Rev 42:6277–6289. https://doi.org/10.1039/c3cs60021a
Hermanova S, Zarevúcká M, Bouša D, Pumera M, Sofer Z (2015) Graphene oxide immobilized enzymes show high thermal and solvent stability. Nanoscale 7(13):5852–5858. https://doi.org/10.1039/c5nr00438
Hillyer JF, Albrecht RM (2001) Gastrointestinal persorption and tissue distribution of differently sized colloidal gold nanoparticles. J Pharm Sci 90:1927–1936. https://doi.org/10.1002/jps.1143
Hisaki I, Xin C, Takahashi K, Nakamura T (2019) Designing hydrogen-bonded organic frameworks (HOFs) with permanent porosity. Angew Chem Int Ed 58:11160–11170. https://doi.org/10.1002/anie.201902147
Holyavka MG, Artyukhov VG, Sazykina SM, Nakvasina MA (2017) Physical, chemical, and kinetic properties of trypsin-based heterogeneous biocatalysts immobilized on ion-exchange fiber matrices. Pharm Chem J 51:702–706. https://doi.org/10.1007/s11094-017-1678-0
Holyavka MG, Kondratyev MS, Lukin AN, Agapov BL, Artyukhov VG (2019) Immobilization of inulinase on KU-2 ion-exchange resin matrix. Int J Biol Macromol 138:681–692. https://doi.org/10.1016/j.ijbiomac.2019.07.132
Holyavka MG, Kondratyev MS, Samchenko AA, Kabanov AV, Komarov VM, Artyukhov VG (2016) In silico design of high-affinity ligands for the immobilization of inulinase. Comput Biol Med 71:198–204. https://doi.org/10.1016/j.compbiomed.2016.02.015
Homaei A, Saberi D (2015) Immobilization of α-amylase on gold nanorods: an ideal system for starch processing. Process Biochem 50:1394–1399. https://doi.org/10.1016/j.procbio.2015.06.002
Hu B, Huang Qr (2013) Biopolymer based nano-delivery systems for enhancing bioavailability of nutraceuticals. Chin J Polym Sci 31:1190–1203. https://doi.org/10.1007/s10118-013-1331-7
Hu C, Bai Y, Hou M, Wang Y, Wang L, Cao X, Chan C-W, Sun H, Li W, Ge J, Ren K (2020) Defect-induced activity enhancement of enzyme-encapsulated metal-organic frameworks revealed in microfluidic gradient mixing synthesis. Sci Adv. https://doi.org/10.1126/sciadv.aax5785
Hu H, Li P, Wang Z, Du Y, Kuang G, Feng Y, Jia S, Cui J (2022) glutamate oxidase-integrated biomimetic metal-organic framework hybrids as cascade nanozymes for ultrasensitive glutamate detection. J Agric Food Chem 70(12):3785–3794. https://doi.org/10.1021/acs.jafc.2c01639
Hu M, Giapis KP, Poulikakos D (2011) Interfacial mixing during annealing of zinc oxide nanoparticle junctions. Appl Phys Lett 98:211904–211907. https://doi.org/10.1063/1.3593487
Huang Q, Yu H, Ru Q (2010) Bioavailability and delivery of nutraceuticals using nanotechnology. J Food Sci 75:50–57. https://doi.org/10.1111/j.1750-3841.2009.01457.x
Huang S, Chen G, Ouyang G (2022) Confining enzymes in porous organic frameworks: from synthetic strategy and characterization to healthcare applications. Chem Soc Rev 51:6824–6863. https://doi.org/10.1039/d1cs01011e
Huang S, Kou X, Shen J, Chen G, Ouyang G (2020) Armoring the enzymes with metal-organic frameworks (MOFs). Angew Chem Int Ed 59:8786–8798. https://doi.org/10.1002/anie.201916474
Huang W-C, Wang W, Xue C, Mao X (2018) Effective enzyme immobilization onto a magnetic chitin nanofiber composite. ACS Sustain Chem Eng 6(7):8118–8124. https://doi.org/10.1021/acssuschemeng.8b01150
Huang Y, Zhao M, Han S, Lai Z, Yang J, Tan C, Ma Q, Lu Q, Chen J, Zhang X, Zhang Z, Li B, Chen B, Zong Y, Zhang H (2017) Growth of Au nanoparticles on 2D metalloporphyrinic metal-organic framework nanosheets used as biomimetic catalysts for cascade reactions. Adv Mater 29:1700102. https://doi.org/10.1002/adma.201700102
Huffman MA, Fryszkowska A, Alvizo O, Borra-Garske M, Campos KR, Canada KA, Devine PN, Duan D, Forstater JH, Grosser ST, Halsey HM, Hughes GJ, Jo J, Joyce LA, Kolev JN, Liang J, Maloney KM, Mann BF, Marshall NM, McLaughlin M, Moore JC, Murphy GS, Nawrat CC, Nazor J, Novick S, Patel NR, Rodriguez-Granillo A, Robaire SA, Sherer EC, Truppo MD, Whittaker AM, Verma D, Xiao L, Xu Y, Yang H (2019) Design of an in vitro biocatalytic cascade for the manufacture of islatravir. Science 366:1255–1259. https://doi.org/10.1126/science.aay8484
Husain S, Nandi A, Simnani FZ, Saha U, Ghosh A, Sinha A, Sahay A, Samal SK, Panda PK, Verma SK (2023) Emerging trends in advanced translational applications of silver nanoparticles: a progressing dawn of nanotechnology. J Funct Biomater 14:47–76. https://doi.org/10.3390/jfb14010047
Hwang ET, Gu MB (2013) Enzyme stabilization by nano/microsizedhybrid materials. Life Sci 13(1):49–61. https://doi.org/10.1002/elsc.201100225
Hwang ET, Lee S (2019) Multienzymatic cascade reactions via enzyme complex by immobilization. ACS Catal 9:4402–4425. https://doi.org/10.1021/acscatal.8b04921
Iftikhar FJ, Shah A, Akhter MS, Kurbanoglu S, Ozkan SA (2019) Introduction to nanosensors. In new developments in nanosensors for pharmaceutical analysis, сhapter 1. Cambridge, MA, pp 1–46. https://doi.org/10.1016/B978-0-12-816144-9.00001-8
Iijima S (1991) Helical microtubules of graphitic carbon. Nature 354:56–58. https://doi.org/10.1038/354056a0
Illum L, Farraj NF, Davis SS (1994) Chitosan as a novel nasal delivery system for peptide drugs. Pharm Res 11:1186–1189. https://doi.org/10.1023/a:1018901302450
Issaka E, Amu-Darko JNO, Adams M, Yakubu S, Gyimah E, Ali N, Cui J, Bilal M (2023) Zinc imidazolate metal–organic frameworks-8-encapsulated enzymes/nanoenzymes for biocatalytic and biomedical applications. Catal Lett 153:2083–2106. https://doi.org/10.1007/s10562-022-04140-x
Issaka E, Fapohunda FO, Amu-Darko JNO, Yeboah L, Yakubu S, Varjani S, Ali N, Bilal M (2022) Biocharbased composites for remediation of polluted wastewater and soil environments: challenges and prospects. Chemosphere 297:134163. https://doi.org/10.1016/J.CHEMOSPHERE.2022.134163
Jesionowski T, Zdarta J, Krajewska B (2014) Enzyme immobilization by adsorption: a review. Adsorption 20:801–821. https://doi.org/10.1007/s10450-014-9623-y
Jesorka A, Orwar O (2008) Liposomes: technologies and analytical applications. Annu Rev Anal Chem (Palo Alto Calif) 1:801–832. https://doi.org/10.1146/annurev.anchem.1.031207.112747
Ji P, Tan H, Xu X, Feng W (2010) Lipase covalently attached to multiwalled carbon nanotubes as an efficient catalyst in organic solvent. AIChE J 56(11):3005–3011. https://doi.org/10.1002/aic.12180
Ji Y, Wu Z, Zhang P, Qiao M, Hu Y, Shen B, Li B, Zhang X (2021) Enzyme-functionalized magnetic framework composite fabricated by one-pot encapsulation of lipase and Fe3O4 nanoparticle into metal-organic framework. Biochem Eng J 169:107962–107969. https://doi.org/10.1016/j.bej.2021.107962
Jiang H-L, Xu Q (2011) Porous metal-organic frameworks as platforms for functional applications. Chem Commun 47:3351–3370. https://doi.org/10.1039/c0cc05419d
Jiang J, Oberdörster G, Biswas P (2009) Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J Nanopart Res 11(1):77–89. https://doi.org/10.1007/s11051-008-9446-4
Jiang W, Wang X, Yang J, Han H, Li Q, Tang J (2018) Lipase-inorganic hybrid nanoflower constructed through biomimetic mineralization: a new support for biodiesel synthesis. J Colloid Interface Sci 514:102–107. https://doi.org/10.1016/j.jcis.2017.12.025
Jiang Y, Li Z, Zheng S, Xu H, Zhou YJ, Gao Z, Meng C, Li S (2020) Establishing an enzyme cascade for one-pot production of α-olefins from low-cost triglycerides and oils without exogenous H2O2 addition. Biotechnol Biofuels 13(1):52–63. https://doi.org/10.1186/s13068-020-01684-1
Jiang B, Yang К, Zhang L, Liang Z, Peng X, Zhang Y (2014) Dendrimer-grafted graphene oxide nanosheets as novel support for trypsin immobilization to achieve fast on-plate digestion of proteins. Talanta 122:278–284. https://doi.org/10.1016/j.talanta.2014.01.056
