Abstract
Photoreceptor phytochrome A (phyA) plays a key role both in the individual development and in the evolution of higher plants. It acts in three distinct modes — far-red light-induced very low fluence responses (VLFRs), high irradiance responses (HIRs), and red/far-red–reversible low fluence responses (LFRs). Signal transduction from phyA includes its transportation from the cytoplasm into the nucleus and activation of light-responsive genes there. It is also active in the cytoplasm. Two types of phyA speckles were detected upon its light-induced nucleocytoplasmic partitioning and a fraction remained in the cytoplasm. In this review, we present a concept that this complex picture of the phyA action is due, at least partially, to the existence of two phyA types in the cell differing by the structure of the N-terminus, probably, by its serine phosphorylation. These are phosphorylated water-soluble phyA′ and underphosphorylated ambiquitous phyA″ represented by two fractions — water-soluble and membrane-associated. From the analysis of the phyA pools’ activity in the regulation of phyA synthesis, seed germination, seedling establishment, and (proto)chlorophyll biosynthesis it is concluded that phyA″ is responsible for the regulation of seed germination, whereas in seedlings phyA′ mediates the VLFRs, and the water-soluble phyA″ fraction, the HIRs. The membrane-associated phyA″ is likely to be active in cytoplasmic photoregulatory events. Functional interaction between phyA and the defense-related hormone jasmonic acid is also considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction: an emerging theme — diverse modes of phytochrome A action and its polymorphism
Investigation of the photoreceptor phytochrome occupies one of the central positions in contemporary plant photobiology due to its key role both in the individual processes of growth and development and in their evolution in general (Mathews et al. 2003; Casal 2013; Li et al. 2015). It is a biliprotein whose action is based on the photoisomerization of the chromophore that is induced most effectively by red light (R) and is reversed by far-red light (FR) (Braslavsky et al. 1997; Song et al. 1997). This reaction converts the initial physiologically inactive red-absorbing form (Pr), which is synthesized in the cytoplasm, into the active far-red–absorbing form (Pfr). The appearance of the latter is perceived by a plant as an informational signal that, after several steps of intracellular transduction, is realized in the modulation of a light-inducible genes’ activity, and, as a result, in the plant’s transition from the dark development (scotomorphogenesis) to the light development (photomorphogenesis) (Casal et al. 2014).
Light signal transduction by phytochrome can be briefly presented as follows: photoreceptor in the Pfr form is translocated from the cytoplasm into the nucleus (Klose et al. 2015), where it participates in the degradation of the phytochrome interacting factors blocking photomorphogenesis and activates the positive photomorphogenic factors. The latter induces transcriptional activity of the photoresponsive genes and initiation of the photomorphogenic mode of plant development (Sheerin and Hiltbrunner 2017; Lee and Choi 2017). Functional activity of phytochrome in the cytoplasm characterized by fast (seconds, minutes) biophysical and biochemical responses is also found (Hughes 2013).
The character of the photophysiological response mediated by phytochrome is determined by the photoreversibility of the Pr → Pfr photoconversion and by the spectral composition of the actinic light. Indeed, many plant photoresponses reveal themselves under R (induction of seed germination, stem elongation inhibition, cotyledon opening) and its effect can be abolished by FR. However, there are light responses that do not fit into this classical scheme. A key feature distinguishing them from the “classical” ones is their inability to be reverted by FR. On the contrary, the latter activates them with relatively high effectiveness. Detailed investigation of their light fluence and spectral dependences revealed three photoresponse modes — besides the photoreversible low fluence responses (LFRs), there exist the FR-induced nonphotoreversible very low fluence responses (VLFRs) and high irradiance responses (HIRs) (Casal et al. 1998).
Along with the detection of the various photoresponse modes, the heterogeneity of the photoreceptor itself was discovered — the existence of its species differing by light lability (Brockman and Schäfer 1982), immunochemical properties (Shimazaki and Pratt 1985), and spectral and photochemical characteristics (Sineshchekov and Sineshchekov 1987, 1989). A breakthrough was the discovery of a small family of phytochrome genes and their products (Sharrock and Quail 1989), for instance, five in Arabidopsis (Clack et al. 1994) and three in rice (Takano et al. 2005), two of which are the major ones both in content and functionality — light-labile phytochrome A (type I) in etiolated plants and light-stable phytochrome B (type II) in light-grown. Their existence could explain both the heterogeneity of the photoreceptor and the complex picture of its action. Using phytochrome-deficient mutants, it was shown that the LFRs are mediated by phyB, whereas the VLFRs and HIRs, by phyA (Casal et al 1998). The latter two types are functionally different and operate through distinct pathways and/or in different cells (Yanovsky et al. 1997; Casal 2000).
In our group, it was found that the phytochrome system could be even more complex than that. Two types of phytochrome A were detected in mono- and dicotyledons differing by fluorescent and photochemical properties — the post-translationally modified products of the phytochrome A gene, phyA′ and phyA″, and it was shown that they are responsible for the two modes of the phyA photoresponses, the VLFRs and HIRs (see reviews Sineshchekov 1995a, 2010, 2019 and the original papers by the same author cited therein). In this review, we are exploring further this possibility considering their functional roles in the regulation of the key stages of plant development — induction of seed germination and seedling establishment.
The heterogeneity of phytochrome A: distinct molecular species and conformers
Investigations of phytochrome in situ are strongly hampered by its very low content in plant tissues, their high light scattering, and the presence of other pigments in them. The discoverers of phytochrome employed absorption difference spectroscopy, which for long was the only method of its determination in plant tissues (Butler et al. 1959). In our group, low-temperature (T) fluorescence of phytochrome was detected in etiolated plants (Fig. 1) and based on that a highly sensitive and informative method of its in vivo assay was developed (Fig. 2) (see reviews Sineshchekov 1995a, 2010, 2019). With its use, phytochrome in its Pr form was described by fluorescent and photochemical parameters and, proceeding from their temperature dependence, the initial photoisomerization reaction of Pr was characterized as an activation process with an energy barrier Ea in the excited state (Sineshchekov and Sineshchekov 1990; Sineshchekov 1994, 1995a) (Fig. 3). It reflects the degree of freedom of the cis–trans flip-flop in the Pr → lumi-R conversion in the chromophore pocket. At low T (77–85 K), the Pr → Pfr conversion is stopped at the stage of the photoproduct lumi-R. Ea of the Pr and lumi-R states determine the quantum yield of the direct (Pr → lumi-R) and reverse (lumi-R → Pr) photoreactions (Fig. 3) and, hence, the photoequilibrium between Pr and lumi-R at the wavelength of the actinic light. The Pr/lumi-R ratio in the photoequilibrium is measured as the extent of the Pr conversion into lumi-R under saturating R, γ1 (Fig. 2).
Fluorescence emission (λex = 630 nm) and excitation (λem = 700 nm) spectra of phytochrome in the Pr form in stems of etiolated pea seedlings at 77 K and 200 K. Excitation spectrum with the maximum at 375 nm was calculated in the region 350–500 nm with due consideration of green background fluorescence with an excitation maximum at 420 nm. (From Sineshchekov and Sineshchekov 1987, 1989)
Low-temperature (T) fluorescence emission spectra (λex = 632.8 nm) of phytochrome in situ in its initial red light-absorbing Pr form and after its photochemical conversions at low T into lumi-R and at ambient T into the far-red–absorbing form Pfr: 1—etiolated (wheat) coleoptiles at 85 K, 2—the same sample after saturating red illumination (λa = 632.8 nm) (R) at 85 K partially converting Pr into lumi-R, the first stable at low T photoproduct, and 3—the same sample after thawing at 273 K and R illumination converting Pr into Pfr and freezing again at 85 K. Four major parameters can be obtained from these spectra: position of the spectrum (λmax); phytochrome content ([Ptot] ≈ F0); extent of the Pr conversion into lumi-R (γ1 = ΔF1/F0) and the far-red light-absorbing form Pfr (γ2 = ΔF2/F0). The maximum at 685 nm belongs to Pr and shoulder at 704 nm, to lumi-R; Pfr does not fluoresce even at low T. (From Sineshchekov 1994, 2004)
The energy level scheme of the photoisomerization reaction of the initial red-absorbing phytochrome form (Pr) into the first photoproduct (lumi-R) stable at low temperatures via a short-lived unstable orthogonal intermediate (prelumi-R). At 85 K and saturating red illumination (λa = 632.8), there is a photoequilibrium between Pr and lumi-R determined by the rates of the forward (Pr → lumi-R) and reversed (lumi-R → Pfr) photoreactions (corresponds to spectrum 2 in Fig. 1). The activation barrier in the excited state, Ea for Pr and Ea′ for lumi-R, determines the photochemical properties of Pr and lumi-R and the extent of the Pr photoconversion to reach a photoequilibrium with lumi-R at low T (γ1 varies for different Pr species from 0 to 0.5). At ambient temperatures, this barrier is easily overcome and the extent of the Pr → Pfr photoconversion remains relatively constant, 0.75–0.85. (From Sineshchekov and Sineshchekov 1990; Sineshchekov 1994, 1995a)
Two phenomenological phytochrome types — photoactive at low T Pr′ (with γ1′ = 0.5) and inactive Pr″ (γ1″ = 0) — were detected. With these individual γ1 parameters, their content and proportion could be determined from experimental γ1 values of a sample as described in (Sineshchekov 1994). Experiments with phytochrome mutants lacking phyB (Sineshchekov 1995b; Sineshchekov et al., 1998, 1999a, b, 2006) and heterologous transgenic systems expressing recombinant phyA (Sineshchekov et al. 2001a, 2013) have shown that both the types belong to phyA, whereas phyB is represented only by Pr″ (Sineshchekov et al. 2000). The two phyAs were characterized by physicochemical parameters and phenomenological properties (Table 1), their content and proportion being strongly dependent on plant species and organ/tissue used, plant age and stage of development, physiological conditions, and environmental factors (light, dehydration, water stress, etc.) (see Table 1 in (Sineshchekov 2019)). Along with the two phyA gene products, heterogeneity within phyA′ was also detected: it comprised several conformers differing by fluorescence and photochemical characteristics (Sineshchekov and Akhobadze 1992).
The heterogeneity is not limited to phyA: two species of the fern Adiantum phy1 (expressed in transgenic Arabidopsis) like the two phyAs were also distinguished (Sineshchekov et al. 2014a) suggesting that this differentiation preceded the evolution of the appearance of the higher plants. The existence of conformers like those of phyA′ was demonstrated in the case of the cyanobacterial phytochrome Cph1 (Sineshchekov et al. 2014b; Sineshchekov and Bekasova 2020). We can thus distinguish three levels of organization of the phytochrome system: besides the products of phytochrome genes, there are another two levels — post-translationally modified species and conformers within one molecular type.
