Abstract
Lipofuscin granules (LGs) are accumulated in the retinal pigment epithelium (RPE) cells. The progressive LG accumulation can somehow lead to pathology and accelerate the aging process. The review examines composition, spectral properties and photoactivity of LGs isolated from the human cadaver eyes. By use of atomic force microscopy and near-field microscopy, we have revealed the fluorescent heterogeneity of LGs. We have discovered the generation of reactive oxygen species by LGs, and found that LGs and melanolipofuscin granules are capable of photoinduced oxidation of lipids. It was shown that A2E, as the main fluorophore (bisretinoid) of LGs, is much less active as an oxidation photosensitizer than other fluorophores (bisretinoids) of LGs. Photooxidized products of bisretinoids pose a much greater danger to the cell than non-oxidized one. Our studies of the fluorescent properties of LGs and their fluorophores (bisretinoids) showed for the first time that their spectral characteristics change (shift to the short-wavelength region) in pathology and after exposure to ionizing radiation. By recording the fluorescence spectra and fluorescence decay kinetics of oxidized products of LG fluorophores, it is possible to improve the methods of early diagnosis of degenerative diseases. Lipofuscin (“aging pigment”) is not an inert “slag”. The photoactivity of LGs can pose a significant danger to the RPE cells. Fluorescence characteristics of LGs are a tool to detect early stages of degeneration in the retina and RPE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lipofuscin granules, or age pigment, are accumulated with aging in retinal pigment epithelium (RPE) cells of human eye and remain there to the end of life, occupying up to 20% of cell cytoplasmic volume (Feeney–Burns et al. 1984; Jung et al. 2077; Yin 1996). The mechanism of LG formation is related to the physiological functions of the retina and RPE.
The retina is the light-sensitive tissue of the eye (Fig. 1a). It consists of several layers of neurons interconnected by synapses (Fig. 1b). The primary light-sensing cells in the retina are the photoreceptor cells, rods and cones. The RPE is the pigmented single-cell layer just outside the retina, firmly attached to the underlying choroid and is in close contact with the photoreceptor cells (Fig. 1b, c). The RPE has several crucial functions for vision, namely, scattered light absorption, epithelial transport, spatial ion buffering, visual cycle, phagocytosis of photoreceptor outer segment membranes, secretion and immune modulation (Strauss 2005).
Scheme of ocular exposure to light (a), the retina and retinal pigment epithelium (RPE) (b), and RPE cell containing LGs (c). Figure was modified from Yakovleva et al. (2022b)
With exposure to light during rhodopsin photolysis, retinoid side products can be produced in photoreceptor cells. Biogenesis of these products occurs when two molecules of all-trans retinal condense with one molecule of phosphatidylethanolamine (Fig. 2) in the photoreceptor membrane (Wolf 2003). Evolution has developed a powerful mechanism that prevents the accumulation of retinoid side products in terminally differentiated photoreceptor cells (Young 1967). Throughout life, the debris of the photoreceptor outer segment apical part, are phagocytized and digested by the RPE cells, while new photoreceptor discs with rhodopsin molecules are synthesized by the photoreceptor inner segments (Kennedy et al. 1995). However, the lysosomal enzyme system of the RPE cell is not effective in degrading of the photoreceptor outer segment debris, because the latter are supposed to contain modified retinoid side products of rhodopsin photolysis as well as modified lipids and proteins. In other words, the lysosomal enzyme system of the RPE cell cannot recognize such modified molecules and do not digest them (Feeney 1973). As a consequence, lipofuscin granules (LGs), containing retinoid derivatives, are formed in the RPE cells (Fig. 1c).
LGs have been long believed to be just a cell metabolism by-product. It turned, however, that they are photochemically active. As we have shown in the early 1990s, LGs can generate oxygen reactive species (ROS) upon photoexcitation with visible light, which account for their phototoxicity (Boulton et al. 1993). The main photo-inducible generators of ROS in LGs are retinoid side products. The features of these compounds, such as photosensitizing, have been studied in detail (Boulton et al. 1993; Sparrow et al. 2000; Rozanowska et al. 1995, 1998, 2005; Avalle et al. 2005). There is the correlation between LG accumulation in the RPE cells and development of degenerative retinal diseases, including such a severe and widespread retinopathy as age-related macular degeneration AMD (Holz et al. 2004; Katz 2002; Sparrow and Boulton 2005).
LGs are heterogeneous, composed of mixtures of proteins and lipids, including more than 21 different fluorescent compounds (Warburton et al. 2005; Bazan et al. 1990; Eldred and Katz 1988; Ng et al. 2008; Sparrow et al. 2009). The structure and fluorescence properties of LGs were analyzed by atomic force microscopy (AFM) and near-field microscopy (Yakovleva et al. 2016; Petrukhin et al. 2005; Warburton et al. 2005; Clancy et al. 2000). Figure 3a shows an image of aggregated LGs. Single LGs were found to be approximately 0.7–1.0 μm in diameter, with a fairly uniform density. Figure 3b shows the fluorescence emission spectra of aggregated LGs, as determined by near-field microscopy, at the two points marked on Fig. 3a. The LG fluorescence emission spectrum 1 at point 1 (Fig. 3a) is broad and has several emission maxima, ranging approximately from 570 to 625 nm, that correlates with the fluorescence properties of LGs in suspension (Feldman et al. 2015; Boulton et al. 1990; Haralampus-Grynaviski et al. 2003).
Atomic force microscopy (AFM) image of aggregated LGs on a cover slip (a) and near-field microscopy analysis of the fluorescence emission spectra (b). The excitation wavelength was 420 nm. Emission spectrum 1 was recorded from point 1 (a) and corresponded to the aggregated LGs, whereas emission spectrum 2 was recorded from point 2 (a) and corresponded to the part of the slide glass without LGs. OZ represents the height (μm) of the sample (Yakovleva et al. 2016)
Major sources of LG fluorescence are retinoid side products (Kennedy et al. 1995; Sparrow et al. 2012). They are bisretinoids (BisRets) and their oxidation and degradation derivatives (BisRets-OX) (Lamb and Simon 2004; Sakai et al. 1996; Sparrow et al. 2008, 2012; Wang et al. 2006a, b; Wu et al. 2010; Kim et al. 2007; Feldman et al. 2015). N-retinyl-N-retinylidenethanolamine (A2E) (Fig. 2) is the most widely studied BisRet (Lamb and Simon 2004; Sakai et al. 1996; Sparrow et al. 2008).
LGs exhibit distinct fluorescence in the visible region. Based on its measurement, fundus autofluorescence (AF) imaging is a modern noninvasive diagnostic method for revealing age-related base changes and degenerative retinal and RPE pathology. AF allows assessment of the condition, integrity and viability of the photoreceptor/RPE complex (Schmitz-Valckenberg et al. 2007).
This review considers the phototoxic properties of BisRets in LGs, as well as the cytotoxic properties of the BisRets-OX. The review also examines the fluorescent properties of LGs in health and disease for the use of this knowledge in expanding the capabilities of the fundus AF method for diagnosing degenerative diseases of the retina and RPE.
Phototoxic properties of LGs in the RPE cell
It is well known that LGs in the RPE cells under the influence of visible light stimulate the oxidation of both lipids (Yin 1996; Wassel et al. 1999; Dontsov et al. 1999; Dontsov et al. 2005; Dontsov et al. 2012; Nowak 2013) and proteins (Wassell et al., 1999; Rozanowska et al., 2002; Wiktor et al., 2018). This is due to the ability of LGs to produce ROS under the action of visible light (Boulton et al. 1993; Rozanowska et al. 1995, 1998, 2002). LGs most effectively photogenerate ROS in the blue-green region of the spectrum (400–520 nm) (Boulton et al. 1993, 2004; Dontsov et al. 2012). Figure 4 demonstrates data on oxidation of 4,7,10,13,16,19-docosahexaenoic acid (DHA) induced by blue-green light irradiation and sensitized with LG and melanolipofuscin granules from human RPE cells. Both types of granules containing lipofuscin stimulated DHA peroxidation, but LGs were more active in this respect.
DHA (4,7,10,13,16,19-docosahexaenoic acid) peroxidation produced by photoactivation of lipofuscin and melanolipofuscin RPE pigment granules. The argon ion laser was used to generate blue-green light (488.1 nm and 514.5 nm; granule concentration was 1.5 × 10.7 granules/mL). Figure was modified from Dontsov et al. (1999)
The ROS generation by LGs in the RPE under the action of blue light can explain the “blue light hazard” phenomenon for the retina (Rozanowska et al. 1995; Boulton et al. 2004). It has been shown, for example, that in the RPE cells loaded with LGs in vitro and irradiated with blue-green light, there is a significant increase in lipid and protein oxidation, accompanied by such morphological changes as loss of lysosome integrity (Brunk et al. 1995; Nilsson et al. 2003; Sparrow and Boulton 2005; Shamsi and Boulton 2001), damage to the cell membrane and increased vacuolization of the cytoplasm compared to the RPE cells irradiated with long-wavelength (> 550 nm) light (Boulton 2001). LGs in the RPE are also capable of photogeneration of singlet oxygen, the efficiency of which decreases monotonically with increasing wavelength of excitation light (Rozanowska, et al. 1998, 2004; Avalle et al. 2005).