Jin K, Park J, Lee J, Yang KD, Pradhan GK, Sim U, Jeong D, Jang HL, Park S, Kim D, Sung NE, Kim SH, Han S, Nam KT (2014) Hydrated manganese (II) phosphate (Mn3(PO4)2·3H2O) as a water oxidation catalyst. J Am Chem Soc 136:7435–7443. https://doi.org/10.1021/ja5026529
Joshi HM, Bhumkar DR, Joshi K, Pokharkar V, Sastry M (2006) Gold nanoparticlesas carriers for efficient transmucosal insulin delivery. Langmuir 22:300–305. https://doi.org/10.1021/la051982u
Ju J, Li R, Gu S, Leader JK, Wang X, Chen Y, Zheng B, Wu S, Gur D, Sciurba F, Pu J (2014) Impact of emphysema heterogeneity on pulmonary function. PLoS One 9:e113320. https://doi.org/10.1371/journal.pone.0113320
Juang RS, Wu FC, Tseng RL (2001) Solute adsorption and enzyme immobilization on chitosan beads prepared from shrimp shell wastes. Bioresour Technol 80:187–193. https://doi.org/10.1016/s0960-8524(01)00090-6
Kalkan NA, Aksoy S, Aksoy EA, Hasirci N (2012) Preparation of chitosan-coated magnetite nanoparticles and application for immobilization of laccase. J Appl Polym Sci 123:707–716. https://doi.org/10.1002/app.34504
Kazenwadel F, Franzreb M, Rapp B (2015) Synthetic enzyme supercomplexes: coimmobilization of enzyme cascades. Anal Methods 7(10):4030–4037. https://doi.org/10.1039/C5AY00453E
Ke C, Fan Y, Chen Y, Xu L, Yan Y (2016) A new lipase-inorganic hybrid nanoflower with enhanced enzyme activity. RSC Adv 6:19413–19416. https://doi.org/10.1039/x0xx00000x
Khan HA, Shanker R (2015) Editorial Toxicity of Nanomaterialshttps://doi.org/10.1155/2015/521014
Khan IM, Niazi S, Akhtar W, Yue L, Pasha I, Khan MKI, Mohsin A, Iqbal MW, Zhang Y, Wang Z (2023) Surface functionalized AuNCs optical biosensor as an emerging food safety indicator: fundamental mechanism to future prospects. Coord Chem Rev 474:214842. https://doi.org/10.1016/j.ccr.2022.214842
Khorshidi KJ, Lenjannezhadian H, Jamalan M, Zeinali M (2016) Preparation and characterization of nanomagnetic cross-linked cellulase aggregates for cellulose bioconversion. J Chem Technol Biotechnol 91:539–546. https://doi.org/10.1002/jctb.4615
Kilic NM, Singh S, Keles G, Cinti S, Kurbanoglu S, Odaci D (2023) Novel approaches to enzyme-based electrochemical nanobiosensors. Biosensors 13(6):622. https://doi.org/10.3390/bios13060622
Kim K, Kim HJ, Oh DK, Cha SS, Rhee S (2006) Crystal structure of D-psicose 3-epimerase from Agrobacterium tumefaciens and its complex with true substrate D-fructose: a pivotal role of metal in catalysis, an active site for the nonphosphorylated substrate, and its conformational changes. J Mol Biol 361:920–931. https://doi.org/10.1016/j.jmb.2006.06.069
Kim KH, Jeong JM, Lee SJ, Choi BG, Lee KG (2016) Protein-directed assembly of cobalt phosphate hybrid nanoflowers. J Colloid Interface Sci 484:44–50. https://doi.org/10.1016/j.jcis.2016.08.059
Klein MP, Nunes MR, Rodrigues RC, Benvenutti EV, Costa TMH, Hertz PF, Ninow JL (2012) Effect of the support size on the properties of β-galactosidase immobilized on chitosan: advantages and disadvantages of macro and nanoparticles. Biomacromol 13:2456–2464. https://doi.org/10.1021/bm3006984
Kokufuta E, Takahashi K (1986) Adsorption of poly(diallyldimethylammonium chloride) on colloid silica from water and salt solution. Macromolecules 19:351–354. https://doi.org/10.1021/ma00156a019
Kopp W, da Costa TP, Pereira SC, Jr MJ, Giordano RC, Marques RFC, Araujo-Moreira FM, Giordano RLC (2014) Easily handling penicillin g acylase magnetic cross-linked enzymes aggregates: catalytic and morphological studies. Process Biochem 49:38-46.https://doi.org/10.1016/j.procbio.2013.09.024
Kumar A, Sevonkaev I, Goia DV (2014) Synthesis of selenium particles with various morphologies. J Colloid Interface Sci 416:119–123. https://doi.org/10.1016/j.jcis.2013.10.046
Laurienzo P (2010) Marine polysaccharides in pharmaceutical applications: an overview. Mar Drugs 8:2435–2465. https://doi.org/10.3390/md8092435
Lee HR, Chung M, Kim MI, Ha SH (2017) Preparation of glutaraldehyde-treated lipaseinorganic hybrid nanoflowers and their catalytic performance as immobilized enzymes. Enzym Microb Technol 105:24–29. https://doi.org/10.1016/j.enzmictec.2017.06.006
Lee SW, Cheon SA, Kim MI, Park TJ (2015) Organic-inorganic hybrid nanoflowers: types, characteristics, and future prospects. J Nanobiotechnol 13:54. https://doi.org/10.1186/s12951-015-0118-0
Lei Z, Gao C, Chen L, He Y, Ma W, Lin Z (2018) Recent advances in biomolecule immobilization based on self-assembly: organic-inorganic hybrid nanoflowers and metal-organic frameworks as novel substrates. J Mater Chem B 6:1581–1594. https://doi.org/10.1039/c7tb03310a
Leitgeb M, Knez Z, Vasic K (2016) In Micro and Nanotechnologies for Biotechnology: Micro- and nanocarriers for immobilization of enzymes. London, pp 1–38. https://doi.org/10.5772/63129
Li L, Xiang S, Cao S, Zhang J, Ouyang G, Chen L, Su CY (2013) A synthetic route to ultralight hierarchically micro/mesoporous Al(III)-carboxylate metal-organic aerogels. Nat Commun 4:1774-1773. https://doi.org/10.1038/ncomms2757
Li P, Moon SY, Guelta MA, Lin L, Gomez-Gualdron DA, Snurr RQ, Harvey SP, Hupp JT, Farha OK (2016) Nanosizing a metal-organic framework enzyme carrier for accelerating nerve agent hydrolysis. ACS Nano 10:9174–9182. https://doi.org/10.1021/acsnano.6b04996
Li W, Shi J, Chen Y, Liu X, Meng X, Guo Z, Li S, Zhang B, Jiang Z (2023) Nano-sized mesoporous hydrogen-bonded organic frameworks for in situ enzyme immobilization. Chem Eng J 468:143609. https://doi.org/10.1016/j.cej.2023.143609
Li X, Cao Y, Luo K, Sun Y, Xiong J, Wang L, Liu Z, Li J, Ma J, Ge J, Xiao H, Zare RN (2019a) Highly active enzyme–metal nanohybrids synthesized in protein–polymer conjugates. Nat Catal 2(8):718–725. https://doi.org/10.1038/s41929-019-0305-8
Li Y, Wan W (2017) Exploring Polymer Nanofiber Mechanics: a review of the methods for determining their properties. IEEE Nanotechnol Mag 11:16–28. https://doi.org/10.1109/MNANO.2017.2708819
Li Y, Wang Q, Ding Z, Wan D, Nie X, Zhong C (2022) A functionalized magnetic graphene-based MOFs platform as the heterogeneous mimic enzyme sensor for glucose detection. Catal Lett 152:2375–2385. https://doi.org/10.1007/s10562-021-03815-1
Li Y, Wen L, Tan T, Lv Y (2019b) Sequential co-immobilization of enzymes in metal-organic frameworks for efficient biocatalytic conversion of adsorbed CO2 to formate. Front Bioeng Biotechnol 7:394–407. https://doi.org/10.3389/fbioe.2019.00394
Li Z, Zhang Y, Su Y, Ouyang P, Ge J, Liu Z (2014) Spatial co-localization of multienzymes by inorganic nanocrystal-protein complexes. Chem Commun 50:12465–12468. https://doi.org/10.1039/c4cc05478d
Lian X, Fang Y, Joseph E, Wang Q, Li J, Banerjee S, Zhou H-C (2011) Enzyme-MOF (metal-organic framework) composites. Chem Soc Rev 46:3386–3401. https://doi.org/10.1039/c7cs00058h
Lian X, Fang Yu, Joseph E, Wang Q, Li J, Banerjee S, Lollar C, Wanga X, Zhou HC (2017) Enzyme–MOF (metal–organic framework) composites. Chem Soc Rev 46:3386–3401. https://doi.org/10.1039/C7CS00058H
Liang J, Liang K (2020) Multi-enzyme cascade reactions in metal-organic frameworks. Chem Rec 20:1100–1116. https://doi.org/10.1002/TCR.202000067
Liang W, Carraro F, Solomon MB, Bell SG, Amenitsch H, Sumby CJ, White NG, Falcaro P, Doonan CJ (2019) Enzyme encapsulation in a porous hydrogenbonded organic framework. J Am Chem Soc 141:14298–14305. https://doi.org/10.1021/jacs.9b06589
Liao FS, Lo WS, Hsu YS, Wu CC, Wang SC, Shieh FK, Morabito JV, Chou LY, Wu KC, Tsung CK (2017) Shielding against unfolding by embedding enzymes in metal-organic frameworks via a de novo approach. J Am Chem 139:6530–6533. https://doi.org/10.1021/jacs.7b01794
Liao H, Nehl CL, Hafner JH (2006) Biomedical applications of plasmon resonant metal nanoparticles. Nanomedicine (London) 1:201–208. https://doi.org/10.2217/17435889.1.2.201