To the nature of the phytochrome A differentiation
Our experiments with phyA mutants have shown that the site(-s) responsible for the phyA′ formation is at the N-terminal extension (NTE) (Sineshchekov 1995a, b, 2010, 2019). It is likely a serine residue(s) within the 10 N-terminal serines (in rice phyA expressed in Arabidopsis) since their substitution by alanines (phyA SA) resulted in the presence of only phyA″ in the sample (lacking phyA′) (Sineshchekov et al. 2018). Taking into consideration that phytochrome A is a phosphoprotein autophosphorylated in the Pr state in darkness at serines 8 and 18 (in oats) (see review (Hoang et al. 2019)), we could assume that phyA′ formation is the result of phyA″ serine phosphorylation. However, this hypothetical serine(s) is not serines 8 and/or 18 as it was demonstrated in the experiments on phyA mutants with the S/A substitution at positions 8 and 18 (of oat phyA expressed in transgenic Arabidopsis) (Sineshchekov et al. 2019).
The presumed phosphorylation of phyA at the NTE affects the conformation of the chromophore pocket and the photochemical properties of the pigment (Ea of the photoreaction) because of the appearance in the molecule of a charged phosphoryl group. Although the interaction of the NTE with the chromophore in the Pr form of phyA is much less pronounced than in the Pfr form (Song et al. 2018), its effect is seen in changes in the Ea of the Pr → lumi-R isomerization (see above). These changes are likely due to variations in the conformation of the chromophore pocket and steric hindrances to the isomerization. The phyA modification with the formation of the presumably phosphorylated phyA′ from phyA″ may thus change the conformation of the pigment.
Another important outcome could be the changes in the hydrophobicity/hydrophilicity of the pigment. phyA is represented by both the phyA pools, phyA′, and phyA″, in the supernatant from etiolated maize coleoptiles (Sineshchekov et al. 1994) or Arabidopsis hypocotyls (V. Sineshchekov and M. Zeidler, unpublished results), whereas in the sediment, primarily or exclusively by phyA″ in agreement with the data on the existence of the membrane-(protein-) associated phyA fraction (see Lamparter et al. 1992; Terry et al. 1992). phyA″ thus shows amphiphilic properties and can be called an “ambiquitous phyA type,” in analogy with the “ambiquitous enzymes” — having the ability to reversibly bind to subcellular structures (Clegg 1984). Also, deep dehydration of etiolated tissues brings about a disappearance of phyA′ (Sineshchekov 2006) in line with the notion that phyA′ is a hydrophilic protein.
In general, the existence of the two phyA pools so profoundly different in their properties allows an assumption that they perform essentially distinct functions in plants each mediating its “own” photoresponse type, VLFRs, or HIRs. Noteworthy in this connection is that the two phyA pools form different types of nuclear bodies after the phyA light-induced transfer from the cytoplasm (Sineshchekov et al. 2014c). Nagy et al. (2001) distinguished two pools of phyA in the Pfr form one of which makes distinct types of loci in the nucleus and the other is homogeneously distributed in the cytoplasm. It is tempting to connect these phenomena with the existence of the two phyA types — water-soluble phyA′ and phyA″ represented by two fractions, water-soluble and membrane-associated.
Light as a major factor determining the content and activity of the two phyA populations
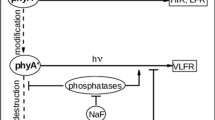
In darkness, the phyA pools’ balance is intimately connected with the kinase/phosphatase equilibrium in the cell. This is pointed at by the fact that the phosphatase inhibition by okadaic and cantharidic acids (Sineshchekov et al. 2013) and by NaF (Sineshchekov et al. 2021a, b) shifts the ratio towards phyA″ due to the accelerated destruction of the labile phyA′. On the other hand, phytochrome kinase substrates (PKS1 and PKS2), which are transphosphorylated by phyA (Lariguet et al. 2003), can contribute to the maintenance of a proper phyA′ /phyA″ ratio (Sineshchekov and Fankhauser 2004). In the double pks1pks2 (Arabidopsis) mutant, the equilibrium between phyA′ and phyA″ shifted considerably towards phyA″. The phyA′ /phyA″ ratio is also affected by the cytosolic pH with the phyA′ proportion reaching a maximum at pH values close to the physiological (Sineshchekov et al. 2013).
The most efficient factor affecting the phytochrome A species is, however, light. Red light brings about a rapid (minutes) and selective destruction of the phyA′ form in etiolated tissues, whereas phyA″ remains relatively constant (Sineshchekov et al. 1999a, b; Sineshchekov and Weller 2004). This results in the shift of the phyA′ /phyA″ ratio strongly towards phyA″ which correlates with the detection of the two phyA fractions differing by destruction kinetics (Clough and Vierstra 1997). From this, one may assume that relatively light-stable phyA″ is a dominating or the only phyA species in light-grown plants.
A more complex picture is observed upon the action of FR: depending on the plant species and illumination conditions there could be a decline of the total phyA content with or without violation of the phyA′ /phyA″ content. In pea grown under constant FR (FRc), [phyA] dropped down considerably; however, the phyA pools’ equilibrium remained essentially the same as in the control (Sineshchekov and Weller 2004). This effect is likely to relate to the inhibition by phyA of its synthesis (Cantón and Quail 1999; Clough et al. 1995). This notion is supported by the fact that in epicotyls of the pea mutant lip, which reveals the deetiolated phenotype in darkness, total phyA content drops down significantly without violation of the phyA′ /phyA″ proportion, too (Sineshchekov et al. 1995). Interestingly, this effect of the mutation was lacking in roots, suggesting that the state of phyA in roots differs from that in epicotyls (for the specificity of the phyA state in roots, see also (Sineshchekov et al. 2021b)).
A strong [phyA] decline (ca 95%) was observed in the coleoptiles of FRc-grown wild-type (WT) rice (Oryza sativa L. cv. Nipponbare) and its phyB mutant (Sineshchekov et al. 2006) suggesting that phyB, as expected, is not involved in this process. (The phyA′ /phyA″ proportion could not be measured because of low residual phyA.) The FRc-induced inhibition of [phyA] was also observed on another rice variety, Oryza sativa L. cv. Nihonmasari, followed by a shift in the phyA′ /phyA″ proportion towards phyA″. FRc regimes differing approx. 50-fold by fluence rate (FRc-high and FRc-low) produced a similar inhibiting effect and pulsed FR (FRp) was ineffective (Sineshchekov et al. 2004b) or less effective (Sineshchekov et al. 2021a) than FRc (Fig. 4).
The total content of phytochrome A in the coleoptiles of the wild-type seedlings of rice (Oryza sativa L. ssp. Japonica cv. Nihonmasari) and its hebiba and cpm2 mutants deficient in the hormone jasmonic acid (JA) (a) and the proportion of its two native types, phyA′ (light grey) /phyA″ (grey) (b). The illumination conditions: far-red constant light FRc of high irradiance (FRc-high, λa = 740 nm, 0.2 μmol m−2 s−1) (favorable for the HIRs), pulsed FR (FRp, 6 min light/54 min dark, 0.2 μmol m−2 s−1) (favorable for the VLFRs) and constant FR of low irradiance (FRc-low, 0.004 μmol m−2 s.−1), which may have the properties of the two response modes (HIR and VLFR). a The effects of the FR light of all the regimes on the phyA content are statistically significant for all the lines. The significant differences between the mutants and the WT are indicated by asterisks, and there is no difference between the mutants. b The statistically significant light effects on the phyA pools' ratio in all the lines are Dark vs. FRp and FRc-high, the statistically significant differences between the mutants and the WT are indicated by asterisks, and between the mutants, by the diamond symbol. (From Sineshchekov et al. 2021a)
Experiments with rice mutants, hebiba, and cpm2, deficient in the defense-related phytohormone jasmonic acid (JA), which is engaged in plants’ responses to biotic and abiotic stress, have shown that it is deeply involved in the FR-induced effects (Sineshchekov et al. 2004b, 2021a). The latter revealed a strong dependence on seedlings’ genotype, their age, and illumination conditions. FRc (HIRs) was as inhibiting in the mutants as in the WT, whereas FRp (the VLFRs) was ineffective in hebiba or more effective than in the WT (Fig. 4). The decline in the phyA content upon FRc was followed by the domination of phyA′ in the mutants and of phyA″ in the WT suggesting that in the mutants there is a stimulation of the phyA″ → phyA′ conversion, which is slowed down by JA in the WT. Besides, in the mutants, the direction of the FRp effect (domination of phyA″ in the phyA′ /phyA″ balance) was opposite to that of FRc (Fig. 4). The observed specificity of the JA influence on the FR effects in the WT can relate to JA participation in phyA destruction documented in (Sineshchekov et al. 2004b; Riemann et al. 2009). In the JA mutants, the light-induced destruction of phyA in its light-labile form phyA′ is slowed down, which brings about the domination of the latter in contrast to the WT.
An unexpected light effect on phyA was observed after light induction of (Arabidopsis) seed germination. White light preillumination of seeds (tungsten lamp,15 min or 3 h) caused a considerable redistribution of the phyA pools in dark-grown seedlings towards phyA′ without violation of the total phyA content (Fig. 5), possibly, via promotion of the phyA″ phosphorylation (Sineshchekov et al. 2014a,c). This effect may relate to that observed by Magliano and Casal (2004) when R preillumination of Arabidopsis seeds induced an inhibitory effect on hypocotyl elongation in seedlings grown in the dark. On the other hand, it may result from the stimulation of seedling development because the total phyA content and the phyA′ /phyA″ proportion increase with the seedling’s age (Sineshchekov 1994; Sineshchekov et al. 2013).
Effect of germination-inducing seed preillumination on the relative content of the two phyA pools, phyA′ and phyA″ (light grey and dark grey, respectively), in the etiolated coleoptiles of wild-type (WT) Arabidopsis (Ler) and its transgenic lines deficient in endogenous phyA but expressing foreign phyA — (1) rice WT phyA (WT phyA) and mutant rice phyA with the substitution of 10 serines at the NTE for alanines (10SA), and (2) oat WT phyA and mutant oat phyA with the S8A, S18A, S8/18A substitutions. The Arabidopsis line expressing phy1 of fern Adiantum capilus veneris (Ad phy1 OX) was also used. Imbibed seeds were preilluminated with white light (W) for 15 min or 3 h to induce germination. Mean values with standard error (SE) are presented. (Modified from Sineshchekov et al. 2014a, b, c, 2018, 2019)
A different light effect was seen in rice coleoptiles, WT and phyB mutant (Sineshchekov et al. 2006). The phyA content was higher in the phyB mutant, compared with WT. This might be the consequence of the inhibitory function of phyB in the dark-grown rice seedlings due to the illumination of seeds during their maturation. This is supported by the fact that the WT coleoptile is shorter than that of the phyB mutant (see below). The proportion of the two phyA pools remains, however, the same in WT and the phyB mutant suggesting that the phyB influence is localized upstream of the posttranslational modification of phyA. Collectively, these different light-induced modifications of the phyA turnover — inhibition of the phyA synthesis, preferential phyA′ degradation, and promotion of the phyA″ conversion into phyA′ — result in the modulation of their photophysiological activity thus contributing to the fine-tuning of the photoreceptor apparatus.