The phototoxicity of LGs is associated with the presence in them of bisretinoid fluorophores that absorb light in the blue region of the spectrum. One of the main LG bisretinoids, A2E, has been shown to be localized not only in LGs, but also in other RPE cell compartments. Although the main amount of A2E is found mainly in lysosomes (Eldred and Lasky 1993; Sparrow et al. 1999; Holz et al. 1999; Schutt et al. 2007), but to a lesser extent, A2E accumulates in the mitochondria, Golgi apparatus, and cytoplasmic membrane (Schutt et al. 2007). The amount of A2E in the RPE cells in vivo can reach 800 pmol/eye (Parish et al. 1998). This level of BisRets in the RPE cells can be obtained in vitro by incubating cells with 15–30 μM A2E (Sparrow et al. 1999; Roberts et al. 2002; Lakkaraju et al. 2007). The RPE cells from donors older than 50 years have significantly higher concentration of A2E (2–3 times more) compared to young donors (Bhosale et al. 2009). Due to chemical structure, A2E can exhibit dark toxicity, acting as an amphiphilic detergent capable of destroying phospholipid membranes (Eldred and Lasky 1993; De and Sakmar 2002; Lakkaraju et al. 2007; Sokolov et al. 2007; Dontsov et al. 2012) and induce cell apoptosis in the dark (Suter et al. 2000). A2E exposed to blue light sensitizes lipid peroxidation (Dontsov et al. 2005, 2012, 2016), causes destruction of lysosomal membranes (Schutt et al. 2000a, b), and inhibits mitochondrial cytochrome oxidase (Suter et al. 2000). The mechanism of the phototoxic action of A2E is associated with its ability to photoproduce ROS. It is known that A2E can photogenerate superoxide radicals (Pawlak et al. 2002, 2003; Gaillard et al. 2004; Broniec et al. 2005) and singlet oxygen (Ragauskaite et al. 2001; Cantrell et al. 2001; Pawlak et al. 2003). However, the efficiency of these processes is not very high compared to other RPE chromophores (all-trans retinal, protoporphyrins) (Bynoe et al. 1998; Pawlak et al. 2003; Maeda et al. 2009; Wielgus et al. 2010). Moreover, A2E is a much less potent sensitizer than LGs in the RPE cells (Rozanowska et al. 1998; Pawlak et al. 2002; Boulton et al. 2004; Dontsov et al. 2005). Apparently, the higher photosensitizing activity of LGs is associated with the presence of other photoreactive substances in them.
A2E in the presence of oxygen is easily oxidized by irradiation with the formation of numerous oxidation products (see below). Photobleached A2E loses its ability to enhance photoinduced lipid peroxidation (Fig. 5).
Photoinduced peroxidation of bovine photoreceptor outer segments (POS) with non-irradiated and pre-irradiated bisretinoid A2E. TBA (thiobarbituric acid) — active products. The concentration of A2E was 100 µM. The samples were irradiated with visible light (390–700 nm) with an energy of 100 mW/ cm2. Photobleached A2E was prepared by irradiating the original bisretinoid with an LED source (wavelength 450 nm) for 60 min. Concentration of POS was 0.2 mg of rhodopsin per 1 mL. Figure was modified from Dontsov et al. (2012)
Figure 5 demonstrates that non-irradiated A2E is a much more effective sensitizer of photooxidation of the outer segments of photoreceptor cells than photobleached A2E. As is known, in the RPE cells, in addition to lipofuscin-containing granules, there are melanin-containing organelles — melanosomes. If the former exhibit photosensitizing properties, enhancing the production of ROS when exposed to blue light, then melanosomes, on the contrary, serve to protect the RPE cells from the damaging effects of light and ROS (Ostrovsky et al. 1987; Wang et al. 2006a, b; Ostrovsky and Dontsov 2019). Age-related changes occurring in the RPE cells contribute to the enhancement of photooxidative processes induced by LGs. This is mainly due to an increase in the number of lipofuscin-containing granules in the RPE cell, as well as a significant drop in the concentration of melanin (Feeney–Burns et al. 1984, 1990; Sarna et al. 2003; Yacout et al. 2019). This leads to weakening of the protective effect of melanin, which includes screening of photosensitive cell structures from excess light (Ostrovsky et al. 2018), antioxidant and antiradical protection (Ostrovsky et al. 1987; Wang et al. 2006a, b; Ostrovsky and Dontsov 2019), as well as the binding of BisRets into inactive complexes (Dontsov et al. 2013; Sakina et al. 2013). A decrease in the effectiveness of antioxidant protection of melanin in the RPE cells with age is apparently associated with its photooxidative destruction in melanolipofuscin granules under the action of superoxide radicals and the formation of degradation products that do not have antioxidant activity and, on the contrary, exhibit prooxidant properties (Zareba et al. 2006; Dontsov et al. 2017; Mahendra et al. 2020; Olchawa et al. 2021).
Cytotoxic properties of LGs in the RPE cell
In the presence of oxygen, LG BisRets themselves can be photo-oxidized to form various products, consisting primarily of epoxides, peroxides, aldehydes, and ketones, which are potentially cytotoxic (Wu et al. 2010; Ben-Shabat et al. 2002; Feldman et al. 2015; Sparrow et al. 2012; Yakovleva et al. 2006; Dontsov et al. 2009; Yoon et al. 2012). The cytotoxic properties of BisRets-OX in LGs have not been fully investigated. The role of these compounds in pathological processes of the RPE remains controversial. Some studies have suggested that highly reactive cytotoxic carbonyl compounds, aldehydes and ketones, are formed during photo-oxidation of BisRets in LGs (Schütt et al. 2000; Sparrow et al. 2000; Wang et al. 2006a, b). By contrast, other studies (Murdaugh et al. 2010, 2011) suggest that BisRet-OX interacts with itself or with A2E, forming products with a higher molecular weight inside LGs. Most of these compounds are also hydrophobic and remain inside LGs, resulting in the concomitant diminution of its reactivity in vivo. Clarifying the roles of BisRets-OX is important to delineate the mechanisms of pathological ocular diseases, especially AMD.
Our prior findings have demonstrated that LG BisRet-OX content is higher in AMD eyes than in normal eyes, which was indicated by changes in the characteristics of LG fluorescence spectra and in the parameters of fluorescence decay kinetic curves (Feldman et al. 2018). Specifically, the fluorescence excitation at 488 nm of samples from eyes with AMD increases the fluorescence intensity of the band at 556 nm, and the contribution of BisRets-OX to total fluorescence increases. However, the pathophysiological, or protective, properties of these products remain controversial, as prior studies have suggested conflicting roles (Murdaugh et al. 2010, 2011), and as BisRets-OX could also potentially become a neutral product eventually. Investigating the potential release of BisRets-OX from LGs into the RPE cell cytoplasm and the assessment of their toxicity to cellular structures is thus fundamental to understanding the pathogenesis of retinal diseases.
The chemical characteristics of BisRets-OX in LGs, which were obtained from healthy donor eyes, have been studied (Yakovleva et al. 2022a). Raman spectroscopy and Time-of-Flight secondary ion mass spectrometry (ToF–SIMS) analysis identified the presence of free-state aldehydes and ketones within LGs (Fig. 6). It has been shown, that these substances are formed as a result of Bis-Ret photooxidation and can accumulate in LGs. This is consistent with prior findings (Wu et al. 2010; Wang et al. 2006a, b).
a Averaged Raman spectra for LG suspensions before (solid line) and after (dotted line) visible light irradiation for 100 min. Three independent experiments were conducted. In each experiment, 25 spectra were obtained (p < 0.05). (b) ToF–SIMS analysis of LG suspensions before irradiation (1) and after visible light irradiation for 2 (2), 10 (3), 40 (4), 100 (5), and 160 (6) min. On the abscissa axis, the numbers correspond to the mass of positive fragment ions, containing carbonyl groups (29: CHO + ; 43: C2H3O + ; 60: C2H4O2 + ; 69: C4H5O +). On the ordinate axis, the relative intensities of the corresponding positive fragment ions are plotted as relative units. Data are presented as means ± SD from nine independent experiments. * p < 0.01 (Yakovleva et al. 2022a)
Together, fluorescence spectroscopy, high-performance liquid chromatography, and mass spectrometry revealed that BisRets-OX have both hydrophilic and amphiphilic properties, allowing their diffusion through LG membrane into the RPE cell cytoplasm (Yakovleva et al. 2022a). These products contain cytotoxic carbonyls, which are thiobarbituric acid (TBA)-active products (Fig. 7).
a Concentrations of TBA-active products in samples of the supernatants from the original LG suspension (1), LG suspension irradiated with visible light for 60 min (2), or LG suspension oxidized by superoxide radicals (3). (b) Fluorescence spectra of the supernatants (the spectrum numbers correspond to the sample numbers in panel A). The fluorescence excitation wavelength was 365 nm. (c) Supernatant content of TBA-active products from LG suspension irradiated with visible light for 90 min (1), and in the aqueous (2) and chloroform (3) fractions. Data are presented as means ± SD from three independent experiments. * p < 0.05 (Yakovleva et al. 2022a)
There are a number of works (Schutt et al. 2003; Ye et al. 2016; Hyttinen et al. 2018; Rózanowska and Rózanowski 2022) where it was shown that the source of TBA-active products in LGs is lipid peroxidation end-products i.e., highly reactive electrophilic aldehydes like malondialdehyde (MDA) and 4-hydroxynonenal (HNE). Some recent studies have shown that LG BisRets are involved in the development of photoinduced glycative stress, not only in RPE tissue, but also in adjacent tissues, in particular, in Bruch’s membrane (Zhou et al. 2015; Thao et al. 2014). It was suggested that the development of glycative stress in the RPE cells is largely associated with the photooxidative destruction of LG BisRets, leading to the formation of water-soluble reactive carbonyls which are extremely cytotoxic molecules (Schleicher et al. 2001). They are assumed to be the main precursors of advanced glycation end products (AGEs) formation (Rowan et al. 2018; Lin et al. 2016) and can be formed by direct oxidative decay of BisRets (Kim et al. 2021). In our work (Dontsov et al. 2022), we have shown that water-soluble carbonyl compounds formed during A2E photooxidation cause modification of serum albumin and hemoglobin. The antiglycation agent, aminoguanidine, has inhibited the process of protein modification. It is assumed that these carbonyl products can initiate the inflammatory processes in the retina and RPE.