Lin C, Xu K, Zheng R, Zheng Y (2019) Immobilization of amidase into a magnetic hierarchically porous metal-organic framework for efficient biocatalysis. Chem Commun 55:5697–5700. https://doi.org/10.1039/c9cc02038a
Liu D, Yang F, Xiong F, Gu N (2016) The smart drug delivery system and its clinical potential. Theranostics 6:1306–1323. https://doi.org/10.7150/thno.14858
Liu J, Ma R-T, Shi Y-P (2020) «Recent advances on support materials for lipase immobilization and applicability as biocatalysts in inhibitors screening methods» - a review. Anal Chim Acta 1101–1166:9–22. https://doi.org/10.1016/j.aca.2019.11.073
Liu X, Liu Y, Wang J, Wei T, Dai Z (2019) Mild hyperthermia-enhanced enzyme-mediated tumor cell chemodynamic therapy. ACS Appl Mater Interfaces 11(26):23065–23071. https://doi.org/10.1021/acsami.9b08257
Liu Y, Cao X, Ge J (2021) Antioxidative composites based on multienzyme systems encapsulated in metal-organic frameworks. ACS Appl Mater Interfaces 13:46431–46439. https://doi.org/10.1021/ACSAMI.1C15506/SUPPL_FILE/AM1C15506_SI_001
Liu Y, Chen J, Du M, Wang X, Ji X, He Z (2017a) The preparation of dual-functional hybrid nanoflower and its application in the ultrasensitive detection of diseaserelated biomarker. Biosens Bioelectron 92:68–73. https://doi.org/10.1016/j.bios.2017.02.004
Liu Y, Guo C, Liu CZ (2015) Enhancing the resolution of (R, S)-2-octanol catalyzed by magnetic crosslinked lipase aggregates using an alternating magnetic field. Chem Eng J 280:36–40. https://doi.org/10.1016/j.cej.2015.05.089
Liu Y, Zhang Y, Li X, Yuan Q, Liang H (2017b) Self-repairing metal-organic hybrid complexes for reinforcing immobilized chloroperoxidase reusability. Chem Commun 53:3216–3219. https://doi.org/10.1039/c6cc10319g
Liu Y, Zou P, Huang J, Cai J (2022) Co-immobilization of glucose oxidase and catalase in porous magnetic chitosan microspheres for production of sodium gluconate. Int J Chem React Eng 20:989–1001. https://doi.org/10.1515/ijcre-2021-0237
Long W-J (2023) Design, performance, and mechanism of cement-based materials with 2D nanomaterials. Nanotechnol Civil Infrastructure 127–159. https://doi.org/10.1016/B978-0-12-817832-4.00001-8
López-Gallego F, Yate L (2015) Selective biomineralization of Co3(PO4)2-sponges triggered by His-tagged proteins: efficient heterogeneous biocatalysts for redox processes. Chem Commun 51:8753–8756. https://doi.org/10.1039/C5CC00318K
Lu J, Weerasiri RR, Lee I (2013) Carbon nanotubes tuned foam structures as novel nanostructured biocarriers for lignocellulose hydrolysis. Biotechnol Lett 35:181–188. https://doi.org/10.1007/s10529-012-1066-5
Lu Z, Wu L, Zhang J, Dai W, Mo G, Ye J (2019) Bifunctional and highly sensitive electrochemical non-enzymatic glucose and hydrogen peroxide biosensor based on NiCo2O4 nanoflowers decorated 3D nitrogen doped holey graphene hydrogel. Mater Sci Eng C 102:708–717. https://doi.org/10.1016/j.msec.2019.04.072
Luesakul U, Komenek S, Puthong S, Muangsin N (2016) Shape-controlled synthesis of cubic-like selenium nanoparticles via the self-assembly method. Carbohydr Polym 153:435–444. https://doi.org/10.1016/j.carbpol.2016.08.004
Lundqvist M, Sethson I, Jonsson B-H (2004) Protein adsorption onto silica nanoparticles: conformational changes depend on the particles’ curvature and the protein stability. Langmuir 20(24):10639–10647. https://doi.org/10.1021/la0484725
Lyu F, Zhang Y, Zare RN, Ge J, Liu Z (2014) One-pot synthesis of protein-embedded metal-organic frameworks with enhanced biological activities. Nano Lett 14:5761–5765. https://doi.org/10.1021/NL5026419/SUPPL_FILE/NL5026419_SI_001
Ma Y-X, Li Y-F, Zhao G-H, Yang L-Q, Wang J-Z, Shan X, Yan X (2012) Preparation and characterization of graphite nanosheets decorated with Fe3O4 nanoparticles used in the immobilization of glucoamylase. Carbon 50:2976–2986. https://doi.org/10.1016/j.carbon.2012.02.080
Magner E (2013) Immobilisation of enzymes on mesoporous silicate materials Chem. Soc Rev 42:6213–6222. https://doi.org/10.1039/c2cs35450k
Majewski MB, Howarth AJ, Li P, Wasielewski MR, Hupp JT, Farha OK (2017) Enzyme encapsulation in metal-organic frameworks for applications in catalysis. Cryst Eng Comm 19:4082–4091. https://doi.org/10.1039/c7ce00022g
Majumdar S, Keller AA (2021) Omics to address the opportunities and challenges of nanotechnology in agriculture. Crit Rev Environ Sci Technol 51:157. https://doi.org/10.1080/10643389.2020.1785264
Makarova AO, Derkach SR, Kadyirov AI, Ziganshina SA, Kazantseva MA, Zueva OS, Gubaidullin AT, Zuev YuF (2022) Supramolecular structure and mechanical performance of k-carrageenan–gelatin gel. Polymers 14(20):4347. https://doi.org/10.3390/polym14204347
Makarova AO, Derkach SR, Khair T, Kazantseva MA, Zuev YuF, Zueva OS (2023) Ion-induced polysaccharide gelation: peculiarities of alginate egg-box association with different divalent cations. Polymers 15:1243. https://doi.org/10.3390/polym15051243
Makshakova O, Antonova M, Bogdanova L, Faizullin D, Zuev Yu (2023) Regulation of intersubunit interactions in homotetramer of glyceraldehyde-3-phosphate dehydrogenases upon its immobilization in protein-kappa-carrageenan gels. Polymers 15:676. https://doi.org/10.3390/polym15030676
Makshakova ON, Bogdanova LR, Makarova AO, Kusova AM, Ermakova EA, Kazantseva MA, Zuev YuF (2022) k-Carrageenan Hydrogel as a Matrix for Therapeutic Enzyme Immobilization. Polymers 14:4071. https://doi.org/10.3390/polym14194071
Makshakova ON, Zuev YuF (2022) Interaction-induced structural transformations in polysaccharide and protein-polysaccharide gels as functional basis for novel soft-matter: a case of carrageenans. Gels 8:287. https://doi.org/10.3390/gels8050287
Malykhina NV, Olshannikova SS, Holyavka MG, Sorokin AV, Lavlinskaya MS, Artyukhov VG, Faizullin DA, Zuev YuF (2022) Preparation of ficin complexes with carboxymethylchitosan and n-(2-hydroxy)propyl-3-trimethylammoniumchitosan and studies of their structural features. Russ J Bioorganic Chem 48:50–60. https://doi.org/10.1134/S1068162022060176
Manning GS (1969) Limiting laws and counterion condensation in polyelectrolyte solutions I. Colligative Properties. J Chem Phys 51:924–933. https://doi.org/10.1063/1.1672157
Mas-Balleste R, Gomez-Navarro C, Gomez-Herrero J, Zamora F (2011) 2D materials: to graphene and beyond. Nanoscale 3:20–30. https://doi.org/10.1039/c0nr00323a
Mayer KM, Hafner JH (2011) Localized surface plasmon resonance sensors. Chem Rev 111:3828–3857. https://doi.org/10.1021/cr100313v
Mayer SF, Kroutil W, Faber K (2001) Enzyme-initiated domino (cascade) reactions. Chem Soc Rev 30:332–339. https://doi.org/10.1039/B105493G
McClements DJ (2011) Edible nanoemulsions: fabrication, properties, and functional performance. Soft Matter 7:2297–2316. https://doi.org/10.1039/c0sm00549e
McNamara LE, McMurray RJ, Biggs MJP, Kantawong F, Oreffo ROC, Dalby VJ (2010) Nanotopographical control of stem cell differentiation. J Tissue Eng 2010:120623–120636. https://doi.org/10.4061/2010/120623
Mehta J, Bhardwaj N, Bhardwaj SK, Kim K-H, Deep A (2016) Recent advances in enzyme immobilization techniques: metal-organic frameworks as novel substrates. Coord Chem Rev 322:30–40. https://doi.org/10.1016/j.ccr.2016.05.007
Meng XG, Guo Y, Hu CW, Zeng XC (2004) Mimic models of peroxidase-kinetic studies of the catalytic oxidation of hydroquinone by H2O2. J Inorg Biochem 98:2107–2113. https://doi.org/10.1016/j.jinorgbio.2004.09.019
Menzies KL, Jones L (2010) The impact of contact angle on the biocompatibility of biomaterials. Optom Vis Sci 87:387–399. https://doi.org/10.1097/OPX.0b013e3181da863e