Functional role of phytochrome A in the regulation of seed germination
Phytochromes affect seed germination inducing or inhibiting it, with phyB and phyA playing a key role in these processes (Casal and Sánchez 1998; Franklin and Quail 2010). Seeds of some plant species can germinate both in light and in darkness, they have sufficient [Pfr] to induce germination (Takaki 2001). phyB regulates germination through detection of the R:FR ratio (LFR) including the conditions under the canopy shade of the deciduous forests (Kendrick 1976; Ballaré and Casal 2000). phyA promotes germination in the VLFR and HIR modes under the conditions of the FR light domination including those of the evergreen forests (Botto et al. 1996; Johnson et al. 1994; Hennig et al. 2001; Seo et al. 2009; Takaki 2001).
In dry seeds, there is a domination of phyB with a very low level of phyA (Tobin and Briggs 1969; Hauser et al. 1997; Somers and Quail 1995). Pre-existing in dry Arabidopsis seeds phyB mediates the R-induced germination 3 h after the start of imbibition — the typical R/FR LFR (Shinomura et al. 1994; Botto et al. 1995). Germination under the regulation of phyA is observed only after its de novo synthesis 48 h after the onset of (Arabidopsis) seed imbibition (Shinomura et al. 1996).
In early works, it was shown that the “seed phytochrome” differs from the “seedling phytochrome” in immunological traits and stability (in Avena sativa) (Spruit and Mancinelli 1969; Hilton and Thomas 1985; Tokuhisa and Quail 1987). Our experiments suggest that the state of the phyA pools in developing embryos may also substantially differ from that in seedlings. In wheat embryos at the stage of their breaking through 15 h after the onset of imbibition, the proportion of phyA′/ phyA″ is approx. 50/50, whereas in coleoptiles 80/20 (Sineshchekov et al. 2001a). In imbibing seeds of pea and bean, we observed the active rise of [phyA] and the concomitant increase of the phyA′ proportion in seeds 8 h after the onset of the imbibition (Sineshchekov et al. 1998) that is much earlier than the onset of the phyA synthesis in Arabidopsis (Shinomura et al. 1996). The phyA′/phyA″ balance with phyA′ domination characteristic for the seedlings establishes after 28–30 h of imbibition (Sineshchekov et al. 1989). Of interest in this context is the effect of dehydration on phyA in etiolated tissues (Sineshchekov 2006). Even a considerable loss of water (up to 75–85% of the initial fresh weight of etiolated coleoptiles of barley and maize) does not bring about noticeable alterations in the total phyA content and the phyA′/phyA″ proportion pointing to relative stability of the phyA′/phyA″ system in this regard. However, extreme dehydration (loss of weight ≈90%) caused a transformation of phyA′ into phyA″, which was not reversed by rehydration. We may thus hypothesize that phyA in dry and imbibing seeds is represented by its phyA″ isoform and this form is active in the regulation of germination.
This assumption is supported by our experiments on transgenic Arabidopsis expressing rice phyA SA represented by phyA″ (Sineshchekov et al. 2018). It was found that the germination rate of these transgenic seeds after preillumination (W, 15 min and 3 h was approx. the same as that of the Arabidopsis line expressing WT rice phyA and the Ler variety (Fig. 6). Earlier, Kneissl et al. (2008) have observed that germination of the PHYA SA-expressing lines was more efficient than the WT PHYA-expressing lines and this effect was stronger under R light suggesting that it can sense the R- and FR-light pulses. Collectively, these data allow a conclusion that phyA″ is active in the stimulation of seed germination under the conditions of HIR or LFR.
White light-induced germination of the wild-type (WT) Arabidopsis and its transgenic lines expressing foreign WT and mutant phytochrome A. (For the description of the Arabidopsis lines used and measuring conditions, see the legend to Fig. 5). (Modified from Sineshchekov et al. 2014a, b, c, 2018, 2019)
Phosphorylation of phyA at serines 8 and 18 (in oat phyA overexpressed in Arabidopsis) does not participate in the differentiation of the photoreceptor into the phyA′ and phyA″ pools (see above and (Sineshchekov et al. 2019)). However, it regulates the functional properties of the photoreceptor (Han et al. 2010). We investigated how this may affect the phyA″ activity in the germination induction. Transgenic Arabidopsis overexpressing WT Avena sativa phyA (AsAox) is characterized by a low germination rate — less than 30% even after 3 h white light (W) pre-illumination (Fig. 6). However, the transgenic Arabidopsis with the mutant oat phyA — S8A, S18A, S8/18A — germinated in darkness (up to 25%) and reached a germination rate of 90–98% even after 15-min preillumination. Thus, this block of phosphorylation by the SA substitution makes phyA″ more functionally effective supporting the notion by Han et al. (2010) that phosphorylation at these sites serves as an instrument of the regulation of phyA activity.
The role of phyA′ in the regulation of seed germination is not quite clear. Of interest in this respect is truncated oat Δ6-12 phyA because according to our data (Sineshchekov et al. 2014c), it is represented by phyA′. Trupkin et al. (2007) have documented that the seeds of Arabidopsis expressing truncated Arabidopsis Δ6-12 phyA (in the phyA background) revealed a weak germination response to a pulse of long-wavelength FR (VLFR) and there were no significant differences among WT, full-length, and Δ6-12 phyA lines suggesting that phyA′ does not play a significant role in the regulation of seed germination.
Several signal transduction components downstream of phyA participating in germination were detected (Kim et al. 2008; Oh et al. 2004; Kneissl et al. 2009). Of interest in the context of our discussion are the Arabidopsis mutants deficient in FHL, FHY1, and related proteins — the phyA partners transporting it into the nucleus (Zeidler et al. 2004; Hiltbrunner et al. 2005). Mutations of fhy1 and fhy3 weaken the HIRs (Whitelam et al. 1993); fhy3-1 retains VLFR but is severely impaired in HIR (Yanovsky et al 2000). Casal et al. (2014) suggest that the mechanism controlling the expression of genes FHL/FHY1 operates under HIR but not under VLFR. We have undertaken experiments on Arabidopsis fhl/fhy1 double mutant seeds with the aim to see if there is any connection of the mutation with the state of the phyA pools in the mutant seedlings. In these experiments, germination in the mutant reached 100% after 15 min of W illumination, whereas in the WT, not more than 10%; the WT seeds germinated with the rate of 100% only after 1 h exposition (L. Koppel, M. Zeidler and V. Sineshchekov, unpublished results). There were no differences in the total phyA content and the phyA′/phyA″ proportion in plant tissues suggesting that the phyA pools’ modification is not connected with the phyA-FHL/FHY1 complex formation and that the observed germination effects are not connected with the changes in the phyA state.
Our experiments on Arabidopsis overexpressing fern Adiantum capillus-veneris phy1 (Sineshchekov et al. 2014a) may have a relation to the understanding of the specificity of the phyA state and action in seeds. The seeds of this transgenic line were characterized by an unexpectedly high germination rate in darkness and after the light pretreatment of 15 min and 3 h (Fig. 6), whereas phy1 was shown earlier to be inactive in seedling growth responses (Okamoto et al. 1997). The latter agrees with our observation that the overexpressed phy1 does not contribute to the stimulation of the phyA′ accumulation in seedlings upon seed preillumination (see above and Fig. 5). Given that phy1 is represented also by the two native pools like those of phyA (phy1′ and phy1″), we may speculate that phy1″ participates in this germination effect as does phyA″.
Phytochrome A photoregulation reactions in seedling growth and establishment
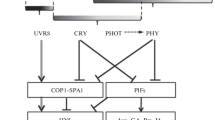
Phytochromes A and B are deeply involved in the regulation of plants’ de-etiolation and seedling establishment, their action being overlapping and redundant, and, depending on the illumination conditions, synergistic or antagonistic (Reed et al. 1994; Casal 2000; Tepperman et al. 2006). phyB is more important under R and W light whereas phyA is a major photoreceptor for the FR-induced responses working in the VLFR and HIR modes. phyA mediates the regulation of growth reactions (Yanovsky et al. 1997; Casal 2000), anthocyanin accumulation (Duek and Fankhauser 2003), CAB biosynthesis induction, and block of greening (Reed et al. 1994; Barnes et al. 1996). It also suppresses gravitropism and stimulates phototropism (Sullivan et al. 2016).
In transgenic plants, the manifestation of the photoresponses depends on the presence of endogenous phytochromes (phyA and phyB) in the host plant, in which phyA, from a mono- or dicot plant, is introduced, and the promoter used. Transgenic plants expressing Avena phyA have shown exaggerated sensitivity to the VLFR conditions, when expressed in rice (Clough et al. 1995), tobacco (Casal et al. 1994; Sineshchekov et al. 1999b), and Arabidopsis (Mazzella et al. 1997). However, more often, the expression of monocot phyA in dicot plants revealed higher sensitivity to the FR-HIR conditions (Boylan and Quail 1989; Cherry et al. 1991; Halliday et al. 1999). The overexpression of an Arabidopsis PHYA in rice or soybean PHYA in Arabidopsis resulted in exaggerated FR-HIRs in growth inhibition by both Rc and FRc (Garg et al. 2006; Wu et al. 2013).
In our works, experimental evidence is being accumulated that the two modes of FR action, VLFR and HIR, are initiated by the different phyA types, phyA′ and phyA″, respectively. It was found that transgenic Arabidopsis plants (in the phyA and phyAphyB background) that expressed mutant rice phyA SA represented primarily or exclusively in the phyA″ form (Sineshchekov et al. 2019) were characterized by the HIR of growth responses (inhibition of hypocotyl elongation and anthocyanin accumulation under FRc) (Kneissl et al. 2008). At the same time, the wild-type rice phyA, which is represented by both phyA types, phyA′, and phyA″, complemented the wild-type Arabidopsis phenotype under FRp (VLFR) and FRc (HIR). Collectively, this allows the attribution of the HIRs to phyA″.
On the other hand, the Δ6-12 phyA, which is represented by phyA′ (Sineshchekov et al. 2014c), is likely to be responsible for the VLFR. Casal et al. (2002) have shown that oat Δ6-12 phyA expressed in transgenic tobacco and Arabidopsis was active in the VLFR of hypocotyl growth inhibition and cotyledon opening whereas the HIR responses were suppressed. Similarly, with the use of a homological system — Arabidopsis with the Δ6–12 deletion in Arabidopsis PHYA — it was shown that the 6–12 aa. region is dispensable for the VLFRs but is necessary for the HIR (Trupkin et al 2007).
Less defined are our results on the assignment of the phyA pools to the different response modes obtained with transgenic potato with modified phyA content (Sineshchekov et al. 1996) and transgenic wheat overexpressing oat phyA (Sineshchekov et al. 2001b). The uncertainty is because of the presence of the two phyAs in these lines. Based on a positive correlation between the manifestation of the HIRs in growth responses and variations of [phyA′] (primarily in the potato and exclusively in the wheat), the HIRs were attributed to phyA′. However, the participation of phyA″ in the phenotype of these plants cannot be excluded because a relatively moderate increase of [phyA] (2–threefold) brings about a saturation of its action (Boylan and Quail 1989). On the other hand, phyA responses can be substantially modified in transgenic plants (Casal et al. 2002). We may speculate in this connection that phyA′ in overexpressors can acquire the properties and functions of phyA″. This assumption is supported by the observation (Clough and Vierstra 1997; Sineshchekov et al. 2001b) that overexpressed phyA is relatively light-stable which is characteristic of phyA″ (see discussion in (Sineshchekov 2019)).