It should be noted that BisRets-OX can be formed not only during BisRet photo-oxidation, but also during the oxidative destruction of BisRets in the dark via non-lipofuscin ROS (Yakovleva et al. 2022a, b). Thus, a significant change in the fluorescent properties of retinoids in the retina and RPE from mouse eye exposed to ionizing radiation (IR) was detected (Yakovleva et al. 2022b). Such changes occur when retinoids are oxidized, suggesting that IR induce oxidation and degradation of retinoids, similar to photo-oxidation of BisRets in the human RPE (Feldman et al. 2015, 2018). However, in the case of IR, in the absence of light, the source of ROS is different. For example, these can be ROS-generated by mitochondria, caused by IR, or water radiolysis (Kobashigawa et al. 2011; Azzam et al. 2012; Belli and Indovina 2020). Thus, LGs can have a damaging effect on the RPE cell through the BisRets-OX formation. Therefore, BisRets-OX are a likely aggravating factor in the progression of various senile eye pathologies.
The RPE cells are constantly exposed to (photo)oxidative stress. This is facilitated by high oxygen consumption and prolonged exposure to light (Beatty et al. 2000). An important factor contributing to the increase in oxidative stress in the RPE cells is the progressive age-related accumulation of LGs (Feeney–Burns et al. 1984; Wing et al. 1978; Delori et al. 2001) and a decrease in the content of melanosomes (Schmidt and Peisch 1986; Sarna et al. 2003; Dontsov et al. 2017; Yacout et al. 2019). These processes lead to the appearance of damaged and modified cellular proteins, lipids, and DNA (Kohen and Nyska 2002). Oxidative stress and inflammation are of great importance in the development of degenerative processes in the RPE cells. So, it is believed that oxidative stress plays a central role in the development of AMD (Beatty et al. 2000; Datta et al. 2017; Abokyi et al. 2020; Ruan et al. 2021). It has been shown that in the RPE cells from donor eyes with AMD, compared with normal eyes, there are increased levels of TBA-active products, protein carbonyls (Totan et al. 2009), a high content of carboxyethylpyrrole in Bruch’s membrane (Crabb et al. 2002; Lu et al. 2009), as well as oxidative damage and dysfunction of mitochondria (Terluk et al. 2015; Blasiak et al. 2013; Golestaneh et al. 2018). Also, in AMD, the accumulation of damaged proteins and disruption of the autophagy process are noted (Mitter et al. 2014). The modified and damaged proteins formed as a result of oxidative stress, which are incapable of repair by heat shock proteins, are directed to the proteasome for purification. However, if the activity of the proteasome is reduced (Zhang et al. 2008; Fernandes et al. 2008), proteins aggregate and can be degraded by autophagy (Fig. 8).
Autophagy is also used by the RPE cells to digest damaged mitochondria (mitophagy). A decrease in the activity of lysosomal enzymes as a result of oxidative stress (Brunk et al. 1995; Nilsson et al. 2003; Sparrow and Boulton 2005) leads to inhibition of the autophagy process. ROS also destroy the integration relationship with proteasomes and autophagy, which ultimately leads to increased accumulation of toxic aggregates, development of chronic inflammation, activation of the complement system, formation of extracellular drusen, and death of the RPE cells (Kinnunen et al. 2012; Ferrington et al. 2016; Moreno-García et al. 2018).
Fluorescence characteristics of LGs as a tool to detect early stages of degeneration in the retina and RPE
LGs exhibit distinct fluorescence in the visible region. BisRets and BisRets-OX are major sources of LG fluorescence. Fundus autofluorescence (FAF) imaging is a noninvasive, prospective diagnostic method based on the detection of LG fluorescence in the RPE cells (Von Ruckmann et al. 1997; Holz et al. 2007; Sparrow et al. 2010a, b). FAF is excited by a wavelength of 488 nm, yielding monochromatic images in the long-wavelength region, starting at 500 nm, resulting from the fluorescence of BisRets and BisRets-OX. FAF can detect early phenotypic changes in RPE, occurring prior to the progression of disease. Analysis of FAF patterns can provide detailed qualitative information that allows the detection of areas of pathology, thereby differentiating among different types of ocular disease. At present, however, it is not possible to quantify the detected changes. The degree of disease progression must be assessed subjectively by an expert, who compares patterns with those of normal eyes.
Efforts are underway to expand the capabilities of this diagnostic method, based on knowledge about the spectral characteristics of LG fluorophores. Increased blue-green autofluorescence of the Bruch’s membrane, relative to the yellow-orange autofluorescence of RPE-associated lipofuscin, is associated with AMD (Marmorstein et al. 2002). In addition, with the use of fluorescence lifetime imaging ophthalmoscopy (FLIO) in vivo, healthy eyes were shown to exhibit different patterns than those of AMD eyes (Sauer et al. 2018a, b, c; Schweitzer et al. 2009). Specific patterns were also detected for retinitis pigmentosa (Andersen et al. 2018; Dysli et al. 2018), Stargardt disease (Dysli et al. 2016), macular telangiectasia type 2 (MacTel) (Sauer et al. 2018a, b, c), and other diseases (Sauer et al. 2018a, b, c). Changes from normal fluorescence parameters have also been observed in the eyes of patients with diabetes (Schweitzer et al. 2015) and Alzheimer’s disease (Jentsch et al. 2014; Sadda et al. 2019). However, it should be noted that none of these publications have explained the nature or underlying mechanism of these differences. Nevertheless, these findings, especially in AMD, were confirmed by experimental research, which has demonstrated that the quantitative and qualitative properties of LG fluorophores change during pathologic development (Feldman et al. 2015, 2018; Wu et al. 2010).
The main drawback of these experimental studies is the inability to analyze LG fluorophore composition in vivo, as more than 20 fluorophores, bisretinoids, and their derivatives have been identified to date. Nevertheless, one of the main objectives of in vivo FAF and FLIO methods is to determine differences in the fluorescence characteristics of the fundus between individuals with ocular pathology and those with healthy eyes.
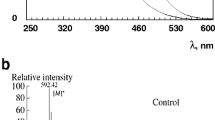
Recently, we have shown that the content of BisRets-OX increases with the development of AMD (Feldman et al. 2018). Because, the fluorescence can show the accumulation of BisRets-OX in LGs, therefore BisRets-OX may be an indicator of AMD progression. Changes in the quantitative and qualitative composition of fluorophores and their spectral characteristics are determined only by the presence or absence of pathological changes in the RPE, but are independent of age. These patterns have characteristic and reproducible features that can be used as diagnostic indicators of visual pathology (Fig. 9a).
a Comparative statistical analysis of spectral characteristics of the RPE cell suspensions from cadaver eyes without (Norm) and with (Pathology) signs of AMD. Fluorescence spectra were averaged for 19 normal eyes (from donors aged 27–74 years), and for 12 AMD eyes (from donors aged 59–88 years). The excitation wavelength was 488 nm, with emission spectra normalized at 592 nm. (b) Fluorescence decay kinetic curves (normalized) of LG fluorophores of the RPE cells from human cadaver eyes. The samples of the RPE cell suspensions were from normal eyes (Norm) from a 74-year-old donor, and the other from eyes with AMD (Pathology) from a 75-year-old donor. Fluorescence was excited at 485 nm (pulse duration, 30 ps), and kinetic curves were recorded at 540 nm. Figure was modified from Feldman et al. (2018)
Moreover, the fluorescence lifetimes were measured by picosecond-resolved time correlated single photon counting technique (Feldman et al. 2018; Yakovleva et al. 2020) (Fig. 9b). It was shown that BisRets-OX exhibited a longer fluorescence lifetime (average value approximately 6 ns) and a shorter wavelength maximum (530–580 nm). Further, these products significantly contributed (more than 30%) to total fluorescence compared to the other fluorophores in LGs. Thus, the contribution of BisRets-OX to autofluorescence decay kinetics is an important characteristic for fluorescence lifetime imaging microscopy data analysis (Feldman et al. 2018; Yakovleva et al. 2020).