Mezzenga R, Schurtenberger P, Burbidge A, Michel M (2005) Understanding foods as soft materials. Nat Mater 4:729–740. https://doi.org/10.1038/nmat1496
Misson M, Zhang H, Jin B (2015) Nanobiocatalyst advancements and bioprocessing applications. J R Soc Interface 12(102):20140891. https://doi.org/10.1098/rsif.2014.0891
Mohajerani A, Burnett L, Smith J, Kurmus H, Milas J, Arulrajah A, Horpibulsuk S, Kadir AA (2019) Materials review nanoparticles in construction materials and other applications, and implications of nanoparticle use. https://doi.org/10.3390/ma12193052
Mora-Huertas CE, Fessi H, Elaissari A (2010) Polymer-based nanocapsules for drug delivery. Int J Pharm 385:113–142. https://doi.org/10.1016/j.ijpharm.2009.10.018
Morris W, Briley WE, Auyeung E, Cabezas MD, Mirkin CA (2014) Nucleic acid-metal organic framework (MOF) nanoparticle conjugates. J Am Chem Soc 136:7261–7264. https://doi.org/10.1021/ja503215w
Morshedi M, Thomas M, Tarzia A, Doonan CJ, White NG (2017) Supramolecular anion recognition in water: synthesis of hydrogen-bonded supramolecular frameworks. Chem Sci 8:3019–3025. https://doi.org/10.1039/c7sc00201g
Motamedi N, Barani M, Lohrasbi-Nejad A, Mortazavi M, Riahi-Medvar A, Varma RS, Torkzadeh-Mahani M (2021) Enhancement of thermostability of Aspergillus flavus urate oxidase by immobilization on the Ni-based magnetic metal-organic framework. Nanomaterials 11:1759–1779. https://doi.org/10.3390/nano11071759
Moyano DF, Rotello VM (2011) Nano meets biology: structure and function at the nanoparticle interface. Langmuir 27:10376–10385. https://doi.org/10.1021/la2004535
Muley AB, Thorat AS, Singhal RS, Babu KH (2018) A tri-enzyme co-immobilized magnetic complex: process details, kinetics, thermodynamics and applications. Int J Biol Macromol 118:1781–1795. https://doi.org/10.1016/j.ijbiomac.2018.07.022
Müller RH, Mäder K, Gohla S (2000) Solid lipid nanoparticles (SLN) for controlled drug delivery-a review of the state of the art. Eur J Pharm Biopharm 50:161–177. https://doi.org/10.1016/s0939-6411(00)00087-4
Munyemana JC, He H, Ding S, Yin J, Xi P, Xiao J (2018) Synthesis of manganese phosphate hybrid nanoflowers by collagen-templated biomineralization. RSC Adv 8:2708–2713. https://doi.org/10.1039/c7ra12628j
Nadar SS, Gawas SD, Rathod VK (2016) Self-assembled organic-inorganic hybrid glucoamylase nanoflowers with enhanced activity and stability. Int J Biol Macromol 92:660–669. https://doi.org/10.1016/j.ijbiomac.2016.06.071
Naha A, Antony S, Nath S, Sharma D, Mishra A, Biju DT, Madhavan A, Binod P, Varjani S, Sindhu R (2023) A hypothetical model of multi-layered cost-effective wastewater treatment plant integrating microbial fuel cell and nanofiltration technology: a comprehensive review on wastewater treatment and sustainable remediation. Environ Pollut 121274. https://doi.org/10.1016/j.envpol.2023.121274
Negahdary M, Heli H (2018) Applications of nanoflowers in biomedicine. Recent Pat Nanotechnol 12:22–33. https://doi.org/10.2174/1872210511666170911153428
Nematian T, Shakeri A, Salehi Z, Saboury AA (2020) lipase immobilized on functionalized superparamagnetic few-layer graphene oxide as an efficient nanobiocatalyst for biodiesel production from Chlorella vulgaris bio-oil. Biotechnol Biofuels 13:57–72. https://doi.org/10.1186/s13068-020-01688-x
Netto CGCM, Toma HE, Andrade LH (2013) Superparamagnetic nanoparticles as versatile carriers and supporting materials for enzymes. J Mol Catal B: Enzym 85–86:71–92. https://doi.org/10.1016/j.molcatb.2012.08.010
Neupane S, Patnode K, Li H, Baryeh K, Liu G, Hu J, Chen B, Pan Y, Yang Z (2019) Enhancing enzyme immobilization on carbon nanotubes via metal-organic frameworks for large-substrate biocatalysis. ACS Appl Mater Interfaces 11(12):12133–12141. https://doi.org/10.1021/acsami.9b01077
Nguyen MM, Carlini AS, Chien MP, Sonnenberg S, Luo C, Braden RL, Osborn KG, Li Y, Gianneschi NC, Christman KL (2015) Enzyme-responsive nanoparticles for targeted accumulation and prolonged retention in heart tissue after myocardial infarction. Adv Mater 27:5547–5552. https://doi.org/10.1002/adma.201502003
Ni W, Zhang P, Long L, Ding S (2022) Engineering and linker-mediated co-immobilization of carotenoid cleavage oxygenase with phenolic acid decarboxylase for efficiently converting ferulic acid into vanillin. Process Biochem 122:67–77. https://doi.org/10.1016/j.procbio.2022.08.020
Nicoletti G, Cipolatti EP, Valerio A, Carbonera NTG, Soares NS, Theilacker E, Ninow JL, de Oliveira D (2015) Evaluation of different methods for immobilization of Candida antarctica lipase B (CalB lipase) in polyurethane foam and its application in the production of geranyl propionate. Bioprocess Biosyst Eng 38:1739–1748. https://doi.org/10.1007/s00449-015-1415-6
Nonsuwan P, Puthong S, Palaga T, Muangsin N (2018) Novel organic/inorganic hybrid flower-like structure of selenium nanoparticles stabilized by pullulan derivatives. Carbohydr Polym 184:9–19. https://doi.org/10.1016/j.carbpol.2017.12.029
Novoselov KS, Geim AK, Morozov SV, Jiang D, Katsnelson MI, Grigorieva IV, Dubonos SV, Firsov AA (2005) Two-dimensional gas of massless Dirac fermions in graphene. Nature 438:197–200. https://doi.org/10.1038/nature04233
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field effect in atomically thin carbon films. Science 306:666–669. https://doi.org/10.1126/science.1102896
Obayomi KS, Lau SY, Danquah M, Chiong T, Takeo M (2022) Advances in graphene oxide based nanobiocatalytic technology for wastewater treatment. Environmental Nanotechnology, Monitoring & Management 17:100647. https://doi.org/10.1016/j.enmm.2022.100647
Ó’Fágáin C (2003) Enzyme stabilization-recent experimental progress. Enzyme Microb Technol 33(2–3):137–149. https://doi.org/10.1016/S0141-0229(03)00160-1
Ocsoy I, Dogru E, Usta S (2015) A new generation of flowerlike horseradish peroxides as a nanobiocatalyst for superior enzymatic activity. Enzym Microb Technol 75:25–29. https://doi.org/10.1016/j.enzmictec.2015.04.010
Olshannikova SS, Redko YuA, Lavlinskaya MS, Sorokin AV, Holyavka MG, Yudin NE, Artyukhov VG (2022) Study of the proteolytic activity of ficin associates with chitosan nanoparticles. Condensed Matter and Interphases 24:523–528. https://doi.org/10.17308/kcmf.2022.24/10556
Padilla-Martínez SG, Martínez-Jothar L, Sampedro JG, Tristan F, Pérez E (2015) Enhanced thermal stability and PH behavior of glucose oxidase on electrostatic interaction with polyethylenimine. Int J Biol Macromol 75:453–459. https://doi.org/10.1016/j.ijbiomac.2015.02.005
Pakchin PS, Ghanbari H, Saber R, Omidi Y (2018) Electrochemical immunosensor based on chitosan-gold nanoparticle/carbon nanotube as a platform and lactate oxidase as a label for detection of CA125 oncomarker. Biosens Bioelectron 122:68–74. https://doi.org/10.1016/j.bios.2018.09.016
Palanisamy S, Ramaraj SK, Chen S-M, Yang TCK, Yi-Fan P, Chen T-W, Velusamy V, Selvam S (2017) A novel laccase biosensor based on laccase immobilized graphene-cellulose microfiber composite modified screen-printed carbon electrode for sensitive determination of catechol. Sci Rep 7(1):1–12. https://doi.org/10.1038/srep41214
Patel SK, Otari SV, Li J, Kim DR, Kim SC, Cho BK, Kalia VC, Kang YC, Lee JK (2018) Synthesis of crosslinked protein-metal hybrid nanoflowers and its application in repeated batch decolorization of synthetic dyes. J Hazard Mater 347:442–450. https://doi.org/10.1016/j.jhazmat.2018.01.003
Paukov M, Kramberger C, Begichev I, Kharlamova M, Burdanova M (2023) Functionalized fullerenes and their applications in electrochemistry, solar cells, and nanoelectronics. Materials 16:1276–1297. https://doi.org/10.3390/ma16031276