Mutations in the phyA molecule, which do not affect the formation of the phyA pools, have an impact on the manifestation of the different photoresponse modes, most pronounced in the case of the HIRs. The amino acid substitutions R194V and C581T in pea phyA increased the de-etiolation phenotype under FRc and Rc without violation of the phyAʹ/phyAʹʹ balance (Weller et al. 2004; Sineshchekov and Weller 2004). Mutant plants expressing phyA with the substitutions E777K and R384K become insensitive to or severely impaired in the HIR (Yanovsky et al. 2002; Mateos et al. 2006).
Of interest is the fact that transgenic Arabidopsis expressing oat phyA with a reduced kinase activity reveal limited photoresponses to FR — both HIR and VLFR (Shin et al. (2016), whereas those with an enhanced phyA kinase activity are hypersensitive under FR (short hypocotyls and expanded cotyledons) (Hoang et al 2021).
Mutations at S8A and S18A in oat phyA expressed in Arabidopsis, at the sites involved in the phyA autophosphorylation, bring about hypersensitivity to FRc and FRp, which is interpreted to result from the higher light stability of the mutated phyA (Han et al. 2010). There were no significant changes in the phyAʹ/phyAʹʹ ratio in this Arabidopsis line suggesting that the effect is not connected with changes in their content but, rather, in their higher stability (Sineshchekov et al. 2021a). Boylan and Quail (1989) pointed to the higher stability of the heterologous (oat) phyA than the endogenous tomato phyA in transgenic tomato plants characterized by a higher de-etiolation phenotype.
The character of the phyA growth reactions may strongly depend on its functional interaction with the hormonal system as revealed by the experiments with rice mutants (hebiba and cpm2) (Riemann et al 2003, 2013; Sineshchekov et al 2004b, 2021a). For the WT rice, there is a differentiation of the photoregulation effects on different organs: the growth of coleoptiles and seminal roots is inhibited by the VLFR conditions, whereas for mesocotyls, by FR-HIR (Takano et al 2001; Shimizu et al. 2010). The JA mutants, however, show a sign reversal in these effects. Whereas WT coleoptile growth is elevated in the dark and efficiently inhibited by R and FR, mutant coleoptiles are arrested in growth if they remain in the dark but expand rapidly upon illumination (Fig. 7). Our recent experiments on hebiba and cpm2 (Sineshchekov et al. 2021a) have confirmed this sign reversal, in particular, phyA suppresses roots’ growth under FRp in the mutants but not in the WT. They also have shown that the manifestation of a photoresponse depends on the age of the seedlings and on the illumination conditions (Fig. 7). For instance, the coleoptiles remain unresponsive to all the light regimes which are explained in agreement with Xie et al. (2007) and Shimizu et al. (2010) by the earlier age of the seedlings as compared to that in Sineshchekov et al. (2004b). Collectively, the data on JA mutant features suggest that JA reduces the phyA functional activity primarily in its phyA″ form mediating the HIRs.
The length of the coleoptiles, mesocotyls, and roots of dark-grown 5-day-old rice (Oryza sativa L. ssp. Japonica cv. Nihonmasari) seedlings and of its hebiba and cpm2 mutants deficient in the hormone jasmonic acid (JA) and of the seedlings of the same lines grown under far-red light (see light conditions in the caption to Fig. 4). Statistically significant light effects are seen in the wild type in the case of mesocotyls; roots undergo significant growth inhibition only under FRc-high (Student’s t-test; P < 0.05). Statistically significant light effects are seen: in hebiba—for coleoptiles under FRc-low, mesocotyls under all the light conditions, and the roots under FRp; in cpm2—for coleoptiles under FRp and FRc-high, and mesocotyls and roots under all the light conditions. The asterisks indicate the values of the mutants, which are significantly different from those of the wild type, and the diamonds point similarly to the values of cpm2 significantly different from those of hebiba. (From Sineshchekov et al. 2021a)
The role of phytochrome A in the regulation of (proto)chlorophyll biosynthesis
The phyA heterogeneity may also have a close relationship to the complexity of the regulation of chlorophyll (Chl) biosynthesis and the formation of photosynthetic structures (see the review Sineshchekov and Belyaeva 2019). One of the outcomes of this regulation is the synchronization of the accumulation of potentially dangerous photoreactive pigment species with the formation of the protein matrix providing effective channeling of excess light energy into heat. The Chl biosynthesis path comprises two major steps: (1) the dark stage — from the synthesis of aminolevulinic acid (ALA) to the appearance of protochlorophyllide forms (Pchlide633 and Pchlide655), and (2) the light stage — photoreduction of Pchlide655 into chlorophyllide, Chlide. Pchlide633 is a dark precursor of the active Pchlide655; it is incapable of photoconversion into Chlide but is likely to be the major cause of the appearance of harmful reactive oxygen species. Pchlide655 is a component of the tripartite complex comprising also photoenzyme protochlorophyllide oxidoreductase (POR) and NADPH (Pchlide–POR–NADPH) capable of photoconversion into Chlide. The photoconversion of Pchlide655 into Chlide is a complex process comprising two successive photoreactions and several dark stages. phyA was shown to be involved in the regulation of both the dark and the light stages of the process — from ALA to Pchlide655 and from Pchlide655 to Chlide.
Phenomenological manifestations of the regulation of the (proto)chlorophyll biosynthesis by phytochrome A include positive and negative effects and are experimentally observed as (1) potentiation of greening — the reduction of the lag of chlorophyll synthesis by short (hours) periods of FR before the transfer of a seedling to W (Lifschitz et al. 1990; Yanovsky et al. 1997) and (2) blocking of greening by prolonged FR (days) illumination of a seedling before transfer to W (Apel 1981; Batschauer and Apel 1984; Runge et al. 1996; Barnes et al. 1996; Armstrong et al. 1995; Luccioni et al. 2002). The modes of the phyA photoregulation of these effects fall well into the two categories — the VLFRs and HIRs.
Yanovsky et al. (1997, 2000) observed both the VLFR and HIR of potentiation of the greening of etiolated seedlings of Arabidopsis Ler ecotype. Also, in the experiments on Chl biosynthesis blocking on a fhy3-1 mutant, they showed that it retains the VLFRs but is severely impaired in the HIRs suggesting divergent signal pathways for the two modes of action in the Chl biosynthesis regulation.
Blocking of greening under FRp and FRc was followed with the use of Arabidopsis impaired in PKS1 or PKS2 (phytochrome kinase substrate) — pks1, pks2, and pks1 pks2 double mutants and transgenics overexpressing the PKS1 or PKS2 gene in (Lariguet et al. 2003). pks1 and pks2 showed enhanced responses to FRp but not to FRc. These results indicate that PKS1 and PKS2 specifically affected the VLFR pathway of phyA but not the HIR of phyA. This contrasts with the effect of the fhy3-1 mutation, which affects the HIR but not the VLFR (Yanovsky et al. 2000). This is yet another argument for the notion of the distinct pathways of the two modes of phyA-mediated regulation.
In our experiments, it was confirmed that the phyA regulation of Chl synthesis is primarily the HIR and that phyB may interfere with the action of phyA. In coleoptiles of WT rice and its phyB mutant grown under FRc and FRp, the effect of the light treatment was evaluated by direct measurements of Pchlide633 and Pchlide655 fluorescence proportional to their content in tissues (Sineshchekov et al. 2006). Under FRp (the VLFR conditions), there were no significant changes in the levels of both forms of protochlorophyllide in the WT, whereas a considerable decrease was observed for Pchlide633 (40%) and Pchlide655 (ca 100%) in the phyB mutant. In seedlings grown under FRc (the HIR conditions), the inhibitory effect of phyA on [Pchlide633] was much higher — [Pchlide633] decreased by threefold in WT and by sevenfold in the phyB mutant, and [Pchlide655] was almost completely absent in both the lines.
Kneissl et al. (2008) confirmed that phyA is responsible for suppressing Pchlide accumulation under FR using Arabidopsis deficient in phyB and both phyA and phyB and that phyB negatively affects the action of phyA. Of particular importance in the context of the current review is their observation that the rice mutant phyA SA ectopically expressed in phyB or phyAphyB Arabidopsis was considerably less efficient than the WT rice phyA in the Pchlide biosynthesis suppression under FRp (VLFR), whereas the effect of FRc (HIR) was similar in both the lines. Given that phyA SA is represented primarily or exclusively by the phyA″ species (i.e., lacking phyA′) (Sineshchekov et al. 2018), the above observation indicates that phyA″ is responsible for this HIR effect and phyA′, for the VLFR, and that phyB suppresses the phyA′ action.
In line with this assignment of the two different modes of the Chl biosynthesis regulation by phyA to the two phyA pools are the data on Δ6-12 phyA of oat expressed in Arabidopsis (Casal et al. 2002) The truncated phyA was hyperactive for the FRp blocking greening upon transfer to W in Arabidopsis (the VLFR), whereas the effect of FRc (the HIR) was reduced compared with the full-length (FL) phyA. Trupkin et al. (2007) have carried out similar experiments in a more physiological context — on transgenic Arabidopsis expressing Δ6-12 Arabidopsis PHYA. They showed normal responses to FRp and impaired responses to FRc. Besides, reduced light stability of deleted phyA was observed and the authors explain the reduced phyA physiological activity by the enhanced destruction of the mutated phyA. The data by Casal et al. (2002) can be thus straightforwardly explained as a manifestation of the functions of the phyA′ type — the dominating or the only phyA species present in the transgenic Arabidopsis expressing Δ6-12 (oat) phyA (Sineshchekov et al. 2014c). This may hold also in the case of Δ6-12 phyA of Arabidopsis (Trupkin et al. 2007) because of the location of the deletion and the fact that the truncated phyA has higher light lability what is the property of phyA′.
The phyA regulation of Chl biosynthesis and its dependence on the cellular context are becoming even more complicated by the fact that its sign and extent depend on plant species and organ/tissue (Sineshchekov et al. 2004a). Pchlide655 content in the upper stems of FRc-grown seedlings of pea and tobacco increased approx. tenfold as compared with the dark-grown. In the upper stems of Arabidopsis and tomato, the positive effect of FRc was low, 1.2- to 1.5-fold, and the negative effect of FR was seen in cotyledons. The regulation of Pchlide633 in contrast to Pchlide655 was positive independent of the plant species and tissue.