Based on the data obtained, we can deduce that the specific pattern observed in AMD eyes in vivo using FLIO could be due to accumulation of BisRets-OX in LGs (Sauer et al. 2018a, b, c; Schweitzer et al. 2009). Thus, we suggest this as an additional approach to expand the diagnostic capabilities of the FLIO method. Instead of using the averaged fluorescence lifetimes for different eye tissues with fluorescence excitation at 468 nm (Schweitzer et al. 2007), we propose to focus only on LG fluorophores from the RPE with fluorescence excitation at 488 nm (similar to the FAF method) in order to determine the contribution of BisRets-OX to total fluorescence or determine the averaged fluorescence lifetime, because the higher BisRet-OX content in the RPE from AMD eyes increased the average fluorescence lifetime (Feldman et al. 2018; Yakovleva et al. 2020).
Earlier, we have shown that LG BisRets can be oxidized not only in the presence of light, but also by ROS of a non-lipofuscin nature (Yakovleva et al. 2022a, b). In other words, the spectral properties of LG fluorophores can be markers of oxidative stress which can initiate degenerative processes in the retina and RPE. Thus, our findings contribute to progress in the creation of rapid testing of the oxidative stress development in living organisms, and contribute to development of a predictive criterion for increased AMD risk in future.
Thus, there is a possibility to improve the FAF and FLIO techniques to obtain additional information from the total fluorescence patterns. Quantitative determination of increases in BisRets-OX in LGs may be used to establish quantitative diagnostic criteria for degenerative processes in the retina and RPE even in the absence of visible manifestation of the disease.
Conclusion
Lipofuscin was discovered by Virchow R. as early as 1847, and the term “lipofuscin” itself has been used since 1912. Earlier, lipofuscin was traditionally referred to as intracellular and inert “slag” as a marker of aging. It is known that the progressive accumulation of lipofuscin can somehow lead to pathology and accelerate the aging process. However, the mechanism of pathogenetic action of lipofuscin remained unknown until recently.
The nature of the lipofuscin accumulation in cells is associated with the destruction of cell organelles that have not been utilized by lysosomes. In the case of the RPE, these are mainly nondigested fragments of phagocytosed outer segments of photoreceptors. The accumulation of LGs is explained by the absence of enzymes in the cell that can degrade it. So far, there are no effective ways to both slow down the LG accumulation in the cell and remove it from the cell. Despite some encouraging experimental results on the removal of LGs from RPE cells, they have not reached clinical use.
Significant interest in the pathogenetic role of lipofuscin arose in the early nineties after we discovered the photoactivity of lipofuscin granules isolated from human cadaver eyes (Ostrovsky et al. 1992; Boulton et al. 1993). It turned out that LGs are not an inert “slag”, but extracellular structures capable of ROS generating under the action of visible light.
Approximately, at the same time and later, active development and improvement of optical methods for recording fundus autofluorescence began. It was about short-wavelength autofluorescence as a non-invasive diagnostic method for detecting LGs, a by-product of the visual cycle, which accumulates in the RPE cells with age or disease.
Over the past decades, we have conducted research both in the direction of the action of light on LGs, and in the direction of changes in the spectral characteristics of the LGs fluorescence in pathology and under the action of ionizing radiation. The results of these studies significantly expanded our understanding of both the photoactivity and fluorescence of LGs and the fluorophores (bisretinoids) contained in them. The main results of these studies are as follows.
First, our studies of the structure and fluorescence properties of LGs by AFM and near-field microscopy revealed the fluorescent heterogeneity of LGs. This means that twenty or more LG fluorophores are unevenly distributed in the granule. It would of course be interesting to compare this distribution in non-photooxidized (normal) and photooxidized (pathological) LGs.
Secondly, our study of the phototoxicity of LGs which are photo-inducible generators of ROS, showed the following. LGs and melanolipofuscin granules of human RPE cells are capable of photoinduced oxidation of lipids. At the same time, LGs are more active than melanolipofuscin granules. Another rather unexpected result is that A2E, as the main fluorophore (BisRet) of LGs capable of photogeneration of ROS, was much less active as an oxidation photosensitizer than other fluorophores (BisRets) of LGs. In this regard, it would be important to establish which BisRet or group of BisRet fluorophores represent the greatest danger in terms of phototoxicity. Fundamentally important for understanding the mechanisms of LG phototoxicity is the fact that BisRets-OX pose a much greater danger to the cell than, non-oxidized products. Moreover, the BisRets-OX formed in the dark as a result of BisRet oxidative degradation also have significant toxicity for the cell.
Thirdly, our studies of the fluorescent properties of LGs and their fluorophores (BisRets and BisRets-OX) showed for the first time that their spectral characteristics change, namely, they shift to the short-wavelength region, in pathology (AMD) and after exposure to ionizing radiation (gamma-rays and protons).
It is important to emphasize the promise of recording not only changes in the spectra, but also the decay kinetics of FAF for the early diagnosis of degenerative diseases of the retina. These changes are associated precisely with the formation and accumulation of LG BisRets-OX. In other words, by recording the fluorescence decay kinetics of oxidized products of LG BisRets-OX, it is possible to significantly improve the methods of early diagnosis of degenerative diseases, primarily AMD.
Thus, there is no need to talk about any inertness of lipofuscin (“aging pigment”) as an inert “slag”. The photoactivity of lipofuscin can pose a significant danger to the RPE cells. Based on this, it is necessary to observe the well-known light hygiene measures for the senile and diseased eye (sunglasses, colored intraocular lenses, etc.). The search for pharmacological agents that prevent the toxicity of LG fluorophores (BisRets) and their oxidation products is extremely relevant. It would also be very important to find a drug capable of delaying the formation of LGs into the cells or removing (destroying) LGs from the cell.
References
Abokyi S, To C-H, Lam TT, Tse DY (2020) Central role of oxidative stress in age-related macular degeneration: evidence from a review of the molecular mechanisms and animal models. Oxid Med Cell Longev 2020:7901270. https://doi.org/10.1155/2020/7901270
Andersen KM, Sauer L, Gensure RH, Hammer M, Bernstein PS (2018) Characterization of retinitis pigmentosa using fluorescence lifetime imaging ophthalmoscopy (FLIO). Transl Vision Sci Technol 7(3):20. https://doi.org/10.1167/tvst.7.3.20
Avalle LB, Dillon J, Tari S, Gaillard ER (2005) A new approach to measuring the action spectrum for singlet oxygen pro-duction by human retinal lipofuscin. Photochem Photobiol 81:1347–1350. https://doi.org/10.1562/2005-05-17-RN-531
Azzam EI, Jay-Gerin JP, Pain D (2012) Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett 327:48–60. https://doi.org/10.1016/j.canlet.2011.12.012
Bazan HEP, Bozan NG, Feeney-Burns L, Berman ER (1990) Lipids in human lipofuscin-enriched subcellular fractions of two age populations. Comparison with rod outer segments and neural retina. Invest Ophthalmol vis Sci 31:1433–1443
Beatty S, Koh H, Phil M, Henson D, Boulton M (2000) The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol 45:115–134. https://doi.org/10.1155/2020/7901270