Pavlidis IV, Patila M, Bornscheuer UT, Gournis D, Stamatis H (2014) Graphene-based nanobiocatalytic systems: recent advances and future prospects. Trends in Biotechnol 32:312–320. https://doi.org/10.1016/j.tibtech.2014.04.004
Peng M, Li H, Luo Z, Kong J, Wan Y, Zheng L, Zhang Q, Niu H, Vermorken A, Van de Ven W, Chen C, Zhang X, Li F, Guo L, Cui Y (2015) Dextran-coated superparamagnetic nanoparticles as potential cancer drug carriers in vivo. Nanoscale 7:11155–11162. https://doi.org/10.1039/c5nr01382h
Pospiskova K, Safarik I (2015) Magnetically responsive enzyme powders. Magn Magn Mater 380:197–200. https://doi.org/10.1016/j.jmmm.2014.10.037
Pourmadadi M, Rahmani E, Rajabzadeh-Khosroshahi M, Samadi A, Behzadmehr R, Rahdar A, Ferreira LFR (2023) Properties and application of carbon quantum dots (CQDs) in biosensors for disease detection: a comprehensive review. J Drug Delivery Sci Technol 80:104156–104170. https://doi.org/10.1016/j.jddst.2023.104156
Povedano E, Cincotto FH, Parrado C, Díez P, Sánchez A, Canevari TC, Machado SAS, Pingarrón JM, Villalonga R (2017) Decoration of reduced graphene oxide with rhodium nanoparticles for the design of a sensitive electrochemical enzyme biosensor for 17β-estradiol. Biosens Bioelectron 89:343–351. https://doi.org/10.1016/j.bios.2016.07.018
Qiu H, Li Y, Ji G, Zhou G, Huang X, Qu Y, Gao P (2009) Immobilization of lignin peroxidase on nanoporous gold: enzymatic properties and in situ release of H2O2 by co-immobilized glucose oxidase. Bioresour Technol 100:3837–3842. https://doi.org/10.1016/j.biortech.2009.03.016
Quin MB, Wallin KK, Zhang G, Schmidt-Dannert C (2017) Spatial organization of multi-enzyme biocatalytic cascades. Org Biomol Chem 15(20):4260–4271. https://doi.org/10.1039/c7ob00391a
Rai SK, Narnoliya LK, Sangwan RS, Yadav SK (2018) Self-assembled hybrid nanoflowers of manganese phosphate and L-arabinose isomerase: a stable and recyclable nanobiocatalyst for equilibrium level conversion of D-galactose to Dtagatose. ACS Sustain Chem Eng 6:6296–6304. https://doi.org/10.1021/acssuschemeng.8b00091
Raita M, Arnthong J, Champreda V, Laosiripojana N (2015) Modification of magnetic nanoparticle lipase designs for biodiesel production from palm oil. Fuel Process Technol 134:189–197. https://doi.org/10.1016/j.fuproc.2015.01.032
Ramos AP, Cruz MAE, Tovani CB, Ciancaglini P (2017) Biomedical applications of nanotechnology. Biophys Rev 9:79–89. https://doi.org/10.1007/s12551-016-0246-2
Razumovsky SD, Efremenko EN, Makhlis TA, Senko OV, Bikhovsky MY, Podmasterev VV, Varfolomeev SD (2008) Effect of immobilization on the main dynamic characteristics of the enzymatic oxidation of methane to methanol by bacteria Methylosinus sporium B-2121. Russ Chem Bull, Int Ed 57:1633–1636. https://doi.org/10.1007/s11172-008-0211-8
Redko YuA, Olshannikova SS, Holyavka MG, Lavlinskaya MS, Sorokin AV, Artyukhov VG (2022) Development of a method for obtaining bromelain associates with chitosan micro- and nanoparticles. Pharm Chem J 56:984–988. https://doi.org/10.1007/s11094-022-02737-5
Reis CLB, de Sousa EYA, de Franca SJ, Oliveira RC, dos Santos JCS (2019) Design of immobilized enzyme biocatalysts: drawbacks and opportunities. Quim Nova 42:768–783. https://doi.org/10.21577/0100-4042.20170381
Ren S, Wang Z, Bilal M, Feng Y, Jiang Y, Jia S, Cui J (2020) Co-immobilization multienzyme nanoreactor with co-factor regeneration for conversion of CO2. Int J Biol Macromol 155:110–118. https://doi.org/10.1016/j.ijbiomac.2020.03.177
Ren SZ, Li CH, Jiao XB, Jia SR, Jiang YJ, Bilal M, Cui J (2019) Recent progress in multienzymes co-immobilization and multienzyme system applications. Chem Eng J 373:1254–1278. https://doi.org/10.1016/j.cej.2019.05.141
Rezaei B, Shoushtari AM, Rabiee M, Uzun L, Mak WC, Turner APF (2018) An electrochemical immunosensor for cardiac Troponin I using electrospun carboxylated multi-walled carbon nanotube-whiskered nanofibres. Talanta 182:178–186. https://doi.org/10.1016/j.talanta.2018.01.046
Rouhani S, Azizi S, Kibechu RW, Mamba BB, Msagati TAM (2020) Laccase immobilized Fe3O4-graphene oxide nanobiocatalyst improves stability and immobilization efficiency in the green preparation of sulfa drugs. Catalysts 10(4):459–474. https://doi.org/10.3390/catal10040459
Rulı́šek L, Vondrášek J (1998) Coordination geometries of selected transition metal ions (Co2+, Ni2+, Cu2+, Zn2+, Cd2+, and Hg2+) in metalloproteins. J Inorg Biochem 71:115–127. https://doi.org/10.1016/s0162-0134(98)10042-9
Sahoo B, Sahu SK, Pramanik P (2011) A novel method for the immobilization of urease on phosphonate grafted iron oxide nanoparticle. J Mol Catal B: Enzym 69:95–102. https://doi.org/10.1016/j.molcatb.2011.01.001
Saji VS, Choe HC, Young KWK (2010) Nanotechnology in biomedical applications-a review. Int J Nano Biomater 3:119–139. https://doi.org/10.1504/IJNBM.2010.037801
Sargazi G, Afzali D, Ebrahimi AK, Badoei-Dalfard A, Malekabadi S, Karami Z (2018) Ultrasound assisted reverse micelle efficient synthesis of new Ta-MOF@Fe3O4 core/shell nanostructures as a novel candidate for lipase immobilization. Mater Sci Eng C 93:768–775. https://doi.org/10.1016/j.msec.2018.08.041
Senko O, Gladchenko M, Maslova O, Efremenko E (2019) Long-term storage and use of artificially immobilized anaerobic sludge as a powerful biocatalyst for conversion of various wastes including those containing xenobiotics to biogas. Catalysts 9:326–346. https://doi.org/10.3390/catal9040326
Shaarani S, Jahim J, Rahman RA, Idris A, Munir A, Murad IR (2016) Silanized maghemite for cross-linked enzyme aggregates of recombinant xylanase from Trichoderma reesei. J Mol Catal B: Enzym 133:65–76. https://doi.org/10.1016/j.molcatb.2016.07.006
Shahedi M, Habibi Z, Yousefi M, Brask J, Mohammadi M (2021) Improvement of biodiesel production from palm oil by coimmobilization of Thermomyces lanuginosa lipase and Candida antarctica lipase B: optimization using response surface methodology. Int J Biol Macromol 170:490–502. https://doi.org/10.1016/j.ijbiomac.2020.12.181
Shalova IN, Naletova IN, Saso L, Muronetz VI, Izumrudov VA (2007) Interaction of polyelectrolytes with proteins, 3 influence of complexing polycations on the thermoaggregation of oligomeric enzymes. Macromol Biosci 7:929–939. https://doi.org/10.1002/mabi.200700052
Shanta AS, Al Mamun KA, Islam SK, McFarlane N, Hensley DK (2017) Carbon nanotubes, nanofibers and nanospikes for electrochemical sensing: A review. Int J High Speed Electron Syst 26(3):1740008. https://doi.org/10.1142/S0129156417400080
Sharifi M, Sohrabi MJ, Hosseinali SH, Hasan A, Kani PH, Talaei AJ, Karim AY, Nanakali NMQ, Salihi A, Aziz FM, Yan B, Khan RH, Saboury AA, Falahati M (2020) Enzyme immobilization onto the nanomaterials: application in enzyme stability and prodrug-activated cancer therapy. Int J Biol Macromol 143:665–676. https://doi.org/10.1016/j.ijbiomac.2019.12.064
Sheldon RA (2010) Cross-linked enzyme aggregates as industrial biocatalysts. Org Process Res Dev 15:213–223. https://doi.org/10.1021/op100289f
Sheldon RA, Basso A, Brady D (2021) New frontiers in enzyme immobilisation: robust biocatalysts for a circular bio-based economy. Chem Soc Rev 50:5850–5862. https://doi.org/10.1039/d1cs00015b
Sheldon RA, Woodley JM (2018) Role of biocatalysis in sustainable chemistry. Chem Rev 118:801–838. https://doi.org/10.1021/acs.chemrev.7b00203
Shende P, Kasture P, Gaud RS (2018) Nanoflowers: the future trend of nanotechnology for multi-applications. Artif Cells Nanomed Biotechnol 46:413–422. https://doi.org/10.1080/21691401.2018.1428812
Shikama Y, Kitazawa JI, Yagihashi N, Uehara O, Murata Y, Yajima N, Wada R, Yagihashi S (2010) Localized amyloidosis at the site of repeated insulin injection in a diabetic patient. Intern Med 49:397–401. https://doi.org/10.2169/internalmedicine.49.2633