More to that, the phyA regulation of Chl accumulation depends on the hormonal status of the plant. In experiments on coleoptiles of rice hebiba deficient in JA, the content of Pchlide633 and Pchlide655 in the dark was higher in the mutant pointing to the inhibitory effect of the hormone on their biosynthesis in the WT. In seedlings grown under FR, the sign of the Pchlide655 regulation was found to depend on the mode of the light action (VLFR or HIR). In the wild type, FRp (VLFR) was stimulating whereas FRc (HIR) inhibiting (Fig. 8). In the mutant, both FRp and FRc stimulated the Pchlide655 biosynthesis, i.e., the sign of the FRc effect has changed from the negative in the WT to the positive in hebiba. Under FRc, [Pchlide633] remained the same as in the dark in the WT and grew in hebiba.
The content of the two protochlorophyllides ([Pchlide655] + [Pchlide.633]) in the coleoptiles of the wild-type seedlings of rice (Oryza sativa L. ssp. Japonica cv. Nihonmasari) and its hebiba and cpm2 mutants. The pigments were determined by fluorescence emission spectra. The effects of the illumination of the different regimes on the protochlorophyllides' content are not statistically significant in the wild type but they are in the mutants (indicated by an asterisk). For the illumination conditions, see the legend in Fig. 4. (From Sineshchekov et al. 2021a)
In our recent experiments on the same mutant hebiba and a similar mutant line cpm2, we observed a different picture (Sineshchekov et al. 2021a). The proportion of the two Pchlide species was rather conserved, it varied within a very narrow limit. So, the FR effects were evaluated by changes in the total Pchlide content, [Pchlide633] + [ Pchlide655]. In the WT, the FRc and FRp effects were insignificant, whereas in the mutants FRp was inhibiting and FRc stimulating (Fig. 8). This is at variance with the data on the WT rice (of a different var.) in (Sineshchekov et al. 2006), when FRc brought about a complete block of [Pchlide655] and a considerable decline in [Pchlide633], and on hebiba in (Sineshchekov et al. 2004b), when the FR effects were different for the two Pchlide species. The reversion of the sign of the FRc effect was also observed in the mutant (see above). This variability of the FR effects on Chl synthesis even in the same plant (rice) suggests their dependence on the physiological status of the plant, possibly, on its age. Differences in the illumination regimes and light spectrum may also affect the observed results (see the discussion in Sineshchekov et al. 2021a). Thus, phyA can differentially affect the biosynthesis of Pchlide under the VLFR and HIR conditions, and JA counteracts this action in WT (Fig. 9). The suppression of the phyA action by JA may include phyA destruction (see above), and the modulation by JA of the level of the phyA transporters into the nucleus — FHY1 and FHL (Liu and Wang 2020). In general, our data on phyA regulation of Chl biosynthesis in the JA mutants are in line with the notion that the signals from phyA and JA are mutually antagonistic (see reviews Hsieh and Okamoto 2014; Sineshchekov and Belyaeva 2019).
Hypothetical model of the relationship between phytochrome A pools (phyA′ and phyA″) and phytohormone jasmonic acid (JA) signaling in etiolated 5-day-old seedlings of rice grown under far-red illumination (FR) of different regimes — FR constant (FRc, the HIRs) and FR pulsed (FRp, the VLFRs). Thin arrows indicate signaling and thicker arrows, metabolic processes. “High” and “low” stand for the light signaling under FRc of different (by approx. 50-fold) fluences. Lines with arrowheads correspond to positive regulation, lines with blunt ends, to negative regulation. As one can see from the scheme, almost all the effects of JA on the light signals from phyA′ (FRp, VLFR) and phyA″ (FRc, the HIRs) are inhibiting, except for the three effects, when JA promotes phyA signaling – the phyA″ inhibition of phyA biosynthesis and root growth, and the stimulation of phyA′ destruction. Coleoptiles’ growth is not affected because of seedlings’ young age. (From Sineshchekov et al. 2021a)
To the functions of phyA in the cytoplasm
The difference in the hydrophilicity/hydrophobicity of the two phyA pools (see above) may relate to their functional activity. Given that the cytosol is also the site of phyA action (see discussion in Rösler et al. 2007; Jaedicke et al. 2012; Hughes 2013), we may hypothesize that the membrane-(protein-) associated fraction of phyA″ (phyA″m) is the most likely candidate for the initiation of the phyA-specific cytoplasmic responses. Rösler et al. (2007) have shown that the phyA-induced cytosolic photoresponses — R-enhanced phototropism, abrogation of gravitropism, and inhibition of hypocotyl elongation in blue light — are essentially the same in the WT Arabidopsis and in the fhl/fhy1 mutant defective in the light-induced nuclear import of phyA, that is they are identified as phyA-specific cytoplasmic responses. In our opinion, the soluble phyA′ and phyA″ fractions participate in the nuclear regulatory effects (see above), whereas phyA″m, in the cytosolic. This notion is supported by the fact that a small fraction of the phytochrome population is associated with phototropin and with PKS1, and that this association is required for phototropism (Lariguet et al 2006; Jaedicke et al 2012). It is tempting to hypothesize that the phyA″m pool could be responsible for the fast photoregulation effects in the cytoplasm, such as modulation of ion transport, electric potentials, and cytoplasm fluidity. This needs, however, direct experimental verification.
Conclusions
Phytochrome A grants plant’s survival under the conditions of deep vegetative shade when the ambient light is shifted towards domination of the far-red, photosynthetically impotent component. Its regulatory action critical at the very early stages of plant development includes two modes of the FR-induced photoresponses — the VLFRs and HIRs. This complex character of the phyA action is due, at least, partially, to the structural heterogeneity of the photoreceptor — the existence of its two native types, phyA′ and phyA″. phyA is synthesized in the cytoplasm in the unphosphorylated phyA″ form, which converts into phyA′, possibly, via serine phosphorylation at the NTE. phyA′ is water-soluble whereas phyA″ reveals ambiquitous properties — it is found in the liquid phase and associated with a membrane (phyA″m). The three different native phyA species correlate with the three distinct phyA complex formations observed after its light-induced nucleocytoplasmic partitioning — two types of speckles in the nucleus and a cytoplasmic fraction. In seeds, phyA is likely to be present primarily in the phyA″ form, where it participates in the regulation of seed germination. In seedlings, the two phyA species mediate different modes of photoresponses acting in the nucleus — phyA′ initiates the VLFRs, and phyA″, the HIRs, and, probably, the LFRs (Fig. 10). phyA″m may be responsible for the cytosolic activity of phyA including the regulation of photo- and gravitropism In this review, this attribution is demonstrated by the key photoregulation processes — light induction of seed germination, growth responses during de-etiolation, and Chl biosynthesis.
Working scheme of the state and functions of the native phytochrome A pools in etiolated seedlings. In seeds, phyA is likely to be present in the phyA″ form and participates in the regulation of seed germination. De novo synthesized phyA in germinating seeds and growing seedlings is initially in the phyA″ form, which possesses amphiphilic properties and is present in the cell in water-soluble and membrane- (protein-) associated (phyA″m) fractions. In darkness, phyA″ is converted into the water-soluble phyA′ form, possibly, via serine phosphorylation at the N-terminus of the molecule. Upon illumination, the water-soluble phyA′ and phyA″ are transported into the nucleus forming there two different types of nuclear speckles and inducing different modes of photoresponses, the VLFRs and HIRs, respectively. phyA″m in the Pfr form remains in the cytoplasm and initiates fast biochemical processes. Pointed arrows indicate the stimulation effects; blunt-ended arrows indicate the inhibitory effects
The sign and extent of the phyA photoresponses may depend on the plant’s species and organ/tissue, its developmental state, and hormonal status. This is evident, in particular, in the case of the phyA regulation of Chl synthesis when, under certain conditions, instead of its suppression there was its stimulation. The reversion of the sign of the phyA regulation of Pchlide655 accumulation was also observed in the rice mutant hebiba deficient in JA. It differentially affects the two phyA response types, the HIRs (mediated by phyA′′) and the VLFRs (mediated by phyA′), reducing primarily the phyA′′ effects (HIR). This may relate to JA participation in phyA destruction and suppression of the FR-induced phyA transport into the nucleus.
Several key questions in the concept of the structural and functional heterogeneity of phyA remain yet to be solved. This is, first, exact chemical differences between phyA′ and phyA″ granting their physicochemical and functional distinctions. The most straightforward approach here would be to get the two pools separately in a heterologous system and investigate them in vitro with the use of analytical chemistry and crystallography. Second, the exact functions and the mechanisms of action of the three native phyA pools are to be further investigated with the use of transgenic plants or mutants deficient in phyA′ or phyA″. And finally, this structural and functional heterogeneity of phyA is to be considered while exploring its interactions with the hormonal system.