Belli M, Indovina L (2020) The response of living organisms to low radiation environment and its implications in radiation protection. Front Public Health 8:601711. https://doi.org/10.3389/fpubh.2020.601711
Ben-Shabat S, Itagaki Y, Jockusch S, Sparrow JR, Turro NJ, Nakanishi K (2002) Formation of a nona-oxirane from A2E, a lipofuscin fluorophore related to macular degeneration, and evidence of singlet oxygen involvement. Angew Chem Int Ed Engl 41:814–817. https://doi.org/10.1002/1521-3773(20020301)41:5%3c814::aid-anie814%3e3.0.co;2-2
Bhosale P, Serban B, Bernstein PS (2009) Retinal carotenoids can attenuate formation of A2E in the retinal pigment epithelium. Arch Biochem Biophys 483(2):175–181. https://doi.org/10.1016/j.abb.2008.09.012
Blasiak J, Glowacki S, Kauppinen A, Kaarniranta K (2013) Mitochondrial and nuclear DNA damage and repair in age-related macular degeneration. Int J Mol Sci 14:2996–3010. https://doi.org/10.3390/ijms14022996
Boulton M, Docchio F, Dayhaw-Barker P, Ramponi R, Cubeddu R (1990) Age-related changes in the morphology, absorption and fluorescence of melanosomes and lipofuscin granules of the retinal pigment epithelium. Vision Res 30:1291–1303. https://doi.org/10.1016/0042-6989(90)90003-4
Boulton M, Dontsov A, Jarvis-Evans J, Ostrovsky M, Svistunenko D (1993) Lipofuscin is a photoinducible free radical generator. J Photochem Photobiol B Biol 19:201–204. https://doi.org/10.1016/1011-1344(93)87085-2
Boulton M, Rozanowska M, Rozanowski B, Wess T (2004) The photoreactivity of ocular lipofuscin. Photochem Photobiol Sci 3(8):759–764. https://doi.org/10.1039/B400108G
Broniec A, Pawlak A, Sarna T, Wielgus A, Roberts JE, Land EJ, Truscott TG, Edge R, Navaratnam S (2005) Spectroscopic properties and reactivity of free radical forms of A2E. Free Radic Biol Med 38:1037–1046. https://doi.org/10.1016/j.freeradbiomed.2004.12.023
Brunk UT, Wihlmark U, Wrigstad A, Roberg K, Nilsson SE (1995) Accumulation of lipofuscin within retinal pigment epithelial cells results in enhanced sensitivity to photo-oxidation. Gerontology 41:201–212. https://doi.org/10.1159/000213743
Bynoe LA, Del Priore LV, Hornbeck R (1998) Photosensitization of retinal pigment epithelium by protoporphyrin IX. Graefes Arch Clin Exp Ophthalmol 236(3):230–233. https://doi.org/10.1007/s004170050069
Cantrell A, McGarvey DJ, Roberts J, Sarna T, Truscott TG (2001) Photochemical studies of A2-E. J Photochem Photobiol, B 64(2–3):162–165. https://doi.org/10.1016/S1011-1344(01)00224-X
Crabb JW, Miyagi M, Gu X, Shadrach K, West KA, Sakaguchi H, Kamei M, Hasan A, Yan L, Rayborn ME, Salomon RG, Hollyfield JG (2002) Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci USA 99:14682–14687. https://doi.org/10.1073/pnas.222551899
Clancy CMR, Krogmeier JR, Pawlak A, Rozanowska M, Sarna T, Dunn RC, Simon J (2000) Atomic force microscopy and near-field scanning optical microscopy measurements of single human retinal lipofuscin granules. J Phys Chem B 104:12098–12100. https://doi.org/10.1021/jp0030544
Datta S, Cano M, Ebrahimi K, Wang L, Handa JT (2017) The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog Retin Eye Res 60:201–218. https://doi.org/10.1016/j.preteyeres.2017.03.002
Davies S, Elliott MH, Floor E, Truscott TG, Zareba M, Sarna T, Shamsi FA, Boulton ME (2001) Photocytotoxicity of lipofuscin in human retinal pigment epithelial cells. Free Radic Biol Med 31(2):256–265. https://doi.org/10.1016/S0891-5849(01)00582-2
De S, Sakmar TP (2002) Interaction of A2E with model membranes. Implications to the pathogenesis of age-related macular degeneration. J Gen Physiol 120:147–157. https://doi.org/10.1085/jgp.20028566
Delori FC, Goger DG, Dorey CK (2001) Age-related accumulation and spatial distribution of lipofuscin in RPE of normal subjects. Invest Ophthalmol vis Sci 42:1855–1866
Dontsov AE, Glickman RD, Ostrovsky MA (1999) Retinal pigment epithelium pigment granules stimulate the photo-oxidation of unsaturated fatty acids. Free Radic Biol Med 26:1436–1446. https://doi.org/10.1016/S0891-5849(99)00003-9
Dontsov AE, Koromyslova AD, Sakina NL (2013) Lipofuscin component A2E does not reduce antioxidant activity of DOPA-melanin. Bull Exp Biol Med 154(5):624–627. https://doi.org/10.1007/s10517-013-2015-6
Dontsov A, Koromyslova A, Ostrovsky M, Sakina N (2016) Lipofuscins prepared by modification of photoreceptor cells via glycation or lipid peroxidation show the similar phototoxicity. World J Exp Med 6(4):63–71. https://doi.org/10.5493/wjem.v6.i4.63
Dontsov AE, Sakina NL, Bilinska B, Krzyzanowski L, Feldman TB, Ostrovsky MA (2005) Comparison of photosensitizing effect of lipofuscin granules from retinal pigment epithelium of human donor eyes and their fluorophore A2E. Dokl Biochem Biophys 405:458–460. https://doi.org/10.1007/s10628-005-0139-y
Dontsov AE, Sakina NL, Golubkov AM, Ostrovsky MA (2009) Light-induced release of A2E photooxidation toxic products from lipofuscin granules of human retinal pigment epithelium. Dokl Biochem Biophys 425:98–101. https://doi.org/10.1134/S1607672909020112
Dontsov AE, Sakina NL, Ostrovsky MA (2012) Comparative study of the dark and light induced toxicity of lipofuscin granules from human retinal pigment epithelium and their chromophore A2E on the cardiolipin liposome model. Rus Cheml Bull Intl Ed 61:442–448. https://doi.org/10.1007/s11172-012-0061-2
Dontsov AE, Sakina NL, Ostrovsky MA (2017) Loss of melanin by eye retinal pigment epithelium cells is associated with its oxidative destruction in melanolipofuscin granules. Biochemistry (mosc) 82:916–924. https://doi.org/10.1134/S0006297917080065
Dontsov A, Yakovleva M, Trofimova N, Sakina N, Gulin A, Aybush A, Gostev F, Vasin A, Feldman T, Ostrovsky M (2022) Water-soluble products of photooxidative destruction of the bisretinoid A2E cause proteins modification in the dark. Int J Mol Sci 23:1534. https://doi.org/10.3390/ijms23031534
Dysli C, Schürch K, Pascal E, Wolf S, Zinkernagel MS (2018) Fundus autofluorescence lifetime patterns in retinitis pigmentosa. Invest Ophthalmol vis Sci 59:1769–1778. https://doi.org/10.1167/iovs.17-23336
Dysli C, Wolf S, Hatz K, Zinkernagel MS (2016) Fluorescence lifetime imaging in Stargardt disease: potential marker for disease progression. Invest Ophthalmol vis Sci 57:832–841. https://doi.org/10.1167/iovs.15-18033
Eldred GE, Katz ML (1988) Fluorophores of the human retinal pigment epithelium: separation and spectral characterization. Exp Eye Res 47:71–86. https://doi.org/10.1016/0014-4835(88)90025-5
Eldred GE, Lasky MR (1993) Retinal age pigments generated by self-assembling lysosomotropic detergents. Nature 361(6414):724–726. https://doi.org/10.1038/361724a0
Feeney L (1973) The phagosomal system of the pigment epithelium: a key to retinal disease. Invest Ophthalmol vis Sci 12:635–638
Feeney-Burns L, Burns RP, Gao C-L (1990) Age-related macular changes in humans over 90 years old. Am J Ophthalmol 109:265–278. https://doi.org/10.1016/S0002-9394(14)74549-0
Feeney-Burns L, Hilderbrand ES, Eldridge S (1984) Aging human RPE: morphometric analysis of macular, equatorial, and peripheral cells. Invest Ophthalmol vis Sci 25:195–200
Feldman TB, Yakovleva MA, Arbukhanova PM, Borzenok SA, Kononikhin AS, Popov IA, Nikolaev EN, Ostrovsky MA (2015) Changes in spectral properties and composition of lipofuscin fluorophores from human retinal pigment epithelium with age and pathology. Anal Bioanal Chem 407:1075–1088. https://doi.org/10.1007/s00216-014-8353-z
Feldman TB, Yakovleva MA, Larichev AV, Arbukhanova PM, Ash R, Borzenok SA, Kuzmin VA, Ostrovsky MA (2018) Spectral analysis of fundus autofluorescence pattern as a tool to detect early stages of degeneration in the retina and retinal pigment epithelium. Eye 32:1440–1448. https://doi.org/10.1038/s41433-018-0109-0
Fernandes AF, Zhou J, Zhang X, Bian Q, Sparrow J, Taylor A, Pereira P, Shang F (2008) Oxidative inactivation of the proteasome in retinal pigment epithelial cells. A potential link between oxidative stress and upregulation of interleukin-8. J Biol Chem 283(30):20745–20753. https://doi.org/10.1074/jbc.M800268200