Shola OK, Yon JLS, Danquah M, Chiong T, Takeo M (2022) Advances in graphene oxide based nanobiocatalytic technology for wastewater treatment. Environ Nanotechnol Monit Manage 17:100647. https://doi.org/10.1016/j.enmm.2022.100647
Shukla SK, Mishra AK, Arotiba OA, Mamba BB (2013) Chitosan-based nanomaterials: a state-of-the-art review. Int J Biol Macromol 59:46–58. https://doi.org/10.1016/j.ijbiomac.2013.04.043
Singh V, Kaul S, Singla P, Kumar V, Sandhir R, Chung JH, Garg P, Singhal NK (2018) Xylanase immobilization on magnetite and magnetite core/shell nanocomposites using two different flexible alkyl length organophosphonates: linker length and shell effect on enzyme catalytic activity. Int J Biol Macromol 115:590–599. https://doi.org/10.1016/j.ijbiomac.2018.04.097
Somturk B, Hancer M, Ocsoy I, Özdemir N (2015) Synthesis of copper ion incorporated horseradish peroxidase-based hybrid nanoflowers for enhanced catalytic activity and stability. Dalton Trans 44:13845–13852. https://doi.org/10.1039/c5dt01250c
Soozanipour A, Taheri-Kafrani A, Isfahani AL (2015) Covalent attachment of xylanase on functionalized magnetic nanoparticles and determination of its activity and stability. Chem Eng J 270:235–243. https://doi.org/10.1016/j.cej.2015.02.032
Souto EB, Müller RH (2007) Lipid nanoparticles (solid lipid nanoparticles and nanostructured lipid carriers) for cosmetic, dermal, and transdermal applications. drugs and the pharmaceutical sciences. a series of textbooks and monographs. Executive Editor James Swarbrick. PharmaceuTech, Inc. Pinehurst, North Carolina. https://doi.org/10.1201/9781420008449.ch14
Stefani M, Dobson CM (2003) Protein aggregation and aggregate toxicity: new insights into protein folding, misfolding diseases and biological evolution. J Mol Med 81:678–699. https://doi.org/10.1007/s00109-003-0464-5
Stern A, Rotem D, Popov I, Porath D (2012) Quasi 3D imaging of DNA-gold nanoparticle tetrahedral structures. J Phys Condens Matter 24:164203–164209. https://doi.org/10.1088/0953-8984/24/16/164203
Stine KJ (2017) Enzyme immobilization on nanoporous gold: A review. Biochem Insights 10:1178626417748607. https://doi.org/10.1177/1178626417748607
Stocke NA, Arnold SM, Hilt JZ (2015) Responsive hydrogel nanoparticles for pulmonary delivery. J Drug Deliv Sci Technol 29:143–151. https://doi.org/10.1016/j.jddst.2015.06.013
Su Y, Zhou X, Long Y, Li W (2018) Immobilization of horseradish peroxidase on amino-functionalized carbon dots for the sensitive detection of hydrogen peroxide. Microchim Acta 185(2):1–8. https://doi.org/10.1007/s00604-017-2629-x
Sun J, Ge J, Liu W, Lan M, Zhang H, Wang P, Wang Y, Niu Z (2014) Multi-enzyme coembedded organic-inorganic hybrid nanoflowers: synthesis and application as a colorimetric sensor. Nanoscale 6:255–262. https://doi.org/10.1039/c3nr04425d
Sur S, Rathore A, Dave V, Reddy KR, Chouhan RS, Sadhu V (2019) Recent developments in functionalized polymer nanoparticles for efficient drug delivery system. Nano-Struct & Nano-Objects 20:100397–100418. https://doi.org/10.1016/j.nanoso.2019.100397
Syed F, Ali K, Asad MJ, Fraz MG, Khan Z, Imran M, Taj R, Ahmad A (2016) Preparation and characterization of a green nano-support for the covalent immobilization of glucoamylase from Neurospora sitophila. J Photochem Photobiol B 162:309–317. https://doi.org/10.1016/j.jphotobiol.2016.07.002
Takimoto A, Shiomi T, Ino K, Tsunoda T, Kawai A, Mizukami F, Sakaguchi K (2008) Encapsulation of cellulase with mesoporous silica (SBA-15). Microporous Mesoporous Mater 116:601–606. https://doi.org/10.1016/j.micromeso.2008.05.046
Talekar S, Ghodake V, Ghotage T, Rathod P, Deshmukh P, Nadar S, Mulla M, Ladole M (2012) Novel magnetic cross-linked enzyme aggregates (magnetic CLEAs) of alpha amylase. Bioresour Technol 123:542–547. https://doi.org/10.1016/j.biortech.2012.07.044
Tan H, Feng W, Ji P (2012) Lipase immobilized on magnetic multi-walled carbon nanotubes. Bioresour Technol 115:172–176. https://doi.org/10.1016/j.biortech.2011.10.066
Tan WY, Gopinath SCB, Anbu P, Yaakub ARW, Subramaniam S, Chen Y, Sasidharan S (2023) Bio-enzyme hybrid with nanomaterials: a potential cargo as sustainable biocatalyst. Sustainability 15(9):7511. https://doi.org/10.3390/su15097511
Tang J, Liu J, Zheng Q, Li W, Sheng J, Mao L, Wang M (2021a) In-situ encapsulation of protein into nanoscale hydrogen-bonded organic frameworks for intracellular biocatalysis. Angew Chem Int Ed 60:22315–22321. https://doi.org/10.1002/anie.202105634
Tang Z, Li X, Tong L, Yang H, Wu J, Zhang X, Song T, Huang S, Zhu F, Chen G, Ouyang G (2021b) A biocatalytic cascade in an ultrastable mesoporous hydrogenbonded organic framework for point-of-care biosensing. Angew Chem Int Ed 60:23608–23613. https://doi.org/10.1002/anie.202110351
Tang ZX, Qian JQ, Shi LE (2007) Characterizations of immobilized neutral lipase on chitosan nano-particles. Mater Lett 61:37–40. https://doi.org/10.1016/j.matlet.2006.04.048
Thakur K, Attri C, Seth A (2011) Nanocarriers-based immobilization of enzymes for industrial application. Biotech 11:1–12. https://doi.org/10.1007/s13205-021-02953-y
Thandavan K, Gandhi S, Sethuraman S, Rayappan JBB, Krishnan UM (2013) A novel nano-interfaced superoxide biosensor. Sens Actuators, B 176:884–892. https://doi.org/10.1016/j.snb.2012.09.031
Tran DT, Chen CL, Chang JS (2012) Immobilization of Burkholderia sp. lipase on a ferric silica nanocomposite for biodiesel production. J Biotechnol 158:112–119. https://doi.org/10.1016/j.jbiotec.2012.01.018
Ubbink J, Burbidge A, Mezzenga R (2008) Food structure and functionality: a soft matter perspective. Soft Mater 4:1569–1581. https://doi.org/10.1039/b802183j
Uddin MJ, Werfel TA, Crews BC, Gupta MK, Kavanaugh TE, Kingsley PhJ, Boyd K, Marnett LJ, Duvall CL (2016) Fluorocoxib a loaded nanoparticles enable targeted visualization of cyclooxygenase-2 in inflammation and cancer. Biomaterials 92:71–80. https://doi.org/10.1016/j.biomaterials.2016.03.028
Valerio A, Nicoletti G, Cipolatti EP, Ninow JL, Araujo PHH, Sayer C, de Oliveira D (2015) Kinetic study of Candida antarctica lipase b immobilization using poly (methyl methacrylate) nanoparticles obtained by miniemulsion polymerization as support. Appl Biochem Biotechnol 175:2961–2971. https://doi.org/10.1007/s12010-015-1478-5
Valls-Chivas Á, Gómez J, Garcia-Peiro JI, Hornos F, Hueso JL (2017) Enzyme-iron oxide nanoassemblies: a review of immobilization and biocatalytic applications. Catalysts 13(6):980. https://doi.org/10.3390/catal13060980
Vashist SK, Luong JHT (2015) Recent advances in electrochemical biosensing schemes using graphene and graphene-based nanocomposites. Carbon 84:519–550. https://doi.org/10.1016/j.carbon.2014.12.052
Vauthier C, Bouchemal K (2009) Methods for the preparation and manufacture of polymeric nanoparticles. Pharm Res 26:1025–1058. https://doi.org/10.1007/s11095-008-9800-3
Velasco-Lozano S (2020) Co-immobilization and colocalization of multi-enzyme systems for the cell-free biosynthesis of aminoalcohols. ChemCatChem 12(11):3030–3041. https://doi.org/10.1002/cctc.201902404
Verma ML, Puri M, Barrow CJ (2016) Recent trends in nanomaterials immobilised enzymes for biofuel production. Crit Rev Biotechnol 36:108–119. https://doi.org/10.3109/07388551.2014.928811
Vertegel AA, Richard W, Siegel A, Dordick JS (2004) Silica nanoparticle size influences the structure and enzymatic activity of adsorbed lysozyme. Langmuir 20(16):6800–6807. https://doi.org/10.1021/la0497200
Virgen-Ortíz JJ, Dos Santos JCS, Berenguer-Murcia Á, Barbosa O, Rodrigues RC, Fernandez-Lafuente R (2017) Polyethylenimine: a very useful ionic polymer in the design of immobilized enzyme biocatalysts. J Mater Chem B 5:7461–7490. https://doi.org/10.1039/c7tb01639e