References
Apel K (1981) The protochlorophyllide holochrome of barley (Hordeum vulgare, L.). Phytochrome induced decrease of translatable mRNA coding for the NADPH: protochlorophyllide oxidoreductase. Eur J Biochem 120:89–93. https://doi.org/10.1111/j.1432-1033.1981.tb05673.x
Armstrong GA, Runge S, Frick G, Sperling U, Apel K (1995) Identification of NADPH: protochlorophyllide oxidoreductases A and B: a branched pathway for light-dependent chlorophyll biosynthesis in Arabidopsis thaliana. Plant Physiol 108:1505–1517. https://doi.org/10.1104/pp.108.4.1505
Ballaré CL, Casal JJ (2000) Light signals perceived by crop and weed plants. Field Crop Res 67(2):149–160. https://doi.org/10.1016/s0378-4290(00)00090-3
Barnes SA, Nishizawa NK, Quaggio RB, Whitelam GC, Chua N-H (1996) Far-red light blocks greening of Arabidopsis seedlings via phytochrome A-mediated change in plastid development. Plant Cell 8:601–615. https://doi.org/10.1105/tpc.8.4.601
Batschauer A, Apel K (1984) An inverse control by phytochrome of the expression of two nuclear genes in barley (Hordeum vulgare L.). Eur J Biochem 143:593–597. https://doi.org/10.1111/j.1432-1033.1984.tb08411.x
Botto JF, Sánchez RA, Casal JJ (1995) Role of phytochrome B in the induction of seed germination by light in Arabidopsis thaliana. J Plant Physiol 146:307–312. https://doi.org/10.1016/S0176-1617(11)82059-6
Botto JF, Sanchez RA, Whitelam GC, Casal JJ (1996) Phytochrome A mediates the promotion of seed germination by very low fluences of light and canopy shade light in Arabidopsis. Plant Physiol 110:439–444. https://doi.org/10.1104/pp.110.2.439
Boylan MT, Quail PH (1989) Oat phytochrome is biologically active in transgenic tomatoes. Plant Cell 1:765–773. https://doi.org/10.1105/tps.1.8.765
Braslavsky SE, Gärtner W, Schaeffner K (1997) Phytochrome photoconversion. Plant Cell Environ 20:700–706. https://doi.org/10.1046/j.1365-3040.1997.d01-101.x
Brockman J, Schäfer E (1982) Analysis of Pfr destruction in Amarantus caudatus L. – evidence for two pools of phytochrome. Photochem Photobiol 35:555–558. https://doi.org/10.1111/j.1751_1097.1982.tb02608.x
Butler WL, Norris KH, Siegelman HW, Hendricks SB (1959) Detection, assay, and preliminary purification of the pigment controlling photoresponsive development in plants. PNAS USA 45:1703–1708. https://doi.org/10.1073/pnas.45.12.1703
Cantón FR, Quail PH (1999) Both phyA and phyB mediate light-imposed repression of PHYA gene expression in Arabidopsis. Plant Physiol 121:1207–1215. https://doi.org/10.1104/pp.121.4.1207
Casal JJ (2000) Phytochromes, cryptochromes, phototropin: photoreceptor interactions in plants. Photochem Photobiol 71:1–11. https://doi.org/10.1562/0031-8655(2000)0710001PCPII2.0.CO2
Casal JJ (2013) Photoreceptor signaling networks in plant responses to shade. Ann Review Plant Biol 64:403–427. https://doi.org/10.1146/annurev-arplant-050312-120221
Casal JJ, Sánchez RA (1998) Phytochromes and seed germination. Seed Sci Res 8:317–329. https://doi.org/10.1017/S0960258500004256
Casal JJ, Sánchez RA, Vierstra RD (1994) Avena phytochrome A overexpressed in transgenic tobacco seedlings differentially affects red/far-red reversible and very-low-fluence responses (cotyledon unfolding) during de-etiolation. Planta 192:306–309. https://doi.org/10.1007/BF00198564
Casal JJ, Sánchez RA, Botto JF (1998) Modes of action of phytochromes. J Exp Bot 49:127–138. https://doi.org/10.1093/jxb/49.319.127
Casal JJ, Davis SJ, Kirchenbauer D, Viczian A, Yanovsky MJ, Clough RC, Vierstra RD (2002) The serine-rich N-terminal domain of oat phytochrome A helps regulate light responses and subnuclear localization of the photoreceptor. Plant Physiol 129:1127–1137. https://doi.org/10.1104/pp.010977
Casal JJ, Candia AN, Sellaro R (2014) Light perception and signaling by phytochrome A. J Exp Bot 65:2835–2845. https://doi.org/10.1093/jxb/ert379
Cherry JR, Hershey HP, Vierstra RD (1991) Characterization of tobacco expressing functional oat phytochrome: domains responsible for the rapid degradation of Pfr are conserved between monocots and dicots. Plant Physiol 96:775–785. https://doi.org/10.1104/pp.96.3.775
Clack T, Mathews S, Sharrock RA (1994) The phytochrome apoprotein family in Arabidopsis is encoded by five genes: the sequences and expression of PHYD and PHYE. Plant Mol Biol 25:413–427. https://doi.org/10.1007/BF00043870
Clegg JS (1984) Properties and metabolism of the aqueous cytoplasm and its boundaries. Am J Physiol Regul Integr Comp Physiol 246:R133–R151. https://doi.org/10.1152/ajpregu.1984.245.2.R133
Clough RC, Vierstra RD (1997) Phytochrome degradation. Plant Cell Environ 20:713–721. https://doi.org/10.1046/j.1365_3040.1997.d01-107.x
Clough RC, Casal JJ, Jordan ET, Christou P, Vierstra RD (1995) Expression of functional oat phytochrome A in transgenic rice. Plant Physiol 109:1039–1045. https://doi.org/10.1104/pp.109.3.1039
Duek PD, Fankhauser C (2003) HFR1, a putative bHLH transcription factor, mediates both phytochrome A and cryptochrome signalling. Plant J 34:827–836. https://doi.org/10.1046/j.1365-313X.2003.01770.x
Franklin KA, Quail PH (2010) Phytochrome functions in Arabidopsis development. J Exp Bot 61:11–24. https://doi.org/10.1104/pp.109.3.1039
Garg AK, Sawers RJ, Wang H, Kim JK, Walker JM, Brutnell T, Wu RJ (2006) Light-regulated overexpression of an Arabidopsis phytochrome A gene in rice alters plant architecture and increases grain yield. Planta 223:627–636. https://doi.org/10.1007/s00425-005-0101-3
Halliday KJ, Bolle C, Chua NH, Whitelam GC (1999) Overexpression of rice phytochrome A partially complements phytochrome B deficiency in Arabidopsis. Planta 207:401–409. https://doi.org/10.1007/s004250498
Han YJ, Kim HS, Kim YM, Shin AY, Lee SS, Bhoo SH, Kim JI (2010) Functional characterization of phytochrome autophosphorylation in plant light signaling. Plant Cell Physiol 51:596–609. https://doi.org/10.1093/pcp/pcq025
Hauser BA, Pratt LH, Cordonnier-Pratt MM (1997) Absolute quantification of five phytochrome transcripts in seedlings and mature plants of tomato (Solanum lycopersicum L.). Planta 201(3):379–387. https://doi.org/10.1007/s004250050080
Hennig L, Poppe SU, Martin A, Schafer E (2001) Negative interference of endogenous phytochrome B with phytochrome A function in Arabidopsis. Plant Physiol 125:1036–1044. https://doi.org/10.1104/pp.125.2.1036
Hiltbrunner A, Viczián A, Bury E, Tscheuschler A, Kircher S, Tóth R, Schäfer E (2005) Nuclear accumulation of the phytochrome A photoreceptor requires FHY1. Curr Biol 15:2125–2130. https://doi.org/10.1016/j.cub.2005.10.042
Hilton JR, Thomas B (1985) A comparison of seed and seedling phytochrome in Avena sativa L. using monoclonal antibodies. J Exp Bot 36:1937–1946. https://doi.org/10.1093/jxb/36.12.1937
Hoang QT, Han YJ, Kim JI (2019) Plant phytochromes, and their phosphorylation. Int J Mol Sci 20:3450. https://doi.org/10.3390/ijms20143450
Hoang QT, Cho J, Choi DM, Shin AY, Kim JA, Han YJ, and Kim JI (2021) Protein kinase activity of phytochrome A positively correlates with photoresponses in Arabidopsis. Front Plant Sci 1621. https://doi.org/10.3389/fpls.2021.706316
Hsieh HL, Okamoto H (2014) Molecular interaction of jasmonate and phytochrome A signaling. J Exp Bot 65:2847–2857. https://doi.org/10.1093/jxb/eru230
Hughes J (2013) Phytochrome cytoplasmic signaling. Annu Rev Plant Biol 64:377–402. https://doi.org/10.1146/annurev-arplant-050312-120045
Jaedicke K, Lichtenthäler AL, Meyberg R, Zeidler M, Hughes J (2012) A phytochrome–phototropin light signaling complex at the plasma membrane. PNAS USA 109:12231–12236. https://doi.org/10.1073/pnas.1120203109
Johnson E, Bradley M, Harberd NP, Whitelam GC (1994) Photoresponses of light-grown phyA mutants of Arabidopsis (phytochrome A is required for the perception of daylength extensions). Plant Physiol 105:141–149. https://doi.org/10.1104/pp.105.1.141
Kendrick RE (1976) Photocontrol of seed germination. Sci Prog (1933-):347–367. https://www.jstor.org/stable/4340371
Kerckhoffs LHJ, Schreuder MEL, Tuinen AV, Koornneef M, Kendrick RE (1997) Phytochrome control of anthocyanin biosynthesis in tomato seedlings: analysis using photomorphogenic mutants. Photochem Photobiol 65:374–381. https://doi.org/10.1111/j.1751-1097.tb08573.x
Kim DH, Yamaguchi S, Lim S, Oh E, Park J, Hanada A, Choi G (2008) SOMNUS, a CCCH-type zinc finger protein in Arabidopsis, negatively regulates light-dependent seed germination downstream of PIL5. Plant Cell 20:1260–1277. https://doi.org/10.1105/tpc.108.058859
Klose C, Viczián A, Kircher S, Schäfer E, Nagy F (2015) Molecular mechanisms for mediating light-dependent nucleo/cytoplasmic partitioning of phytochrome photoreceptors. New Phytol 206:965–971. https://doi.org/10.1111/nph.13207
Kneissl J, Shinomura T, Furuya M, Boll C (2008) A rice phytochrome A in Arabidopsis: the role of the N-terminus under red and far-red light. Mol Plant 1:84–102. https://doi.org/10.1093/mp/ssm010
Kneissl J, Wachtler V, Chua N, and Bolle C (2009) OWL1: an Arabidopsis J-domain protein involved in perception of very low light fluences. Plant Cell 3212–3225. https://doi.org/10.1105/tpc.109.066472
Lamparter T, Lutterbuse P, Schneider-Poetsch HAW, Hertel R (1992) A study of membrane-associated phytochrome: hydrophobicity test and native size determination. Photochem Photobiol 56:697–707. https://doi.org/10.1111/j.1751-1097.1992.tb02224.x
Lariguet P, Boccalandro HE, Alonso JM, Ecker JR, Chory J, Casal JJ, Fankhauser C (2003) A growth regulatory loop that provides homeostasis to phytochrome A signaling. Plant Cell 15:2966–2978. https://doi.org/10.1105/tpc.014563
Lariguet P, Schepens I, Hodgson D, Pedmale UV, Trevisan M, Kami C et al (2006) PHYTOCHROME KINASE SUBSTRATE 1 is a phototropin 1 binding protein required for phototropism. PNAS USA 103:10134–10139. https://doi.org/10.1073/pnas.0603799103
Lee N, Choi G (2017) Phytochrome-interacting factor from Arabidopsis to liverwort. Curr Opin Plant Biol 35:54–60. https://doi.org/10.1016/j.pbi.2016.11.004
Li FW, Melkonian M, Rothfels CJ, Villarreal JC, Stevenson DW, Graham SW, Wong GK, Pryer KM, Mathews S (2015) Phytochrome diversity in green plants and the origin of canonical plant phytochromes. Nat Commun 6:7852. https://doi.org/10.1038/ncomms8852.PMID:26215968;PMCID:PMC4525182
Lifschitz S, Gepstein S, Horwitz BA (1990) Phytochrome regulation of greening in wild type and long-hypocotyl mutants of Arabidopsis thaliana. Planta 2:234–238. https://doi.org/10.1007/BF02411544
Liu Y, Wang H (2020) JA modulates phytochrome a signaling via repressing FHY3 activity by JAZ proteins. Plant Signal Behav 15:1726636. https://doi.org/10.1080/15592324.2020.1726636
Luccioni LG, Oliverio KA, Yanovsky MJ, Boccalandro HE, Casal JJ (2002) Brassinosteroid mutants uncover fine tuning of phytochrome signaling. Plant Physiol 128:173–181. https://doi.org/10.1104/pp.010668
Magliano TA, Casal JJ (2004) Pre-germination seed–phytochrome signals control stem extension in dark-grown Arabidopsis seedlings. Photochem Photobiol Sci 3:612–616. https://doi.org/10.1039/b315511k