Ferrington DA, Sinha D, Kaarniranta K (2016) Defects in retinal pigment epithelial cell proteolysis and the pathology associated with age-related macular degeneration. Prog Retin Eye Res 51:69–89. https://doi.org/10.1016/j.preteyeres.2015.09.002
Gaillard ER, Avalle LB, Keller LM, Wang Z, Reszka KJ, Dillon JP (2004) A mechanistic study of the photooxidation of A2E, a component of human retinal lipofuscin. Exp Eye Res 79(3):313–319. https://doi.org/10.1016/j.exer.2004.05.005
Golestaneh N, Chu Y, Xiao Y-Y, Stoleru GL, Theos AC (2018) Dysfunctional autophagy in RPE, a contributing factor in age-related macular degeneration. Cell Death Dis 8(1):e2537. https://doi.org/10.1038/cddis.2016.453
Haralampus-Grynaviski NM, Lamb LE, Clancy CM, Skumatz C, Burke JM, Sarna T, Simon JD (2003) Spectroscopic and morphological studies of human retinal lipofuscin granules. Proc Natl Acad Sci U S A 100(6):3179–3184. https://doi.org/10.1073/pnas.0630280100
Holz FG, Pauleikhoff D, Klein R, Bird AC (2004) Pathogenesis of lesions in late age-related macular disease. Am J Ophthalmol 137:504–510. https://doi.org/10.1016/j.ajo.2003.11.026
Holz FG, Schmitz-Valckenberg S, Spaide RF, Bird AC (2007) Atlas of fundus autofluorescence imaging. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-540-71994-6
Holz FG, Schutt F, Kopitz J, Eldred GE, Kruse FE, Volcker HE, Cantz M (1999) Inhibition of lysosomal degradative functions in RPE cells by a retinoid component of lipofuscin. Invest Ophthalmol vis Sci 40(3):737–743
Hyttinen JMT, Viiri J, Kaarniranta K, Błasiak J (2018) Mitochondrial quality control in AMD: does mitophagy play a pivotal role? Cell Mol Life Sci 75:2991–3008. https://doi.org/10.1007/s00018-018-2843-7
Jentsch S, Schweitzer D, Schmidtke KU, Peters S, Dawczynski J, Bar KJ, Hammer M (2014) Retinal fluorescence lifetime imaging ophthalmoscopy measures depend on the severity of Alzheimer’s disease. Acta Ophthalmol 93:e241–e247. https://doi.org/10.1111/aos.12609
Jung T, Bader N, Grune T (2007) Lipofuscin: formation, distribution, and metabolic consequences. Ann NY Acad Sci 1119:97–111. https://doi.org/10.1196/annals.1404.008
Katz M (2002) Potential role of retinal pigment epithelial lipofuscin accumulation in age-related macular degeneration. Arch Gerontol Geriatric 34:359–370. https://doi.org/10.1016/S0167-4943(02)00012-2
Kennedy CJ, Rakoczy PE, Constable IJ (1995) Lipofuscin of the retinal pigment epithelium: a review. Eye 9:763–771. https://doi.org/10.1038/eye.1995.192
Kim SR, Jang YP, Jockusch S, Fishkin NE, Turro NJ, Sparrow JR (2007) The all-trans-retinal dimer series of lipofuscin pigments in retinal pigment epithelial cells in a recessive Stargardt disease model. Proc Natl Acad Sci USA 104:19273–19278. https://doi.org/10.1073/pnas.0708714104
Kim HJ, Montenegro D, Zhao J, Sparrow JR (2021) Bisretinoids of the retina: photo-oxidation, iron-catalyzed oxidation, and disease consequences. Antioxidants 10(9):1382. https://doi.org/10.3390/antiox10091382
Kinnunen K, Petrovski G, Moe MC, Berta A, Kaarniranta K (2012) Molecular mechanisms of retinal pigment epithelium damage and development of age-related macular degeneration. Acta Ophthalmol 90:299–309. https://doi.org/10.1111/j.1755-3768.2011.02179.x
Kobashigawa S, Suzuki K, Yamashita S (2011) Ionizing radiation accelerates Drp1-dependent mitochondrial fission, which involves delayed mitochondrial reactive oxygen species production in normal human fibroblast-like cells. Biochem Biophys Res Commun 414:795–800. https://doi.org/10.1016/j.bbrc.2011.10.006
Kohen R, Nyska A (2002) Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol 30:620–650. https://doi.org/10.1080/01926230290166724
Lakkaraju A, Finnemann SC, Rodriguez-Boulan E (2007) The lipofuscin fluorophore A2E perturbs cholesterol metabolismin retinal pigment epithelial cells. Proc Natl Acad Sci USA 104(26):11026–11031. https://doi.org/10.1073/pnas.0702504104
Lamb LE, Simon JD (2004) A2E: a component of ocular lipofuscin. Photochem Photobiol 79:127–136. https://doi.org/10.1111/j.1751-1097.2004.tb00002.x
Lin J-A, Wu C-H, Lu C-C, Hsia S-M, Yen G-C (2016) Glycative stress from advanced glycation end products (AGEs) and dicarbonyls: an emerging biological factor in cancer onset and progression. Mol Nutr Food Res 60:1850–1864. https://doi.org/10.1002/mnfr.201500759
Lu L, Gu X, Hong L, Laird J, Jaffe K, Choi J, Crabb J, Salomon RG (2009) Synthesis and structural characterization of carboxyethylpyrrole-modified proteins: mediators of age-related macular degeneration. Bioorg Med Chem 17(21):7548–7561. https://doi.org/10.1016/j.bmc.2009.09.009
Maeda A, Maeda T, Golczak M, Chou S, Desai A, Hoppel CL, Matsuyama S, Palczewski K (2009) Involvement of all-trans-retinal in acute light-induced retinopathy of mice. J Biol Chem 284:15173–15183. https://doi.org/10.1074/jbc.M900322200
Mahendra CK, Tan LTH, Pusparajah P, Htar TT, Chuah L, Lee VS, Low LE, Tang SY, Chan K-G, Goh BH (2020) Detrimental effects of UVB on retinal pigment epithelial cells and its role in age-related macular degeneration. Oxid Med Cell Longev 2020:1904178. https://doi.org/10.1155/2020/1904178
Marmorstein AD, Marmorstein LY, Sakaguchi H, Hollyfield JG (2002) Spectral profiling of autofluorescence associated with lipofuscin, bruch’s membrane, and sub-RPE deposits in normal and AMD eyes. Invest Ophthalmol vis Sci 43:2435
Mitter SK, Song C, Qi X, Mao H, Rao H, Akin D, Lewin A, Grant M, Dunn W Jr, Ding J, Bowes Rickman C, Boulton M (2014) Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy 10(11):1989–2005. https://doi.org/10.4161/auto.36184
Moreno-García A, Kun A, Calero O, Medina M, Calero M (2018) An overview of the role of lipofuscin in age-related neurodegeneration. Front Neurosci 12:464. https://doi.org/10.3389/fnins.2018.00464
Murdaugh LS, Avalle LB, Mandal S, Dill AE, Dillon J, Simon JD, Gaillard ER (2010) Compositional studies of human RPE lipofuscin. J Mass Spectrom 45:1139–1147. https://doi.org/10.1002/jms.1795
Murdaugh LS, Mandal S, Dill AE, Dillon J, Simon D, Gaillard ER (2011) Compositional studies of human RPE lipofuscin: mechanisms of molecular modifications. J Mass Spectrom 46:90–95. https://doi.org/10.1002/jms.1865
Ng KP, Gugiu B, Renganathan K, Davies MW, Gu X, Crabb JS, Kim SR, Różanowska MB, Bonilha VL, Rayborn ME, Salomon RG, Sparrow JR, Boulton ME, Hollyfield JG, Crabb JW (2008) Retinal pigment epithelium lipofuscin proteomics. Mol Cell Proteomics 7:1397–1405. https://doi.org/10.1074/mcp.M700525-MCP200
Nilsson SE, Sundelin SP, Wihlmark U, Brunk UT (2003) Aging of cultured retinal pigment epithelial cells: oxidative reactions, lipofuscin formation and blue light damage. Doc Ophthalmol 106(1):13–16. https://doi.org/10.1023/a:1022419606629
Nowak JZ (2013) Oxidative stress, polyunsaturated fatty acids-derived oxidation products and bisretinoids as potential inducers of CNS diseases: focus on age-related macular degeneration. Pharmacol Rep 65(2):288–304. https://doi.org/10.1016/s1734-1140(13)71005-3
Olchawa MM, Szewczyk GM, Zadlo AC, Krzysztynska-Kuleta OI, Sarna TJ (2021) The effect of aging and antioxidants on photoreactivity and phototoxicity of human melanosomes: an in vitro study. Pigment Cell Melanoma Res 34:670–682. https://doi.org/10.1111/pcmr.12914
Ostrovsky MA, Dontsov AE, Sakina NL, Boulton M, Jarvis-Evans J (1992) The ability of lipofuscin granules of the retinal pigment epithelium of the human eye a photosensitized peroxidation lipid by the action of visible light. Sensor Systems 6:51–54
Ostrovsky MA, Dontsov AE (2019) Vertebrate eye melanosomes and invertebrate eye ommochromes as antioxidant cell organelles. Biology Bulletin 46:105–116
Ostrovsky MA, Sakina NL, Dontsov AE (1987) An antioxidative role of ocular screening pigments. Vision Res 27:893–899. https://doi.org/10.1016/0042-6989(87)90005-8
Ostrovsky MA, Zak PP, Dontsov AE (2018) Vertebrate eye melanosomes and invertebrate eye ommochromes as screening cell organelles. Biology Bulletin 45:570–579. https://doi.org/10.1134/S1062359018060109
Parish CA, Hashimoto M, Nakanishi K, Dillon J, Sparrow J (1998) Isolation and one-step preparation of A2E and iso-A2E, fluorophores from human retinal pigment epithelium. Proc Natl Acad Sci USA 95(25):14609–14613. https://doi.org/10.1073/pnas.95.25.14609