Visakh PM, Bayraktar O, Picó G (2014) Polyelectrolyte: Thermodynamics and Rheology. 1–17. https://doi.org/10.1007/978-3-319-01680-1
Wahab RA, Elias N, Abdullaha F, Ghoshal SK (2020) On the taught new tricks of enzymes immobilization: An allinclusive overview. React Funct Polym 152:104613–104639. https://doi.org/10.1016/j.reactfunctpolym.2020.104613
Wahajuddin AS (2012) Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers. Int J Nanomedicine 7:3445–3471. https://doi.org/10.2147/IJN.S30320
Wan G-Z, Ma X-H, Jin L, Chen J (2021) α-Glucosidase immobilization on magnetic core-shell metal-organic frameworks for inhibitor screening from traditional Chinese medicines. Colloids Surf B: Biointerfaces 205:111847–111856. https://doi.org/10.1016/j.colsurfb.2021.111847
Wang B, Lin RB, Zhang Z, Xiang S, Chen B (2020) Hydrogen-bonded organic frameworks as a tunable platform for functional materials. J Am Chem Soc 142:14399–14416. https://doi.org/10.1021/jacs.0c06473
Wang C, Liao K (2021) Recent advances in emerging metal- and covalent-organic frameworks for enzyme encapsulation. ACS Appl Mater Inter 13:56752–56776. https://doi.org/10.1021/acsami.1c13408
Wang J, Chen Y, Chen B, Ding J, Xia G, Gao C, Cheng J, Jin N, Zhou Y, Li X, Tang M, Wang XM (2010) Pharmacokinetic parameters and tissue distribution of magnetic Fe3O4 nanoparticles in mice. Int J Nanomedicine 5:861–866. https://doi.org/10.2147/IJN.S13662
Wang L, Jiang R (2011) Reversible his-tagged enzyme immobilization on functionalized carbon nanotubes as nanoscale biocatalyst. Methods Mol Biol 743:95–106. https://doi.org/10.1007/978-1-61779-132-1_8
Wang L, Zhi W, Lian D, Wang Y, Han J, Wang Y (2019) HRP@ZIF-8/DNA Hybrids: Functionality Integration of ZIF-8 via Biomineralization and Surface Absorption. ACS Sustainable Chem Eng 7:14611–14620. https://doi.org/10.1021/ACSSUSCHEMENG.9B02348
Wang LB, Wang YC, He R, Zhuang A, Wang X, Zeng J, Hou JG (2013) A new nanobiocatalytic system based on allosteric effect with dramatically enhanced enzymatic performance. J Am Chem Soc 135:1272–1275. https://doi.org/10.1021/ja3120136
Wang X, Shi J, Li Z, Zhang S, Wu H, Jiang Z, Yang C, Tian C (2014) Facile one-pot preparation of chitosan/calcium pyrophosphate hybrid microflowers. ACS Appl Mater Interfaces 6:14522–14532. https://doi.org/10.1021/am503787h
Wang Z, Liu Y, Li J, Meng G, Zhu D, Cui J, Jia S (2022) Efficient immobilization of enzymes on amino functionalized MIL-125-NH2 metal organic framework. Biotechnol Bioprocess Eng 27:135–144. https://doi.org/10.1007/s12257-020-0393-y
Wei B, Xu H, Cheng L, Yuan Q, Liu C, Gao H, Liang H (2021) Highly selective entrapment of his-tagged enzymes on superparamagnetic zirconium-based MOFs with robust renewability to enhance pH and thermal stability. ACS Biomater Sci Eng 7:3727–3736. https://doi.org/10.1021/acsbiomaterials.1c00780
Wei W, Yi Y, Song J, Chen X, Li J, Li J (2022) Tunable graphene/nitrocellulose temperature alarm sensors. ACS Appl Mater Interfaces 14:13790–13800. https://doi.org/10.1021/acsami.2c02340
White NG (2021) Amidiniumcdots, three dots, centeredcarboxylate frameworks: predictable, robust, water-stable hydrogen bonded materials. Chem Commun 57:10998–11008. https://doi.org/10.1039/d1cc04782e
Wick P, Louw-Gaume AE, Kucki M, Krug HF, Kostarelos K, Fadeel B, Dawson KA, Salvati A, Vázquez E, Ballerini L, Tretiach M, Benfenati F, Flahaut E, Gauthier L, Prato M, Bianco A (2014) Classification framework for graphene-based materials. Angew Chem Int Ed 53(30):7714–7718. https://doi.org/10.1002/anie.201403335
Wied P, Carraro F, Bolivar JM, Doonan CJ, Falcaro P, Nidetzky B (2022) Combining a genetically engineered oxidase with hydrogen-bonded organic frameworks (HOFs) for highly efficient biocomposites. Angew Chem Int Ed 61:16. https://doi.org/10.1002/anie.202117345
Williams L, Adams W (2007) Nanotechnology demystified. New York, U.S.A. https://doi.org/10.1036/0071460233
Wu H, Li X, Liu W, Chen T, Li Y, Zheng W, Man CWY, Wong MK, Wong KH (2012) Surface decoration of selenium nanoparticles by mushroom polysaccharides-protein complexes to achieve enhanced cellular uptake and antiproliferative activity. J Mater Chem 22:9602–9610. https://doi.org/10.1039/C2JM16828F
Wu Z, Shan H, Jiao Y, Huang S, Wang X, Liang K, Shi J (2022) Covalent organic networks for in situ entrapment of enzymes with superior robustness and durability. Chem Eng J 450:138446. https://doi.org/10.1016/j.cej.2022.138446
Wu ZF, Wang Z, Zhang Y, Ma YL, He CY, Li H, Chen L, Huo QS, Wang L, Li ZQ (2016) Amino acids-incorporated nanoflowers with an intrinsic peroxidase-like activity. Sci Rep 6:22412–22418. https://doi.org/10.1038/srep22412
Wu ZL, Liu ZX, Yuan YH (2017) Carbon dots: materials, synthesis, properties and approaches to long-wavelength and multicolor emission. J Mater Chem B 5(21):3794–3809. https://doi.org/10.1039/C7TB00363C
Wu X, Hou M, Ge J (2015) Metal-organic frameworks and inorganic nanoflowers: a type of emerging inorganic crystal nanocarriers for enzyme immobilization. Catal Sci Technol 5:5077–5508. https://doi.org/10.1039/C5CY01181G
Xie J, Huang J, Li X, Sun S, Chen X (2009) Iron oxide nanoparticle platform for biomedical applications. Curr Med Chem 16:1278–1294. https://doi.org/10.2174/092986709787846604
Xie W, Ma N (2010) Enzymatic transesterification of soybean oil by using immobilized lipase on magnetic nano-particles. Biomass Bioenergy 34:890–896. https://doi.org/10.1016/j.biombioe.2010.01.034
Xu J, Cao P, Fan Z, Luo X, Yang G, Qu T, Gao J (2022) Rapid screening of lipase inhibitors in Scutellaria baicalensis by using porcine pancreatic lipase immobilized on magnetic core-shell metal-organic frameworks. Molecules 27:3475–3489. https://doi.org/10.3390/molecules27113475
Xu W, Jiao L, Wu Y, Hu L, Gu W, Zhu C (2021) Metal-organic frameworks enhance biomimetic cascade catalysis for biosensing. Adv Mater 33:2005172. https://doi.org/10.1002/ADMA.202005172
Yaari Z, Cheung JM, Baker HA, Frederiksen RS, Jena PV, Horoszko CP, Jiao F, Scheuring S, Luo M, Heller DA (2020) Nanoreporter of an enzymatic suicide inactivation pathway. Nano Lett 20(11):7819–7827. https://doi.org/10.1021/acs.nanolett.0c01858
Yan S, Chen X, Wu J, Wang P (2012) Ethanol production from concentrated food waste hydrolysates with yeast cells immobilized on corn stalk. Appl Microbiol Biotechnol 94:829–838. https://doi.org/10.1007/s00253-012-3990-7
Yang F, Tang Q, Zhong X, Bai Y, Chen T, Zhang Y, Li Y, Zheng W (2012) Surface decoration by Spirulina polysaccharide enhances the cellular uptake and anticancer efficacy of selenium nanoparticles. Int J Nanomedicine 7:835–844. https://doi.org/10.2147/IJN.S28278
Yang J, Wang J, Hou B, Huang X, Wang T, Bao Y, Hao H (2020) Porous hydrogenbonded organic frameworks (HOFs): From design to potential applications. Chem Eng J 399:125873–125938. https://doi.org/10.1016/j.cej.2020.125873
Yang J, Zhang T, Tian C, Zhu Y, Zeng Y, Men Y, Chen P, Sun Y, Ma Y (2019) Multi-enzyme systems and recombinant cells for synthesis of valuable saccharides: advances and perspectives. Biotechnol Adv 37:107406–107430. https://doi.org/10.1016/j.biotechadv.2019.06.005
Ye R, Zhu C, Song Y, Song J, Fu S, Lu Q, Yang X, Zhu MJ, Du D, Li H, Lin Y (2016) One-pot bioinspired synthesis of all-inclusive protein-protein nanoflowers for point-of-care bioassay: detection of E. coli O157: H7 from milk. Nanoscale 8:18980–18986. https://doi.org/10.1039/c6nr06870g
Yim EKF, Darling EM, Kulangara K et al (2010) Nanotopographyinduced changes in focal adhesions, cytoskeletal organization, and mechanical properties of human mesenchymal stem cells. Biomaterials 31:1299–1306. https://doi.org/10.1016/j.biomaterials.2009.10.037