Mateos JL, Luppi JP, Ogorodnikova OB, Sineshchekov VA, Yanovsky MJ, Braslavsky SE, Casal JJ (2006) Functional and biochemical analysis of the N-terminal domain of phytochrome A. J Biol Chem 281:34421–34429. https://doi.org/10.1074/jbc.M603538200
Mathews S, Burleigh JG, Donoghue MJ (2003) Adaptive evolution in the photosensory domain of phytochrome A in early angiosperms. Mol Biol Evol 20:1087–1097. https://doi.org/10.1093/molbev/msg123
Mazzella MA, Alconada Magliano TM, Casal JJ (1997) Dual effect of phytochrome A on hypocotyl growth under continuous red light. Plant, Cell Environ 20(2):261–267. https://doi.org/10.1046/j.1365-3040.1997.d01-62.x
Nagatani A, Reed JW, Chory J (1993) Isolation and initial characterization of Arabidopsis mutants that are deficient in phytochrome A. Plant Physiol 102:269–277. https://doi.org/10.1104/pp.102.1.269
Nagy F, Kircher S, Schafer E (2001) Intracellular trafficking of photoreceptors during light-induced signal transduction in plants. J Cell Sci 114:475–480. https://doi.org/10.1242/jcs.114.3.475
Neff MM, Chory J (1998) Genetic interactions between phytochrome A, phytochrome B, and cryptochrome 1 during Arabidopsis development. Plant Physiol 118:27–35. https://doi.org/10.1104/pp.118.1.27
Oh E, Kim J, Park E, Kim JI, Kang C, Cho G (2004) PIL5, a phytochrome-interacting basic helix-loop-helix protein, is a key negative regulator of seed germination in Arabidopsis thaliana. Plant Cell 16:3045–3058. https://doi.org/10.1105/tpc.104.025163
Okamoto H, Sakamoto K, Tomizawa K, Nagatani A, Wada M (1997) Photoresponses of transgenic Arabidopsis overexpressing the fern Adiantum capillus-veneris PHY1. Plant Physiol 115:79–85. https://doi.org/10.1104/pp.115.1.79
Reed JW, Nagatani A, Elich TD, Fagan M, Chory J (1994) Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol 104:1139–1149. https://doi.org/10.1104/pp.104.4.1139
Rieman M, Haga K, Shimizu T, Okad K, Ando, Mochizuki S, Iino M (2013) Identification of rice Allene Oxide Cyclase mutants and the function of jasmonate for defence against Magnaporthe oryzae. Plant J 74:226–238. https://doi.org/10.1104/pp.103.027490
Riemann M, Müller A, Korte A, Furuya M, Weiler EW, Nick P (2003) Impaired induction of the jasmonate pathway in the rice mutant hebiba. Plant Physiol 133:1820–1830. https://doi.org/10.1007/s00425-009-0891-9
Riemann M, Bouyer D, Hisada A, Müller A, Yatou O, Weiler EW et al (2009) Phytochrome A requires jasmonate for photodestruction. Planta 229:1035–1045. https://doi.org/10.1111/tpj.12115
Rösler J, Klein I, Zeidler M (2007) Arabidopsis fhl/fhy1 double mutant reveals a distinct cytoplasmic action of phytochrome A. Proc Natl Acad Sci 104:10737–10742. https://doi.org/10.1073/pnas.0703855104
Runge S, Sperling U, Frick G, Apel K, Armstrong GA (1996) Distinct roles for light-dependent NADPH:protochlorophyllide oxidoreductases A and B during greening in higher plants. Plant J 9:513–523. https://doi.org/10.1046/j.1365-313X.1996.09040513.x
Seo M, Nambara E, Choi G, Yamaguchi S (2009) Interaction of light and hormone signals in germinating seeds. Plant Mol Biol 69:463–472. https://doi.org/10.1007/s11103-008-9429-y
Sharrock RA, Quail PH (1989) Novel phytochrome sequences in Arabidopsis thaliana: structure, evolution, and differential expression of a plant regulatory photoreceptor family. Genes Dev 3:1745–1757. https://doi.org/10.1101/gad.3.11.1745
Sheerin DJ, Hiltbrunner A (2017) Molecular mechanisms and ecological function of far-red light signalling. Plant, Cell Environ 40:2509–2529. https://doi.org/10.1111/pce.12915
Shimazaki Y, Pratt LH (1985) Immunochemical detection with rabbit polyclonal and mouse monoclonal antibodies of different pools of phytochrome from etiolated and green Avena shoots. Planta 164:333–344. https://doi.org/10.1007/BF00402944
Shimizu H, Shinomura, Yamamoto KT (2010) Similarities and differences between phytochrome-mediated growth inhibition of coleoptiles and seminal roots in rice seedlings. Plant signal behav 5:134–135. https://doi.org/10.4161/psb.5.2.10335
Shin AY, Han YJ, Baek A, Ahn T, Kim SY, Nguyen TS, Kim JI (2016) Evidence that phytochrome functions as a protein kinase in plant light signalling. Nat Commun 7:1–13. https://doi.org/10.1038/ncomms11545
Shinomura T, Nagatani A, Chory J, Furuya M (1994) The induction of seed germination in Arabidopsis thaliana is regulated principally by phytochrome B and secondarily by phytochrome A. Plant Physiol 104:363–371. https://doi.org/10.1104/pp.104.2.363
Shinomura T, Nagatani A, Hanzawa H, Kubota M, Watanabe M, Furuya M (1996) Action spectra for phytochrome A-and B-specific photoinduction of seed germination in Arabidopsis thaliana. Proc Natl Acad Sci 93:8129–8133. https://doi.org/10.1073/pnas.93.15.8129
Shinomura T, Uchida K, Furuya M (2000) Elementary processes of photoperception by phytochrome A for high-irradiance response of hypocotyl elongation in Arabidopsis. Plant Physiol 122:147–156. https://doi.org/10.1104/pp.122.1.147
Sineshchekov VA (1994) Two spectroscopically and photochemically distinguishable phytochromes in etiolated seedlings of monocots and dicots Photochem. Photobiol 59:77–86. https://doi.org/10.1111/j.1751-1097.1994.tb05004.x
Sineshchekov VA (1995) Photobiophysics and photobiochemistry of the heterogeneous phytochrome system. Biochim Biophys Acta BBA-Bioenergetics 1228:125–164. https://doi.org/10.1016/0005-2728(94)00173-3
Sineshchekov VA (1995) Evidence for the existence of two phytochrome A populations. J Photochem Photobiol b: Biol 28:53–55. https://doi.org/10.1016/1011-1344(94)07094-5
Sineshchekov V (2004) Phytochrome A: functional diversity and polymorphism. Photochem Photobiol Sci 3:596–607. https://doi.org/10.1039/B315430K
Sineshchekov VA (2006) Extreme dehydration of plant tissues irreversibly converts the major and variable phyA′ into the minor and conserved phyA ″. J Photochem Photobiol b: Biol 85:85–91. https://doi.org/10.1016/j.jphotobiol.2006.05.003
Sineshchekov V (2019) Two molecular species of phytochrome A with distinct modes of action. Funct Plant Biol 46:118–135. https://doi.org/10.1071/FP18156
Sineshchekov VA, Akhobadze VV (1992) Phytochrome states in etiolated pea seedlings: fluorescence and primary photoreactions at low temperatures. Photochem Photobiol 56:743–749. https://doi.org/10.1111/j.1751-1097.1992.tb02229.x
Sineshchekov VA, Bekasova OD (2020) Two distinct photoprocesses in cyanobacterial bilin pigments: Energy migration in light-harvesting phycobiliproteins versus photoisomerization in phytochromes. Photochem Photobiol 96:750–767. https://doi.org/10.1111/php.13197
Sineshchekov VA, Belyaeva OB (2019) Regulation of chlorophyll biogenesis by phytochrome A. Biochem Mosc 84:491–508. https://doi.org/10.1134/S0006297919050043
Sineshchekov V, Fankhauser C (2004) PKS1 and PKS2 affect the phyA state in etiolated Arabidopsis seedlings. Photochem Photobiol Sci 3:608–611. https://doi.org/10.1039/b315431a
Sineshchekov VA, Sineshchekov AV (1987) Fluorescence of phytochrome in the cells of etiolated pea seedlings. Biophysics 32:116–122
Sineshchekov VA, Sineshchekov AV (1989) Fluorescence of phytochrome in the cells of dark-grown plants and its connection with the phototransformations of the pigment. Photochem Photobiol 49:325–330. https://doi.org/10.1111/j.1751-1097.1989.tb04114.x
Sineshchekov VA, Sineshchekov AV (1990) Different photoactive states of the red phytochrome form in the cells of etiolated pea and oat seedlings. J Photochem Photobiol b: Biol 5:197–217. https://doi.org/10.1016/1011-1344(90)80006-J
Sineshchekov VA, Weller JL (2004) Two modes of the light-induced phytochrome A decline — with and without changes in the proportion of its isoforms (phyA′ and phyA ″): evidence from fluorescence investigations of mutant phyA-3D pea. J Photochem Photobiol b: Biol 75:127–135. https://doi.org/10.1016/j.jphotobiol.2004.05.010
Sineshchekov AV, Koppel LA, Sineshchekov VA, Mokronosov AT (1989) Dynamics of phytochrome content and photoactivity in germinating pea and bean seedlings. Fisiol Rast 36:213–221
Sineshchekov V, Lamparter T, Hartmann E (1994) Evidence for the existence of membrane-associated phytochrome in the cell. Photochem Photobiol 60:516–520. https://doi.org/10.1111/j.1751-1097.1994.tb05143.x
Sineshchekov VA, Frances S, White MJ (1995) Fluorescence and photochemical characterization of phytochrome in de-etiolated pea mutant lip. J Photochem Photobiol b: Biol 28:47–51. https://doi.org/10.1016/1011-1344(94)07093-4
Sineshchekov VA, Heyer AG, Gatz C (1996) Phytochrome states in transgenic potato plants with altered phytochrome A levels. J Photochem Photobiol b: Biol 34:137–142. https://doi.org/10.1016/1011-1344(96)07284-3
Sineshchekov VA, Ogorodnikova OB, Devlin PF, Whitelam GC (1998) Fluorescence spectroscopy and photochemistry of phytochromes A and B in wild-type, mutant and transgenic strains of Arabidopsis thaliana. J Photochem Photobiol b: Biol 42:133–142. https://doi.org/10.1016/S1011-1344(97)00133-4
Sineshchekov VA, Ogorodnikova OB, Weller JL (1999) Fluorescence and photochemical properties of phytochromes A and B in etiolated pea seedlings. J Photochem Photobiol b: Biol 49:204–211. https://doi.org/10.1016/S1011-1344(99)00061-5
Sineshchekov VA, Clough RC, Jordan-Beebe ET, Vierstra RD (1999) Fluorescence analysis of oat phyA deletion mutants expressed in tobacco suggests that the N-terminal domain determines the photochemical and spectroscopic distinctions between phyA′ and phyA ″. Photochem Photobiol 69:728–732. https://doi.org/10.1111/j.1751-1097.1999.tb03354.x
Sineshchekov V, Ogorodnikova O, Thiele A, Gatz C (2000) Fluorescence and photochemical characterization of phytochromes A and B in transgenic potato expressing Arabidopsis phytochrome B. J Photochem Photobiol b: Biol 59:139–146. https://doi.org/10.1016/S1011-1344(00)00151-2
Sineshchekov V, Hennig L, Lamparter T, Hughes J, Gärtner W, Schäfer E (2001a) Recombinant phytochrome A in yeast differs by its spectroscopic and photochemical properties from the major phyA′ and is close to the minor phyA: evidence for post-translational modification of the pigment in plants. Photochem Photobiol 73:692–696. https://doi.org/10.1562/0031-8655(2001)0730692RPAIYD2.0.CO2
Sineshchekov V, Koppel L, Shlumukov L, Barro F, Barcelo P, Lazzeri P, Smith H (2001b) Fluorescence and photochemical properties of phytochromes in wild-type wheat and a transgenic line overexpressing an oat phytochrome A (PHYA) gene: functional implications. Plant, Cell Environment 24:1289–1297. https://doi.org/10.1046/j.1365-3040.2001.00780.x
Sineshchekov V, Belyaeva O, Sudnitsin A (2004) Up-regulation by phytochrome A of the active protochlorophyllide, Pchlide655, biosynthesis in dicots under far-red light J Photochem Photobiol b: Biol 74:47–54. https://doi.org/10.1016/j.jphotobiol.2004.02.001
Sineshchekov VA, Loskovich AV, Riemann M, Nick P (2004) The jasmonate-free rice mutant hebiba is affected in the response of phyA′/phyA′′ pools and protochlorophyllide biosynthesis to far-red light. Photochem Photobiol Sci 3:1058–1062.