Pawlak A, Rozanowska M, Zareba M, Lamb LE, Simon JD, Sarna T (2002) Action spectra for the photo consumption of oxygen by human ocular lipofuscin and lipofuscin extracts. Arch Biochem Biophys 403(1):59–62. https://doi.org/10.1016/S0003-9861(02)00260-6
Pawlak A, Wrona M, Rozanowska M, Zareba M, Lamb LE, Roberts JE, Simon JD, Sarna T (2003) Comparison of the aerobic photoreactivity of A2E with its precursor retinal. Photochem Photobiol 77:253–258. https://doi.org/10.1562/0031-8655(2003)077%3c0253:cotapo%3e2.0.co;2
Petrukhin AN, Astaf’ev AA, Zolotavin PN, Fel’dman TB, Dontsov AE, Sarkisov OM, Ostrovsky MA. (2005) Heterogeneity of structure and fluorescence of single lipofuscin granule from retinal pigment epithelium of human donor eyes: study with the use of atomic force microscopy and near-field microscopy. Dokl Biochem Biophys 405:445–449. https://doi.org/10.1007/s10628-005-0136-1
Ragauskaite L, Heckathorn RC, Gaillard ER (2001) Environmental effects on the photochemistry of A2-E, a component of human retinal lipofuscin. Photochem Photobiol 74(3):483–488. https://doi.org/10.1562/0031-8655(2001)074%3c0483:eeotpo%3e2.0.co;2
Roberts JE, Kukieczak BM, Hu DN, Miller DS, Bilski P, Sik RH, Motten AG, Chignell CF (2002) The role of A2E in prevention or enhancement of light damage in human retinal pigment epithelial cells. Photochem Photobiol 75(2):184–190. https://doi.org/10.1562/0031-8655(2002)075%3c0184:troaip%3e2.0.co;2
Rowan S, Bejarano E, Taylor A (2018) Mechanistic targeting of advanced glycation end-products in age-related diseases. BBA Mol Basis Dis 1864:3631–3643. https://doi.org/10.1016/j.bbadis.2018.08.036
Rozanowska M, Jarvis-Evans J, Korytowski W, Boulton ME, Burke JM, Sarna T (1995) Blue light-induced reactivity of retinal age pigment. In vitro generation of oxygen-reactive species. J Biol Chem 270:18825–18830. https://doi.org/10.1074/jbc.270.32.18825
Rozanowska M, Korytowski W, Rozanowski B, Skumatz C, Boulton ME, Burke JM, Sarna T (2002) Photoreactivity of aged human RPE melanosomes: a comparison with lipofuscin. Invest Ophthalmol vis Sci 43(7):2088–2096
Rozanowska M, Pawlak A, Rozanowski B, Skumatz C, Zareba M, Boulton ME, Burke JM, Sarna T, Simon JD (2004) Age-related changes in the photoreactivity of retinal lipofuscin granules: role of chloroform-insoluble components. Invest Ophthalmol vis Sci 45(4):1052–1060. https://doi.org/10.1167/iovs.03-0277
Rózanowska MB, Rózanowski B (2022) Photodegradation of lipofuscin in suspension and in ARPE-19 cells and the similarity of fluorescence of the photodegradation product with oxidized docosahexaenoate. Int J Mol Sci 23:922. https://doi.org/10.3390/ijms23020922
Różanowska M, Sarna T (2005) Light-induced damage to the retina: role of rhodopsin chromophore revisited. Photochem Photobiol 81:1305–1330. https://doi.org/10.1562/2004-11-13-IR-371
Rozanowska M, Wessels J, Boulton M, Burke JM, Rodgers MAJ, Truscott TG, Sarna T (1998) Blue light-induced singlet oxygen generation by retinal lipofuscin in non-polar media. Free Radic Biol Med 24:1107–1112. https://doi.org/10.1016/S0891-5849(97)00395-X
Ruan Y, Jiang S, Gericke A (2021) Age-related macular degeneration: role of oxidative stress and blood vessels. Int J Mol Sci 22:1296. https://doi.org/10.3390/ijms22031296
Sadda SR, Borrelli E, Fan W, Ebraheem A, Marion KM, Harrington M, Kwon S (2019) A pilot study of fluorescence lifetime imaging ophthalmoscopy in preclinical Alzheimer’s disease. Eye 33:1271–1279. https://doi.org/10.1038/s41433-019-0406-2
Sakai N, Decatur J, Nakanishi K, Eldred GE (1996) Ocular age pigment “A2E”: an unprecedented pyridinium bisretinoid. J Am Chem Soc 118:1559–1560. https://doi.org/10.1021/ja953480g
Sakina NL, Koromyslova AD, Dontsov AE, Ostrovsky MA (2013) RPE melanosomes bind A2E fluorophore of lipofuscin granules and products of its photooxidation. Ross Fiziol Zh Im I M Sechenova 99(5):642–653
Sarna T (1992) New trends in photobiology: properties and function of the ocular melanin—a photobiophysical view. J Photochem Photobiol, B 12:215–258. https://doi.org/10.1016/1011-1344(92)85027-R
Sarna T, Burke JM, Korytowski W, Rózanowska M, Skumatz CM, Zareba A, Zareba M (2003) Loss of melanin from human RPE with aging: possible role of melanin photooxidation. Exp Eye Res 76:89–98. https://doi.org/10.1016/s0014-4835(02)00247-6
Sauer L, Andersen KM, Dysli C, Zinkernagel MS, Bernstein PS, Hammer M (2018a) Review of clinical approaches in fluorescence lifetime imaging ophthalmoscopy. J Biomed Opt 23:091415. https://doi.org/10.1117/1.JBO.23.9.091415
Sauer L, Gensure RH, Andersen KM, Kreilkamp L, Hageman GS, Hammer M, Bernstein PS (2018b) Patterns of fundus autofluorescence lifetimes in eyes of individuals with nonexudative age-related macular degeneration. Invest Ophthalmol Vis Sci 59:AMD65. https://doi.org/10.1167/iovs.17-23764
Sauer L, Gensure RH, Hammer M, Bernstein PS (2018c) Fluorescence lifetime imaging ophthalmoscopy: a novel way to assess macular telangiectasia type 2. Ophthalmol Retina 2:587–598. https://doi.org/10.1016/j.oret.2017.10.008
Schmidt SY, Peisch RD (1986) Melanin concentration in normal human retinal pigment epithelium. Regional variation and age-related reduction. Invest Ophthalmol vis Sci 27:1063–1067
Schleicher ED, Bierhaus A, Haring HU, Nawroth PP, Lehmann R (2001) Chemistry and pathobiology of advanced glycation end products. Contrib Nephrol 131:1–9. https://doi.org/10.1159/000060056
Schmitz-Valckenberg S, Holz FG, Fitzke FW (2007) Perspectives in imaging technologies. In: Holz FG, Schmitz-Valckenberg S, Spaide RF, Bird AC (ed) Atlas of fundus autofluorescence imaging. Berlin: Springer pp. 331–338. https://doi.org/10.1007/978-3-540-71994-6
Schutt F, Bergmann M, Holz FG, Dithmar S, Volcker HE, Kopitz J (2007) Accumulation of A2-E in mitochondrial membranes of cultured RPE cells. Graefes Arch Clin Exp Ophthalmol 245(3):391–398. https://doi.org/10.1007/s00417-006-0376-5
Schutt F, Bergmann M, Holz FG, Kopitz J (2003) Proteins modified by malondialdehyde,4-hydroxynonenal, or advanced glycation end products in lipofuscin of human retinal pigment epithelium. Invest Ophthalmol vis Sci 44:3663–3668. https://doi.org/10.1167/iovs.03-0172
Schutt F, Davies S, Kopitz J, Boulton M, Holz FG (2000a) A retinoid constituent of lipofuscin, A2E, is a photosensitizer in human retinal pigment epithelial cells. Ophthalmologe 97:682–687. https://doi.org/10.1007/s003470070037
Schutt F, Davies S, Kopitz J, Holz FG, Boulton ME (2000b) Photodamage to human RPE cells by A2-E, a retinoid component of lipofuscin. Invest Ophthalmol vis Sci 41(8):2303–2308
Schweitzer D, Deutsch L, Klemm M, Jentsch S, Hammer M, Peters S, Haueisen J, Muller UA, Dawczynski J (2015) Fluorescence lifetime imaging ophthalmoscopy in type 2 diabetic patients who have no signs of diabetic retinopathy J Biomed Opt 20:061106. https://doi.org/10.1117/1.JBO.20.6.061106
Schweitzer D, Quick S, Schenke S, Klemm M, Gehlert S, Hammer M, Jentsch S, Fischer J (2009) Comparison of parameters of time-resolved autofluorescence between healthy subjects and patients suffering from early AMD. Ophthalmology 106:714–722. https://doi.org/10.1007/s00347-009-1975-4
Schweitzer D, Schenke S, Hammer M, Schweitzer F, Jentsch S, Birckner E, Becker W, Bergmann A (2007) Towards metabolic mapping of the human retina. Microsc Res Tech 70:410–419. https://doi.org/10.1002/jemt.20427
Shamsi FA, Boulton M (2001) Inhibition of RPE lysosomal and antioxidant activity by the age pigment lipofuscin. Invest Ophthalmol vis Sci 42(12):3041–3046
Sokolov VS, Sokolenko EA, Sokolov AV, Dontsov AE, Chizmadzhev YuA, Ostrovsky MA (2007) Interaction of pyridinium bis-retinoid (A2E) with bilayer lipid membranes. J Photochem and Photobiol b: Biology 86:177–185. https://doi.org/10.1016/j.jphotobiol.2006.09.006
Sparrow JR, Boulton ME (2005) RPE lipofuscin and its role in retinal pathobiology. Exp Eye Res 80:595–606. https://doi.org/10.1016/j.exer.2005.01.007