Yin H, Xu Z, Bao H, Bai J, Zheng Y (2004) Single crystal trigonal selenium nanoplates converted from selenium nanoparticles. Chem Lett 34:122–123. https://doi.org/10.1246/cl.2005.122
Yin Q, Zhao P, Sa RJ, Chen GC, Lu J, Liu TE, Cao R (2018) An ultra-robust and crystalline redeemable hydrogen-bonded organic framework for synergistic chemo-photodynamic therapy. Angew Chem Int Ed 57:7691–7696. https://doi.org/10.1002/anie.201800354
Yin Y, Xiao Y, Lin G, Xiao Q, Lin Z, Cai Z (2015) An enzyme-inorganic hybrid nanoflower based immobilized enzyme reactor with enhanced enzymatic activity. J Mater Chem B 3:2295–2300. https://doi.org/10.1039/C4TB01697A
Yu B, Zhou Y, Song M, Xue Y, Cai N, Luo X, Yu F (2016) Synthesis of selenium nanoparticles with mesoporous silica drug-carrier shell for programmed responsive tumor targeted synergistic therapy. RSC Adv 6:2171–2175. https://doi.org/10.1039/C5RA21460B
Yu Z, Ji N, Li X, Zhang R, Qiao Y, Xiong J, Liu J, Lu X (2023) Kinetics driven by hollow nanoreactors: an opportunity for controllable catalysis. Angew Chem Int Ed 62(3):e202213612. https://doi.org/10.1002/anie.202213612
Zang L, Qiu J, Wu X, Zhang W, Sakai E, Wei Y (2014) Preparation of magnetic chitosan nanoparticles as support for cellulase immobilization. Ind Eng Chem Res 53:3448–3454. https://doi.org/10.1021/ie404072s
Zdarta J, Jankowska K, Wyszowska M, Kijeńska-Gawrońska E, Zgoła-Grześkowiak A, Pinelo M, Meyer AS, Moszyński D, Jesionowski T (2019) Robust biodegradation of naproxen and diclofenac by laccase immobilized using electrospun nanofibers with enhanced stability and reusability. Mater Sci Eng: C 103:109789. https://doi.org/10.1016/J.MSEC.2019.109789
Zeng J, Xia Y (2012) Hybrid nanomaterials: not just a pretty flower. Nat Nanotechnol 7:415–416. https://doi.org/10.1038/nnano.2012.105
Zeng Y, Zhu Z, Du D, Lin Y (2016) Nanomaterial-based electrochemical biosensors for food safety. J Electroanal Chem 781:147–154. https://doi.org/10.1016/j.jelechem.2016.10.030
Zhang B, Chen J, Wang J, Huyan Y, Zhang H, Zhang Q (2018) Flowerlike BSA/Zn3(PO4)2/Fe3O4 magnetic hybrid particles: preparation and application to adsorption of copper ions. J Chem Eng Data 63:3913–3922. https://doi.org/10.1021/acs.jced.8b00544
Zhang B, Li P, Zhang H, Fan L, Wang H, Li X, Tian L, Chen X, Ali N, Ali Z, Zhang Q (2016a) Papain/ Zn3(PO4)2 hybrid nanoflower: preparation, characterization and its enhanced catalytic activity as an immobilized enzyme. RSC Adv 6:46702–46710. https://doi.org/10.1039/C6RA05308D
Zhang B, Li P, Zhang H, Li X, Tian L, Wang H, Ali N, Ali Z, Zhang Q (2016b) Red-blood-cell-like BSA/Zn3(PO4)2 hybrid particles: preparation and application to adsorption of heavy metal ions. Appl Surf Sci 366:328–338. https://doi.org/10.1016/j.apsusc.2016.01.074
Zhang B, Li P, Zhang H, Wang H, Li X, Tian L, Ali N, Ali Z, Zhang Q (2016c) Preparation of lipase/ Zn3(PO4)2 hybrid nanoflower and its catalytic performance as an immobilized enzyme. Chem Eng J 291:287–297. https://doi.org/10.1016/j.cej.2016.01.104
Zhang L, Liu Z, Xie Q, Li Y, Ying Y, Fu Y (2019) Bioinspired assembly of reduced graphene oxide by fibrin fiber to prepare multi-functional conductive bionanocomposites as versatile electrochemical platforms. Carbon 153:504–512. https://doi.org/10.1016/j.carbon.2019.06.101
Zhang W-W, Yang X-L, Jia J-Q, Wang N, Hu C-L, Yu X-Q (2015a) Surfactant-activated magnetic cross-linked enzyme aggregates (magnetic CLEAs) of Thermomyces lanuginosus lipase for biodiesel production. J Mol Catal B: Enzym 115:83–89. https://doi.org/10.1016/j.molcatb.2015.02.003
Zhang Y, Ge J, Liu Z (2015b) Enhanced activity of immobilized or chemically modified enzymes. ACS Catal 5:4503–4513. https://doi.org/10.1021/acscatal.5b00996
Zhang Y, Xu L, Ge J (2022) Multienzyme system in amorphous metal-organic frameworks for intracellular lactate detection. Nano Lett. https://doi.org/10.1021/ACS.NANOLETT.2C01154
Zhang Z, Zhang Y, He L, Yang Y, Liu S, Wangab M, Fand S, Fu G (2015c) A feasible synthesis of Mn3(PO4)2@ BSA nanoflowers and its application as the support nanomaterial for Pt catalyst. J Power Sources 284:170–177. https://doi.org/10.1016/j.jpowsour.2015.03.011
Zhao XS, Bao XY, Guo W, Lee FY (2006) Immobilizing catalysts on porous materials. Mater Today 9(3):32–39. https://doi.org/10.1016/S1369-7021(06)71388-8
Zhao Z, Tian J, Wu Z, Liu J, Zhao D, Shen W, He L (2013) Enhancing enzymatic stability of bioactive papers by implanting enzyme-immobilized mesoporous silicananorods into paper. Mater J Chem B 1:4719–4722. https://doi.org/10.1039/C3TB20953A
Zheng L, Sun Y, Wang J, Huang H, Geng X, Tong Y, Wang Z (2018) Preparation of a flower-like immobilized D-psicose 3-epimerase with enhanced catalytic performance. Catalysts 8:468–478. https://doi.org/10.3390/catal8100468
Zhong L, Feng Y, Hu H, Xu J, Wang Z, Du Y, Cui J, Jia S (2021) Enhanced enzymatic performance of immobilized lipase on metal organic frameworks with superhydrophobic coating for biodiesel production. J Colloid Interface Sci 602:426–436. https://doi.org/10.1016/j.jcis.2021.06.017
Zhong C, Lei Z, Huang H, Zhang M, Cai Z, Lin Z (2020) One-pot synthesis of trypsin-based magnetic metal-organic frameworks for highly efficient proteolysis. J Mater Chem B 8:4642–4647. https://doi.org/10.1039/c9tb02315a
Zhou Z, Hartmann M (2013) Progress in enzyme immobilization in ordered mesoporous materials and related applications. Chem Soc Rev 42:3894–3912. https://doi.org/10.1039/c3cs60059a
Zhu J, Sun G (2012) Lipase immobilization on glutaraldehyde-activated nanofibrous membranes for improved enzyme stabilities and activities. React Funct Polym 72:839–845. https://doi.org/10.1016/j.reactfunctpolym.2012.08.001
Zhu J, Wen M, Wen W, Du D, Zhang X, Wang S, Lin Y (2018) Recent progress in biosensors based on organic-inorganic hybrid nanoflowers. Biosens Bioelectron 120:175–187. https://doi.org/10.1016/j.bios.2018.08.058
Zhu L, Yang R, Zhai J, Tian C (2007a) Bienzymatic glucose biosensor based on coimmobilization of peroxidase and glucose oxidase on a carbon nanotubes electrode. Biosens Bioelectron 23:528–535. https://doi.org/10.1016/j.bios.2007.07.002
Zhu X, Yuri I, Gan X, Suzuki I, Li G (2007b) Electrochemical study of the effect of nano-zinc oxide on microperoxidase and its application to more sensitive hydrogen peroxide biosensor preparation. Biosens Bioelectron 22:1600–1604. https://doi.org/10.1016/j.bios.2006.07.007
Zielinska A, Carreiro F, Oliveira AM, Neves A, Pires B, Venkatesh DN, Durazzo A, Lucarini M, Eder P, Silva AM, Santini A, Souto EB (2020) Polymeric nanoparticles: production, characterization, toxicology and ecotoxicology. Molecules 25:3731–3751. https://doi.org/10.3390/molecules25163731
Zueva OS, Khair T, Derkach SR, Kazantseva MA, Zuev YuF (2023) Strontium-induced gelation of sodium alginate in the presence of carbon nanotubes: Elemental analysis and gel structure. J Compos Sci 7(7):286. https://doi.org/10.3390/jcs7070286
Funding
This research was funded by the Russian Science Foundation, Project Number 21–74-20053.
Author information
Authors and Affiliations
Contributions
Conceptualization, M. G. H., V. G. A.; methodology and investigation, S.S. G., Yu. A. R., M. S. L., A. V. S.; project administration, M. G. H.; supervision, M. G. H., V. G. A.; funding acquisition, M. G. H.; writing of original draft preparation, review and editing, M. G. H.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Holyavka, M.G., Goncharova, S.S., Redko, Y.A. et al. Novel biocatalysts based on enzymes in complexes with nano- and micromaterials. Biophys Rev 15, 1127–1158 (2023). https://doi.org/10.1007/s12551-023-01146-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12551-023-01146-6