Sineshchekov V, Loskovich A, Inagaki N, Takano M (2006) Two native pools of phytochrome A in monocots: Evidence from fluorescence investigations of phytochrome mutants of rice. Photochem Photobiol 82:1116–1122. https://doi.org/10.1562/2005-12-10-ra-749
Sineshchekov V, Koppel L, Shor E, Kochetova G, Galland P, Zeidler M (2013) Protein phosphatase activity and acidic/alkaline balance as factors regulating the state of phytochrome A and its two native pools in the plant cell. Photochem Photobiol 89:83–96. https://doi.org/10.1111/j.1751-1097.2012.01226.x
Sineshchekov V, Koppel L, Okamoto H, Wada M (2014) Fern Adiantum capillus-veneris phytochrome 1 comprises two native photochemical types similar to seed plant phytochrome A. J Photochem Photobiol b: Biol 130:20–29. https://doi.org/10.1016/j.jphotobiol.2013.10.007
Sineshchekov V, Mailliet J, Psakis G, Feilke K, Kopycki J, Zeidler M, Hughes J (2014) Tyrosine 263 in Cyanobacterial Phytochrome C ph1 Optimizes Photochemistry at the prelumi-R→ lumi-R Step. Photochem Photobiol 90:786–795. https://doi.org/10.1111/php.12263
Sineshchekov V, Sudnitsin A, Ádám É, Schäfer E, Viczián A (2014) phyA-GFP is spectroscopically and photochemically similar to phyA and comprises both its native types, phyA’and phyA”. Photochem Photobiol Sci 13:1671–1679. https://doi.org/10.1039/c4pp00220b
Sineshchekov VA, Koppel LA, Bolle C (2018) Two native types of phytochrome A, phyAʹ and phyAʹʹ, differ by the state of phosphorylation at the N-terminus as revealed by fluorescence investigations of the Ser/Ala mutant of rice phyA expressed in transgenic Arabidopsis. Funct Plant Biol 45:150–159. https://doi.org/10.1071/FP16261
Sineshchekov V, Koppel L, Kim JI (2019) The dephosphorylated S8A and S18A mutants of (oat) phytochrome A comprise its two species, phyA’and phyA’’, suggesting that autophosphorylation at these sites is not involved in the phyA differentiation. Photochem Photobiol Sci 18:1242–1248. https://doi.org/10.1039/c8pp00574e
Sineshchekov V, Koppel L, Riemann M, Nick P (2021a) Phytochrome A and its functional manifestations in etiolated and far-red light-grown seedlings of the wild-type rice and its hebiba and Cpm2 mutants deficient in the defense-related phytohormone jasmonic acid. Photochem Photobiol 97:335–342. https://doi.org/10.1111/php.13340
Sineshchekov V, Shor E, Koppel L (2021b) The phosphatase/kinase balance affects phytochrome A and its native pools, phyA′, and phyA″, in etiolated maize roots: evidence from the induction of phyA′ destruction by a protein phosphatase inhibitor sodium fluoride. Photochem Photobiol Sci 20:1429–1437. https://doi.org/10.1007/s43630-021-00110-1
Sineshchekov VA (2010) Fluorescence and photochemical investigations of phytochrome in higher plants. J Bot 2010:15. https://doi.org/10.1155/2010/358372
Somers DE, Quail PH (1995) Temporal and spatial expression patterns of PHYA and PHYB genes in Arabidopsis. Plant J 7(3):413–427. https://doi.org/10.1046/j.1365-313X.1995.7030413.x
Song PS, Park MH, Furuya M (1997) Chromophore: apoprotein interactions in phytochrome A. Plant, Cell Environ 20:707–712. https://doi.org/10.1046/j.1365-3040.1997.d01-103.x
Song C, Mroginski MA, Lang C, Kopycki J, Gärtner MJ, Hughes J (2018) 3D structures of plant phytochrome A as Pr and Pfr from solid-state NMR: implications for molecular function. Front Plant Sci 9:498. https://doi.org/10.3389/fpls.2018.00498
Spruit CJP, Mancinelli AL (1969) Phytochrome in cucumber seeds. Planta 88:303–310. https://doi.org/10.1007/BF00387458
Su L, Hou P, Song M, Zheng X, Guo L, Xiao Y, Yang J (2015) Synergistic and antagonistic action of phytochrome (Phy) A and PhyB during seedling de-etiolation in Arabidopsis thaliana. Int J Mol Sci 16:12199–12212. https://doi.org/10.3390/ijms160612199
Sullivan S, Hart JE, Rasch P, Walker CH, Christie JM (2016) Phytochrome A mediates blue-light enhancement of second-positive phototropism in Arabidopsis. Front Plant Sci 7:290. https://doi.org/10.3389/fpls.2016.00290
Takaki M (2001) New proposal of classification of seeds based on forms of phytochrome instead of photoblastism. Rev Bras Fisiol Veg 13:104–108. https://doi.org/10.1590/s0103-31312001000100011
Takano M, Kanegae H, Shinomura T, Miyao A, Hirochika H, Furuya M (2001) Isolation and characterization of rice phytochrome A mutants. Plant Cell 13:521–534. https://doi.org/10.1105/tpc.13.3.521
Takano M, Inagaki N, Xie X, Yuzurihara N, Hihara F, Ishizuka T et al (2005) Distinct and cooperative functions of phytochromes A, B, and C in the control of deetiolation and flowering in rice. Plant Cell 17:3311–3325. https://doi.org/10.1105/tpc.105.035899
Tepperman JM, Hwang YS, Quail PH (2006) phyA dominates in transduction of red-light signals to rapidly responding genes at the initiation of Arabidopsis seedling de-etiolation. Plant J 48:728–742. https://doi.org/10.1111/j.1365-313X.2006.02914.x
Terry MJ, Hall JL, Thomas B (1992) The association of type I phytochrome with wheat leaf plasma membranes. J Plant Physiol 140:691–698. https://doi.org/10.1016/S0176-1617(11)81025-4
Tobin EM, Briggs WR (1969) Phytochrome in embrios of Pinus palustris. Plant Physiol 44:148–150. https://doi.org/10.1104/pp.44.1.148
Tokuhisa JG, Quail PH (1987) The levels of two distinct species of phytochrome are regulated differently during germination in Avena sativa L. Planta 172:371–377. https://doi.org/10.1007/BF00398666
Trupkin SA, Debrieux D, Hiltbrunner A, Fankhauser C, Casal JJ (2007) The serine-rich N-terminal region of Arabidopsis phytochrome A is required for protein stability. Plant Mol Biol 63:669–678. https://doi.org/10.1007/s11103-006-9115-x
Vázquez-Yanes C, Orozco-Segovia A (1993) Patterns of seed longevity and germination in the tropical rainforest. Annu Rev Ecol Syst 24:69–87. https://doi.org/10.1146/annurev.es.24.110193.000441
Weller JL, Batge SL, Smith JJ, Kerckhoffs LHJ, Sineshchekov VA, Murfet IC, Reid JB (2004) A dominant mutation in the pea PHYA gene confers enhanced responses to light and impairs the light-dependent degradation of phytochrome A. Plant Physiol 135:2186–2195. https://doi.org/10.1104/pp.103.036103
Whitelam GC, Johnson E, Peng J, Carol P, Anderson ML, Cowl JS, Harberd NP (1993) Phytochrome A null mutants of Arabidopsis display a wild-type phenotype in white light. Plant Cell 5:757–768. https://doi.org/10.1105/tpc.5.7.757
Wu FQ, Fan CM, Zhang XM, Fu YF (2013) The phytochrome gene family in soybean and a dominant negative effect of a soybean PHYA transgene on endogenous Arabidopsis PHYA. Plant Cell Rep 32:1879–1890. https://doi.org/10.1007/s00299-013-1500-8
Xie X, Shinomura T, Inagaki N, Kiyota S, Takano M (2007) Phytochrome-mediated inhibition of coleoptile growth in rice: age-dependency and action spectra. Photochem Photobiol 83:131–138. https://doi.org/10.1562/2006-03-17-ra-850
Yanovsky M, Casal J, Luppi J (1997) The VLF loci, polymorphic between ecotypes Landsberg erecta and Columbia, dissect two branches of phytochrome A signal transduction that correspond to very-low-fluence and high-irradiance responses. Plant J 12:659–667. https://doi.org/10.1046/j.1365-313X.1997.d01-19.x
Yanovsky MJ, Whitelam GC, Casal JJ (2000) fhy3-1 retains inductive responses of phytochrome A. Plant Physiol 123:235–242. https://doi.org/10.1104/pp.123.1.235
Yanovsky MJ, Luppi JP, Kirchbauer D, Ogorodnikova OB, Sineshchekov VA, Adam E, Casal JJ (2002) Missense mutation in the PAS2 domain of phytochrome A impairs subnuclear localization and a subset of responses. Plant Cell 14:1591–1603. https://doi.org/10.1105/tpc.000521
Zeidler M, Zhou Q, Sarda X, Yau CP, Chua NH (2004) The nuclear localization signal and the C-terminal region of FHY1 are required for transmission of phytochrome A signals. Plant J 40:355–365. https://doi.org/10.1111/j.1365-313X.2004.02212.x
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
Human subjects were not involved in the research.
Consent to publication
Individuals were not involved in this study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dedicated to Professor Y. A. Vladimirov on his 90th birthday.
Rights and permissions
About this article
Cite this article
Sineshchekov, V., Koppel, L. Phytochrome A in plants comprises two structurally and functionally distinct populations — water-soluble phyA′ and amphiphilic phyA″. Biophys Rev 14, 905–921 (2022). https://doi.org/10.1007/s12551-022-00974-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12551-022-00974-2