Sparrow JR, Gregory-Roberts E, Yamamoto K, Blonska A, Ghosh SK, Ueda K, Zhou J (2012) The bisretinoids of retinal pigment epithelium. Prog Retin Eye Res 31:121–135. https://doi.org/10.1016/j.preteyeres.2011.12.001
Sparrow JR, Kim SR, Cuervo AM, Bandhyopadhyayand U (2008) A2E, a pigment of RPE lipofuscin, is generated from the precursor, A2PE by a lysosomal enzyme activity. Adv Exp Med Biol 613:393–398. https://doi.org/10.1007/978-0-387-74904-4_46
Sparrow JR, Nakanishi K, Parish CA (2000) The lipofuscin fluorophore A2E mediates blue light-induced damage to retinal pigment epithelial cells. Invest Ophthalmol vis Sci 41:1981–1990
Sparrow JR, Parish CA, Hashimoto M, Nakanishi K (1999) A2E, a lipofuscin fluorophore, in human retinal pigmented epithelial cells in culture. Invest Ophthalmol vis Sci 40:2988–2995 (PMID: 10549662)
Sparrow JR, Wu Y, Kim CY, Zhou J (2010a) Phospholipid meets all-trans retinal: the making of RPE bisretinoids. J Lipid Res 51:247–261. https://doi.org/10.1194/jlr.R000687
Sparrow JR, Wu Y, Nagasaki T, Yoon KD, Yamamoto K, Zhou J (2010b) Fundus autofluorescence and the bisretinoids of retina. Photochem Photobiol Sci 9:1480–1489. https://doi.org/10.1039/C0PP00207K
Strauss O (2005) The retinal pigment epithelium in visual function. Physiol Rev 85:845–881. https://doi.org/10.1152/physrev.00021.2004
Suter M, Reme C, Grimm C, Wenzel A, Jaattela M, Esser P, Kociok N, Leist M, Richter C (2000) Age-related macular degeneration The lipofusion component N retinyl-N-retinylidene ethanolamine detaches proapoptotic proteins from mitochondria and induces apoptosis in mammalian retinal pigment epithelial cells. J Biol Chem 275(50):3L9625-39630. https://doi.org/10.1074/jbc.M007049200
Thao MT, Renfus DJ, Dillon J, Gaillard ER (2014) A2E mediated photochemical modification to fibronectin and its implications to age related changes in Bruch’s membrane. Photochem Photobiol 90:329–334. https://doi.org/10.1111/php.12200
Totan Y, Yağci R, Bardak Y, Ozyurt H, Kendir F, Yilmaz G, Sahin S, Sahin Tiğ U (2009) Oxidative macromolecular damage in age-related macular degeneration. Curr Eye Res 34(12):1089–1093. https://doi.org/10.3109/02713680903353772
Von Ruckmann A, Fitzke FW, Bird AC (1997) In vivo fundus autofluorescence in macular dystrophies. Arch Ophthalmol 115:609–615. https://doi.org/10.1001/archopht.1997.01100150611006
Wang Z, Dillon J, Gaillard ER (2006a) Antioxidant properties of melanin in retinal pigment epithelial cells. Photochem Photobiol 82:474–479. https://doi.org/10.1562/2005-10-21-RA-725
Wang Z, Keller LMM, Dillon J, Gaillard ER (2006b) Oxidation of A2E results in the formation of highly reactive aldehydes and ketones. Photochem Photobiol 82:1251–1257. https://doi.org/10.1562/2006-04-01-RA-864
Warburton S, Southwick K, Hardman RM, Secrest AM, Grow RK, Xin H, Woolley AT, Burton GF, Thulin CD (2005) Examining the proteins of functional retinal lipofuscin using proteomic analysis as a guide for understanding its origin. Mol vis 11:1122–1134
Wassell J, Davies S, Bardsley W, Boulton M (1999) The photoreactivity of the retinal age pigment lipofuscin. J Biol Chem 274(34):23828–23832. https://doi.org/10.1074/jbc.274.34.23828
Wielgus AR, Chignell CF, Ceger P, Roberts JE (2010) Comparison of A2E cytotoxicity and phototoxicity with all-trans-retinal in human retinal pigment epithelial cells. Photochem Photobiol 86(4):781–791. https://doi.org/10.1111/j.1751-1097.2010.00750.x
Wiktor A, Sarna M, Wnuk D, Sarna T (2018) Lipofuscin-mediated photodynamic stress induces adverse changes in nanomechanical properties of retinal pigment epithelium cells. Sci Rep 8:17929. https://doi.org/10.1038/s41598-018-36322-2
Wing G, Blanchard G, Weiter J (1978) The topography and age relationship of lipofuscin concentration in the retinal pigment epithelium. Invest Ophthalmol vis Sci 17:601–607
Wolf G (2003) Lipofuscin and macular degeneration. Nutr Rev 61:342–346. https://doi.org/10.1301/nr.2003.oct.342-346
Wu Y, Yanase E, Feng X, Siegel MM, Sparrow JR (2010) Structural characterization of bisretinoid A2E photocleavage products and implications for age-related macular degeneration. Proc Natl Acad Sci USA 107:7275–7280. https://doi.org/10.1073/pnas.0913112107
Yacout SM, McIlwain KL, Mirza SP, Gaillard ER (2019) Characterization of retinal pigment epithelial melanin and degraded synthetic melanin using mass spectrometry and in vitro biochemical diagnostics. Photochem Photobiol 95:183–191. https://doi.org/10.1111/php.12934
Yakovleva M, Dontsov A, Trofimova N, Sakina N, Kononikhin A, Aybush A, Gulin A, Feldman T, Ostrovsky M (2022a) Lipofuscin granule bisretinoid oxidation in the human retinal pigment epithelium forms cytotoxic carbonyls. Int J Mol Sci 23:222. https://doi.org/10.3390/ijms23010222(a)
Yakovleva MA, Feldman TB, Lyakhova KN, Utina DM, Kolesnikova IA, Vinogradova YV, Molokanov AG, Ostrovsky MA (2022b) Ionized radiation-mediated retinoid oxidation in the retina and retinal pigment epithelium of the murine eye. Radiat Res 197:270–279. https://doi.org/10.1667/RADE-21-00069.1(b)
Yakovleva MA, Gulin AA, Feldman TB, Bel’skich YC, Arbukhanova PM, Astaf’ev AA, Nadtochenko VA, Borzenok SA, Ostrovsky MA, (2016) Time-of-flight secondary ion mass spectrometry to assess spatial distribution of A2E and its oxidized forms within lipofuscin granules isolated from human retinal pigment epithelium. Anal Bioanal Chem 408:7521–7528. https://doi.org/10.1007/s00216-016-9854-8
Yakovleva MA, Sakina NL, Kononikhin AS, Feldman TB, Nikolaev EN, Dontsov AE, Ostrovsky MA (2006) Detection and study of the products of photooxidation of N-Retinylidene-N-retinylethanolamine (A2E), the fluorophore of lipofuscin granules from retinal pigment epithelium of human donor eyes. Dokl Biochem Biophys 409:223–225. https://doi.org/10.1134/S1607672906040089
Ye F, Kaneko H, Hayashi Y, Takayama K, Hwang SJ, Nishizawa Y, Kimoto R, Nagasaka Y, Tsunekawa T, Matsuura T, Yasukawa T, Kondo T, Terasaki H (2016) Malondialdehyde induces autophagy dysfunction and VEGF secretion in the retinal pigment epithelium in age-related macular degeneration. Free Radic Biol Med 94:121–134. https://doi.org/10.1016/j.freeradbiomed.2016.02.027
Yin D (1996) Biochemical basis of lipofuscin, ceroid, and age pigment-like fluorophores. Free Rad Biol Med 21:871–888. https://doi.org/10.1016/0891-5849(96)00175-X
Yoon KD, Yamamoto K, Ueda K, Zhou J, Sparrow JR (2012) A novel source of methylglyoxal and glyoxal in retina: implications for age-related macular degeneration. PLoS ONE 7:e41309. https://doi.org/10.1371/journal.pone.0041309
Young RW (1967) The renewal of the photoreceptor cell outer segments. J Cell Biol 33:61–72. https://doi.org/10.1083/jcb.33.1.61
Zareba M, Szewczyk G, Sarna T, Hong L, Simon JD, Henry MM, Burke JM (2006) Effects of photodegradation on the physical and antioxidant properties of melanosomes isolated from retinal pigment epithelium. Photochem Photobiol 82:1024–1029. https://doi.org/10.1562/2006-03-08-RA-836
Zhang X, Zhou J, Fernandes AF, Sparrow JR, Pereira P, Taylor A, Shang F (2008) The proteasome: a target of oxidative damage in cultured human retina pigment epithelial cells. Invest Ophthalmol vis Sci 49(8):3622–3630. https://doi.org/10.1167/iovs.07-1559
Zhou J, Ueda K, Zhao J, Sparrow JR (2015) Correlations between photodegradation of bisretinoid constituents of retina and dicarbonyl adduct deposition. J Bio Chem 290:27215–27227. https://doi.org/10.1074/jbc.M115.680363
Funding
This work was supported by the Russian Science Foundation (grant number 22–24-00549).
Author information
Authors and Affiliations
Contributions
All authors had the idea for the article and performed the literature search and data analysis; T.B. Feldman, A.E. Dontsov and M.A. Ostrovsky drafted the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Feldman, T.B., Dontsov, A.E., Yakovleva, M.A. et al. Photobiology of lipofuscin granules in the retinal pigment epithelium cells of the eye: norm, pathology, age. Biophys Rev 14, 1051–1065 (2022). https://doi.org/10.1007/s12551-022-00989-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12551-022-00989-9